Abstract
Background
Family history of type 2 diabetes mellitus (T2DM) is one of risk factors for that in future a subject can develop diabetes. Insulin resistance (IR) is important in the pathogenesis of T2DM. There is evidence that oxidative stress plays an important role in the etiology and/or progression of diabetes. Myeloperoxidase (MPO) participates in developing of inflammation. The objective was to investigate if MPO is associated with IR and inflammation in individuals with first-degree relatives of T2DM.
Methods
Cross-sectional study in 84 overweight individuals with family history of T2DM divided in two groups according to IR, group with IR (homeostasis model assessment [HOMA] ≥2.5; n=43) and control group (CG; HOMA <2.5; n=41). Complete clinical history and a venous blood sample were collected for measuring glucose and lipids profile, insulin, interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), MPO, glutathione reductase (GRd), glutathione peroxidase, and superoxide dismutase.
Results
MPO, TNF-α, and IL-6 were higher in patients with IR than in CG (MPO: 308.35 [190.85 to 445.42] vs. 177.35 [104.50 to 279.85], P=0.0001; TNF-α: 13.46 [10.58 to 18.88] vs. 9.39 [7.53 to 11.25], P=0.0001; IL-6: 32.93 [24.93 to 38.27] vs. 15.60 [12.93 to 26.27]; P=0.0001, respectively). MPO was associated with IR (rho de Spearman=0.362, P=0.001). In the analysis of lineal regression, MPO predicts IR (β, 0.263; t, 2.520; P=0.014). In the univariate analysis, MPO had an odds ratio of 9.880 for risk of IR (95% confidence interval, 2.647 to 36.879).
Conclusion
MPO had relation with IR and inflammation parameters in overweight subjects with first-degree relatives of T2DM. We need studies on a casual relationship and molecular mechanisms among the increased serum MPO levels, inflammation markers, and IR.
Keywords: First-degree relatives of type 2 diabetes mellitus, Insulin resistance, Myeloperoxidase
INTRODUCTION
Familial history of type 2 diabetes mellitus (T2DM) is one of the dominant risk factors for that in future a subject can develop diabetes. Both, insulin resistance (IR) and β-cell dysfunction are critically important in the pathogenesis of the hyperglycemia of T2DM [1].
Overweight, obesity and impaired glucose tolerance are commonly factors founded in first degree relatives of diabetic patients [2]. However, genetic predisposition has been demonstrated as the primary cause of T2DM independently of increase in body fat and the IR may be a primary abnormality in the pathogenesis of this disease [3].
There is evidence that oxidative stress and reactive oxygen species (ROS) play an important role in the etiology and/or progression of diabetes [4,5]. Oxidative stress is mediated by the intracellular accumulation of ROS; it has been implicated in pathological processes such as obesity, diabetes, cardiovascular disease, and atherogenic processes. Moreover, the excess of body fat may induce systemic oxidative stress and, in turn, it is associated with an irregular production of adipokines, which contributes to the development of the IR and inflammation [6].
Oxidative stress process is due to the production of ROS and the impairment of antioxidant enzymatic defenses such as superoxide dismutase (SOD) or glutathione peroxidase (GPx) [7].
Free radicals of importance in living organisms include hydroxyl (OH·), superoxide (O2·-) nitric oxide (NO·), and peroxyl (RO2·). Peroxynitrite (ONOO-), hypochlorous acid (HOCl), hydrogen peroxide (H2O2), singlet oxygen (1O2), and ozone (O3) are not free radicals but can easily lead to free-radical reactions in living organisms. The term "reactive oxygen species" is often used to include not only the radicals OH·, RO2·, NO·, and O2·- but also the non-radicals HOCl, 1O2, ONOO-, O3, and H2O2 [8].
Myeloperoxidase (MPO) is a heme enzyme which is the major protein in neutrophils and, to a lesser extent, in monocytes. MPO uses H2O2 to generate HOCl that is a potent bactericidal agent, generating ROS [9]. MPO plays an essential part in the innate immune system by catalyzing the production of HOCl. However, MPO has also been implicated as a very harmful agent in an increasing number of inflammatory-mediated disorders [10]. A strong correlation between serum MPO levels and increased risk of subsequent cardiovascular diseases has been demonstrated in patients with acute coronary syndrome [11]. This is evidence for a role of MPO as mediator of vascular inflammation and the generation of oxidant species, including HOCl in the pathophysiology of inflammatory diseases. Therefore, the aim of this study was to evaluate the association between serum levels of MPO with IR and inflammation in overweight subjects with first-degree relatives with T2DM.
METHODS
Subjects
A cross-sectional case-control study was performed in a period of January to September of 2013. Eighty-four overweight subjects with first-degree relatives with T2DM of Family Medicine Unit (FMU) N° 80 of Mexican Institute of Social Security (IMSS) in Morelia, Michoacán, Mexico were included. The participation of subjects was voluntary, based on the invitation. The characteristics of overweight subjects with familial history of T2DM were both sex, 30 to 60 years old and body mass index (BMI) between 25 and 29.9 kg/m2. Subjects with history of viral infection, drug history or inflammation processes or that they were receiving pharmacological treatment were not included. For analysis, the overweight subjects were divided in two groups, depending on the presence (IR group) or absence of IR (control group [CG]). A value ≥2.5 was of cutoff point for analyzing IR by homeostasis model assessment (HOMA) index [(insulin [µU/mL]×glucose [mmol/L])/22.5] in accordance by Aguilar-Salinas et al. [12] in Mexican population. This cutpoint had been used in previous reports [13,14]. Glucose values between 100 to 125 mg/dL were defined as impaired fasting glucose (IFG) [15]. Triglycerides (TGs) ≥150 mg/dL and cholesterol ≥200 mg/dL were taken as cutoff point for hypertriglyceridemia and hypercholesterolemia, respectively [16].
Methods
A complete clinical history, with anthropometrical measurements and blood pressure were obtained; weight was measured to nearest 0.1 kg and height to nearest 0.1 cm. BMI was calculated according to the Quetelet index [weight (kg)/height (m2)] and percentage of body fat was assessed by a body composition analyzer Model TBF-215 TANITA (TANITA Corporation of America, Arlington Heights, IL, USA). Blood pressure was measured with a mercury sphygmomanometer after 20 minutes rest, in a supine position and a proper cuff placed on the dominant arm in accordance of International Criteria of Joint National Committee 7 [16].
Blood samples were collected after 12 hours fasting from a vein in the antecubital fossa without venous occlusion. In woman the samples were collected during 3 to 5 days of their menstrual cycle. All samples were separated in aliquots and frozen immediately at -70℃ until analysis for quantifying interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), insulin, glutathione reductase (GRd), GPx, and SOD so to avoid interassay variability. Glucose, cholesterol, TG, and high density lipoprotein (HDL), low density lipoprotein (LDL) concentrations were measured immediately using an automatic analyzer (Roche Diagnostics, Mannheim, Germany), the intra-analysis coefficient of variation for these tests was 1%.
IL-6, TNF-α, and MPO were measured by EIA kit (Cayman Chemical Co., Ann Arbor, MI, USA); the sensitivity of assay of IL-6 was 7.8 pg/mL, TNF-α was 1 pg/mL, and MPO was 14 pmol/L. The activity of GRd and GPx were measured by assay kits (Cayman Chemical Co.). SOD was measured by Cayman's SOD assay kit (Cayman Chemical Co.).
Insulin was measured by an immunoenzymatic assay, the minimum concentration detection for insulin was 0.17 µIU/mL (Invitrogen Corp., Camarillo, CA, USA).
This study was approved by the Local Investigation and Ethics Committee of the Social Security of Mexican Institute and the procedures were in accordance with Helsinki Declaration of 1975 and revised 2000. All participants received a detailed explanation of the study and, after reading it, they signed the informed consent.
Statistical analysis
The Kolmogorov-Smirnov test was used to assess the normality of distribution of investigated parameters. Data were expressed as mean±standard deviation. Systolic and diastolic arterial pressure, Insulin, IL-6, TNF-α, MPO, GPx, GRd, and SOD were distributed abnormally. In variables distributed normally, differences between groups were calculated using a Student t-test for independent samples. Differences between groups of variables distributed abnormally were calculated with Mann-Whitney U test. Spearman's rho correlation coefficients were used to analyze bivariate relationships. Linear regression analysis by stepwise and odds ratio (OR) analysis were used to test the association of biochemical parameters (glucose, cholesterol, TGs, LDL, HDL, IL-6, TNF-α, and oxidative stress enzymes) with IR as dependent variable. A P<0.05 was considered statistically significant in all cases. Data were stored and analyzed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Of the 84 subjects studied, 43 subjects (51%) had IR (HOMA index ≥2.5) and 41 subjects (49%) HOMA index normal (HOMA index <2.5). The clinical and biochemical characteristics of two groups are given in Table 1. Age, blood pressure, BMI, waist circumference, and percent of body fat were similar in both groups. Glucose (IR group, 94.45±2.10; CG, 85.05±1.91 mg/dL; P=0.001) and insulin (IR group median, 16.80 [13.37 to 22.80]; CG median, 9.37 [8.37 to 10.51]; P=0.0001) were statistically different between groups. IFG was present in 13 subjects of group with IR and in three subjects or CG, hypertriglyceridemia in 27 subjects of IR group and in 20 subjects in the CG, while hypercholesterolemia in 15 subjects of group with IR and in 19 subjects of CG. The results of oxidative stress enzymes and inflammatory variables are resumed in Table 2. Correlations between MPO and other measured variables about ROS and Inflammation are shown in Table 3. Results of linear regression analysis are given in Table 4. MPO and TNF-α were variables with an independent influence in IR (R2=0.178; F=8.868; P=0.0001). The excluded variables of model were GPx, GRd, SOD, and IL-6.
Table 1. Clinical and biochemical characteristics of overweight subjects with first-degree relatives with type 2 diabetes mellitus.
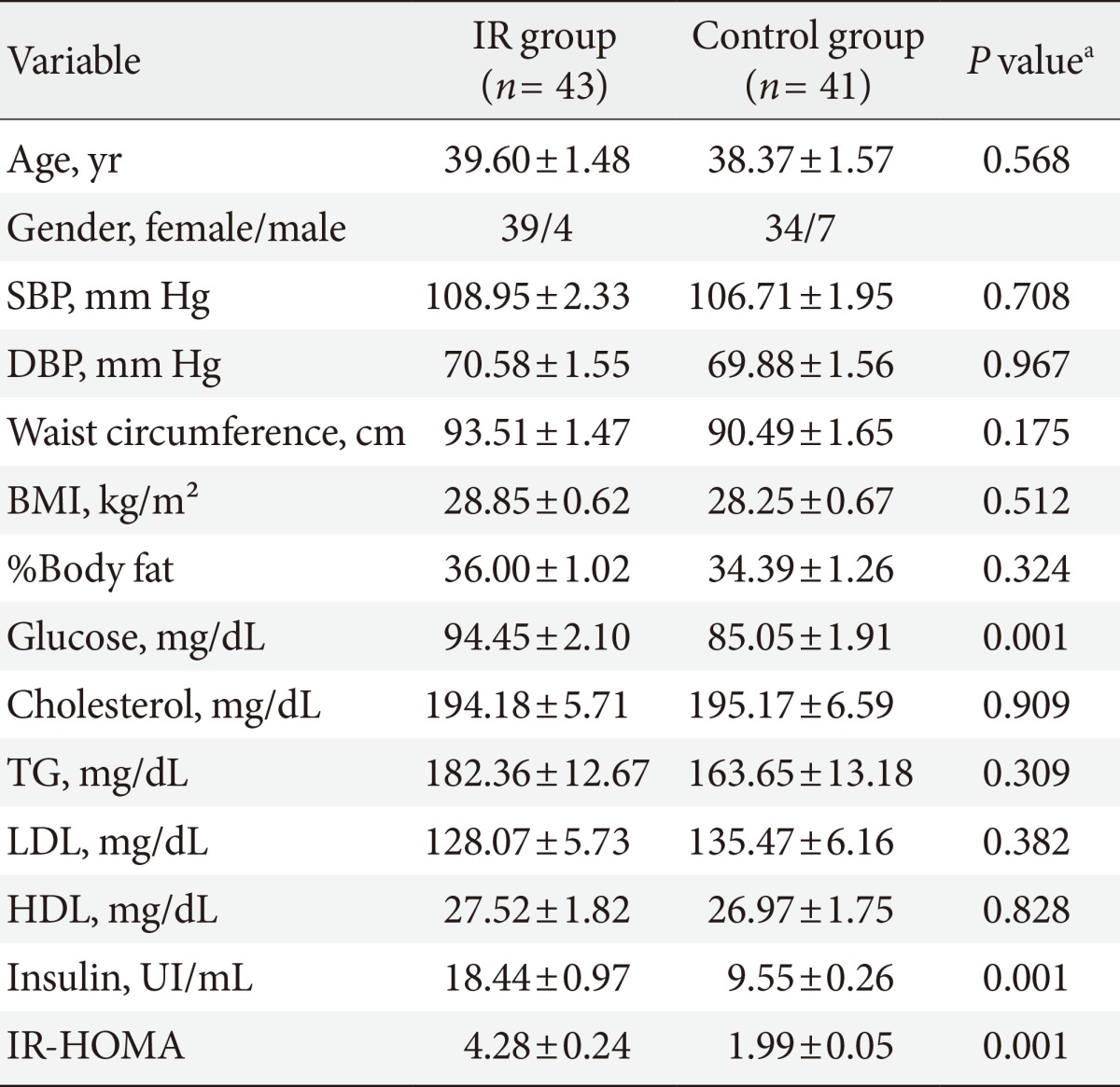
Values are presented as mean±standard deviation.
IR, insulin resistance; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; TG, triglyceride; LDL, low density lipoprotein; HDL, high density lipoprotein; IR-HOMA, insulin resistance evaluated by homeostasis model assessment.
aP<0.05.
Table 2. Oxidative stress enzymes and inflammatory variables in overweight subjects with first-degree relatives with type 2 diabetes mellitus.
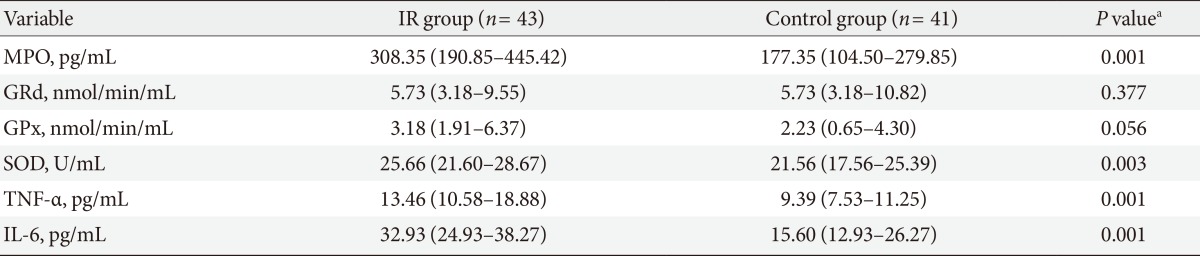
Values are presented as median (interquartile range, Q1-Q3).
IR, insulin resistance; MPO, myeloperoxidase; GRd, gluthathione reductase; GPx, glutathione peroxidase; SOD, superoxide dismutase; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6.
aP<0.05.
Table 3. Correlations between myeloperoxidase and other measured variables about reactive oxygen species and inflammation.
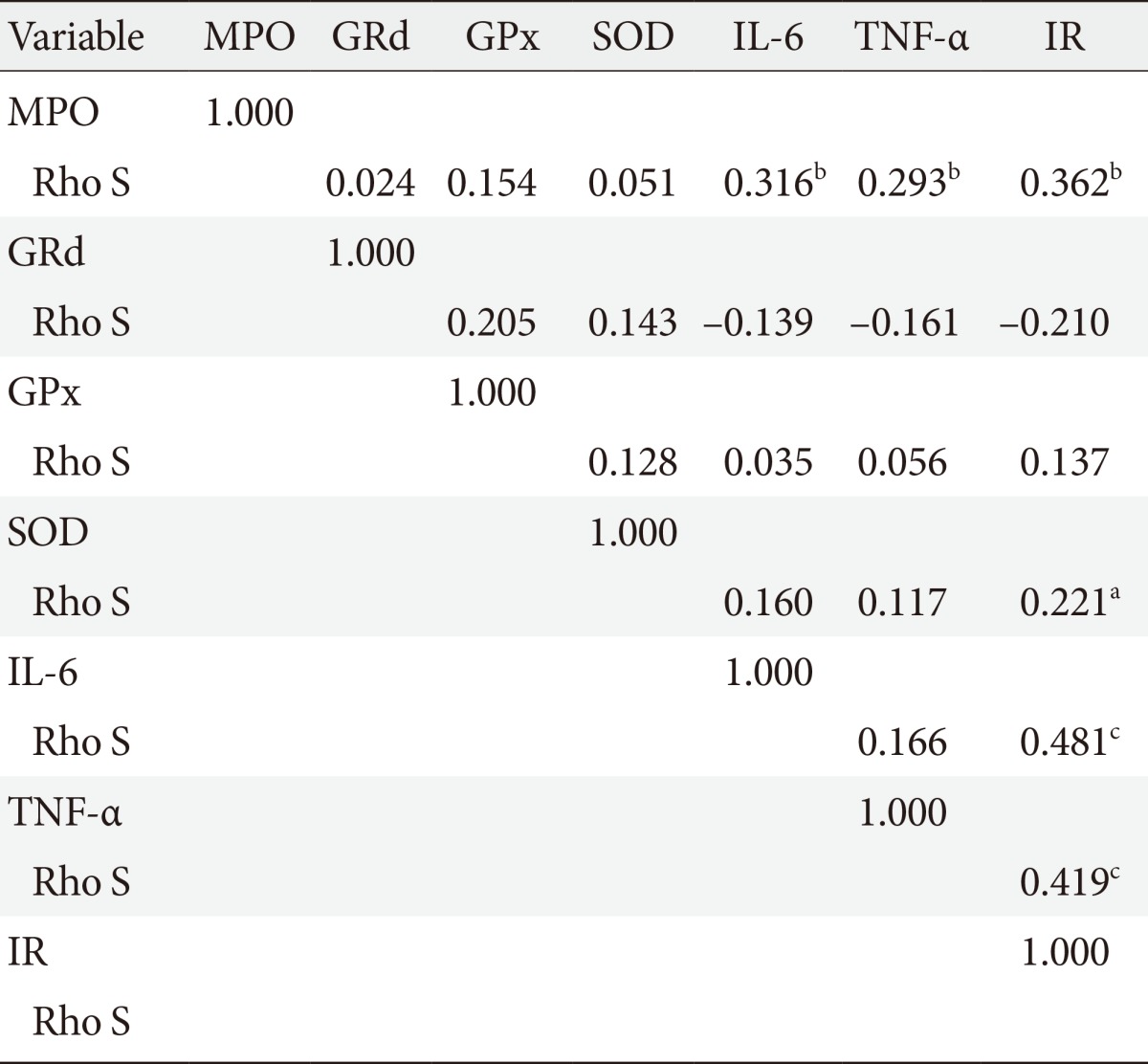
MPO, myeloperoxidase; GRd, gluthathione reductase; GPx, glutathione peroxidase; SOD, superoxide dismutase; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; IR, insulin resistance; Rho S, Spearman rho correlation coefficient.
aP<0.05, bP<0.01, cP<0.001.
Table 4. Regression analysis with insulin resistance predicting variables.
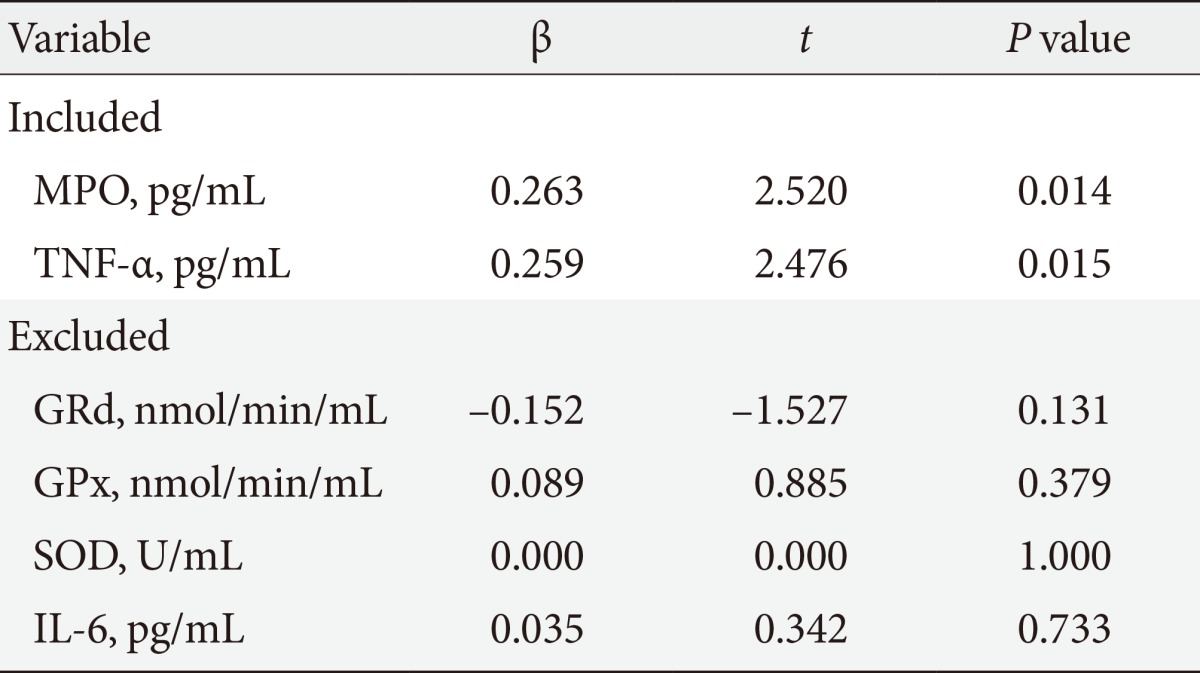
Regression analysis was adjusted by age, body mass index, and gender. MPO, myeloperoxidase; TNF-α, tumor necrosis factor-α; GRd, gluthathione reductase; GPx, glutathione peroxidase; SOD, superoxide dismutase; IL-6, interleukin-6.
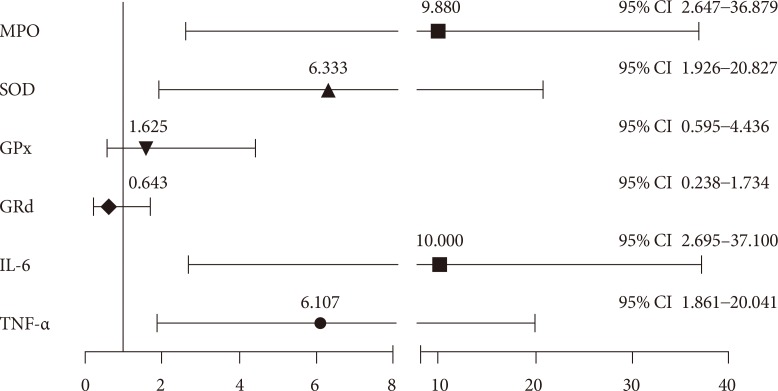
OR analysis was adjusted by age and gender in subjects with overweight and history of first-degree relatives with T2DM, MPO, SOD, IL-6, and TNF-α levels showed a high risk to present IR (MPO: OR, 9.880; 95% confidence interval [CI], 2.647 to 36.879; SOD: OR, 6.333; 95% CI, 1.926 to 20.827; IL-6: OR, 10.000; 95% CI, 2.695 to 37.100; TNF-α: OR, 6.107; 95% CI, 1.861 to 20.041) (Fig. 1).
Fig. 1. Odds ratio and (95% confidence interval [CI]) analysis of variables of oxidative stress and inflammation for insulin resistance in overweight subjects with first-degree relatives with type 2 diabetes mellitus. MPO, myeloperoxidase; SOD, superoxide dismutase; GPx, glutathione peroxidase; GRd, gluthathione reductase; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
DISCUSSION
This study showed that MPO was positively associated with IR and inflammation in overweight subjects with first-degree relatives with T2DM. In other study [17], we corroborated that subjects with first-degree relatives with T2DM had normoglycaemia and hyperinsulinemia that could be early detected but in our FMU N° 80 IMSS, serum insulin is not a routine test in clinic laboratory.
In association with overweight and obesity the prevalence of IR is constantly growing. As a consequence of this fact, T2DM occurs with high frequency in younger age groups [18,19]. Patients with first degree relatives of T2DM may present impaired function of β-cell with normal concentrations of glucose, hypertriglyceridemia, higher body fat percent, abdominal obesity, and these situations were present in both, IR group and in CG which place at subjects with first degree relatives of T2DM in a high risk for future type 2 diabetes [20].
Evans et al. [21] reported that high concentrations of glucose and free fat acids cause oxidative stress and initiates IR in genetically predisposed individuals for diabetes. Oxidative stress is the pathogenic constituent in diabetic endothelial dysfunction. Moreover, overweight and obesity may induce oxidative stress too [22]. In our study, all subjects had overweight, but only MPO and SOD enzymes in subjects with IR and without IR were different. We corroborate the association between IR, MPO, and SOD. Baynes [23] presented evidence that oxidative stress may not early occur in the process of diabetes, but could be an underlying pathogenic factor in the progression of the disease. In contrast, our results show an increase of SOD and MPO enzymes in subjects with IR. Evidence exists that H2O2 generation measured ex vivo in serum of type 2 diabetic patients is significantly higher [24], this start since prepatologic stage (IR) probably because subjects with IR had levels higher activity of SOD. Superoxide is converted to the H2O2 by SOD to oxygen and water by antioxidant enzymes such catalase and GPx. Goyal et al. [25] reported lower activity of GPx in obese diabetic patients and Kornhauser et al. [26] showed in first-degree relatives of patients of T2DM an over expressing of GPx gene as defense mechanisms to protect cell from oxidative stress [27]. Conversely, our results have shown that activity of GPx was not found different between IR group and without IR group in first-degree relatives T2DM.
MPO uses H2O2 to oxidize numerous substrates to hypoclorous acid and is highly expressed in ruptures of human coronary atheroma, and proteins modified by hypoclorous acid are present at high concentrations in these regions of the artery wall [28]. Fu et al. [29] report that hypoclorous acid production by MPO might represent a physiological mechanism that link degradation of matrix proteins by metalloproteinases. Our results report a higher concentration of MPO in IR group, probably as detrimental to β-cells and insulin function. The production of HOCl by MPO could trigger metalloproteases activation, degrade extracellular matrix proteins, and move forward via a complex cascade of cytoskeleton rearrangement brought about by the coordinated action of small Rho family GTPases and consequently damage of β-cell [29,30]. This is a new theme that arising for this study.
Several studies have demonstrated that TNF-α and IL-6 contribute to IR [31,32]. In fact, we accordance with this, TNF-α and IL-6 were significantly higher in IR group in comparison with without IR Group. In accordance with Olza et al. [33] we found a positive correlation between TNF-α, IL-6, and MPO shows that MPO is implicated in inflammation as a mediator of vascular inflammation and further points toward the significance of neutrophil activation and the generation of oxidants species [34,35].
A limitation of this study was the minor participation of men, it was known that the distribution of the body fat is different between man and women and it could have influence in our results, this hypothesis is unlikely because the regression model was adjusted by gender, age, and BMI too.
In conclusion, MPO had relation with IR and with inflammation parameters in overweight subjects with first-degree relatives with T2DM. Overweight subjects with first-degree relatives with T2DM and IR, have an increase for developing T2DM. We need studies on a casual relationship and molecular mechanisms among the increased serum MPO levels, inflammation markers, and IR.
ACKNOWLEDGMENTS
Author would like to thanks to Foundation of Health Research of IMSS for the financial support for this project.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Praveen EP, Sahoo J, Khurana ML, Kulshreshtha B, Khadgawat R, Gupta N, Dwivedi SN, Kumar G, Prabhakaran D, Ammini AC. Insulin sensitivity and beta-cell function in normoglycemic offspring of individuals with type 2 diabetes mellitus: Impact of line of inheritance. Indian J Endocrinol Metab. 2012;16:105–111. doi: 10.4103/2230-8210.91204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haffner SM, Miettinen H, Gaskill SP, Stern MP. Decreased insulin action and insulin secretion predict the development of impaired glucose tolerance. Diabetologia. 1996;39:1201–1207. doi: 10.1007/BF02658507. [DOI] [PubMed] [Google Scholar]
- 3.Straczkowski M, Kowalska I, Stepien A, Dzienis-Straczkowska S, Szelachowska M, Kinalska I, Krukowska A, Konicka M. Insulin resistance in the first-degree relatives of persons with type 2 diabetes. Med Sci Monit. 2003;9:CR186–CR190. [PubMed] [Google Scholar]
- 4.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 5.Ceriello A. New insights on oxidative stress and diabetic complications may lead to a "causal" antioxidant therapy. Diabetes Care. 2003;26:1589–1596. doi: 10.2337/diacare.26.5.1589. [DOI] [PubMed] [Google Scholar]
- 6.Pihl E, Zilmer K, Kullisaar T, Kairane C, Magi A, Zilmer M. Atherogenic inflammatory and oxidative stress markers in relation to overweight values in male former athletes. Int J Obes (Lond) 2006;30:141–146. doi: 10.1038/sj.ijo.0803068. [DOI] [PubMed] [Google Scholar]
- 7.Aruoma OI. Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc. 1998;75:199–212. doi: 10.1007/s11746-998-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 9.van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal. 2009;11:2899–2937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- 10.Nauseef WM. Contributions of myeloperoxidase to proinflammatory events: more than an antimicrobial system. Int J Hematol. 2001;74:125–133. doi: 10.1007/BF02981994. [DOI] [PubMed] [Google Scholar]
- 11.Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, Simoons ML, Hamm CW CAPTURE Investigators. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 12.Aguilar-Salinas CA, Olaiz G, Valles V, Torres JM, Gomez Perez FJ, Rull JA, Rojas R, Franco A, Sepulveda J. High prevalence of low HDL cholesterol concentrations and mixed hyperlipidemia in a Mexican nationwide survey. J Lipid Res. 2001;42:1298–1307. [PubMed] [Google Scholar]
- 13.Taniguchi A, Fukushima M, Sakai M, Kataoka K, Nagata I, Doi K, Arakawa H, Nagasaka S, Tokuyama K, Nakai Y. The role of the body mass index and triglyceride levels in identifying insulin-sensitive and insulin-resistant variants in Japanese non-insulin-dependent diabetic patients. Metabolism. 2000;49:1001–1005. doi: 10.1053/meta.2000.7735. [DOI] [PubMed] [Google Scholar]
- 14.Munguia-Miranda C, Sanchez-Barrera RG, Hernandez-Saavedra D, Cruz-Lopez M. Dyslipidemia prevalence and its relationship with insulin resistance in a population of apparently healthy subjects. Salud Publica Mex. 2008;50:375–382. doi: 10.1590/s0036-36342008000500010. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 16.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Garcia A, Magana-Garns P, Ruiz-Garcia J, Alvarez-Aguilar C. Insulin sensitivity and beta cell function in different glucose tolerance status. Invest Clin. 2006;47:155–166. [PubMed] [Google Scholar]
- 18.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 19.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 20.Cases A. Cardiovascular morbidity and mortality in type 2 diabetes mellitus. Hipertensión. 2002;19:193–196. [Google Scholar]
- 21.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 22.Zengi A, Ercan G, Caglayan O, Tamsel S, Karadeniz M, Simsir I, Harman E, Kahraman C, Orman M, Cetinkalp S, Ozgen G. Increased oxidative DNA damage in lean normoglycemic offspring of type 2 diabetic patients. Exp Clin Endocrinol Diabetes. 2011;119:467–471. doi: 10.1055/s-0031-1275289. [DOI] [PubMed] [Google Scholar]
- 23.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 24.Wierusz-Wysocka B, Wysocki H, Byks H, Zozulinska D, Wykretowicz A, Kazmierczak M. Metabolic control quality and free radical activity in diabetic patients. Diabetes Res Clin Pract. 1995;27:193–197. doi: 10.1016/0168-8227(95)01043-d. [DOI] [PubMed] [Google Scholar]
- 25.Goyal R, Singhai M, Faizy AF. Glutathione peroxidase activity in obese and nonobese diabetic patients and role of hyperglycemia in oxidative stress. J Midlife Health. 2011;2:72–76. doi: 10.4103/0976-7800.92529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kornhauser C, Garcia-Ramirez JR, Wrobel K, Perez-Luque EL, Garay-Sevilla ME, Wrobel K. Serum selenium and glutathione peroxidase concentrations in type 2 diabetes mellitus patients. Prim Care Diabetes. 2008;2:81–85. doi: 10.1016/j.pcd.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Sathiyapriya V, Selvaraj N, Bobby Z, Agrawal A. Perturbation of erythrocyte antioxidant barrier, lipid peroxidation and protein carbonylation in non-diabetic first degree relatives of patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;78:171–175. doi: 10.1016/j.diabres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276:41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 30.Lei XG, Vatamaniuk MZ. Two tales of antioxidant enzymes on beta cells and diabetes. Antioxid Redox Signal. 2011;14:489–503. doi: 10.1089/ars.2010.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monzillo LU, Hamdy O, Horton ES, Ledbury S, Mullooly C, Jarema C, Porter S, Ovalle K, Moussa A, Mantzoros CS. Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes Res. 2003;11:1048–1054. doi: 10.1038/oby.2003.144. [DOI] [PubMed] [Google Scholar]
- 32.Silha JV, Nyomba BL, Leslie WD, Murphy LJ. Ethnicity, insulin resistance, and inflammatory adipokines in women at high and low risk for vascular disease. Diabetes Care. 2007;30:286–291. doi: 10.2337/dc06-1073. [DOI] [PubMed] [Google Scholar]
- 33.Olza J, Aguilera CM, Gil-Campos M, Leis R, Bueno G, Martinez-Jimenez MD, Valle M, Canete R, Tojo R, Moreno LA, Gil A. Myeloperoxidase is an early biomarker of inflammation and cardiovascular risk in prepubertal obese children. Diabetes Care. 2012;35:2373–2376. doi: 10.2337/dc12-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, Topol EJ, Sprecher DL, Hazen SL. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286:2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 35.Ximenes VF, Paino IM, Faria-Oliveira OM, Fonseca LM, Brunetti IL. Indole ring oxidation by activated leukocytes prevents the production of hypochlorous acid. Braz J Med Biol Res. 2005;38:1575–1583. doi: 10.1590/s0100-879x2005001100003. [DOI] [PubMed] [Google Scholar]