Abstract
Background
Thyroid dysfunction (TD) and metabolic syndrome (MetS) are known risk factors for atherosclerotic cardiovascular disease (ASCVD). TD is risk factor for ASCVD mediated by the effects of thyroid hormones on lipid metabolism and blood pressure hence the components of MetS. It is possible that coexistence of these two disease entities and unrecognized TD in patients with MetS might substantially increase ASCVD risk. Moreover, little is known about the relationship between TD and the components of MetS. Thus, the purpose of this study was to evaluate the pattern of TD in patients with MetS and its relationship with components of the MetS.
Methods
A total of 358 previously diagnosed patients with MetS were recruited in the study. The thyroid function test parameters were measured to classify TD at Dhulikhel Hospital-Kathmandu University Hospital, Dhulikhel, Nepal. Statistical analyses were performed using SPSS version 16.0 to evaluate pattern and relationship.
Results
The overall prevalence of TD in patients with MetS was 31.84% with high prevalence of subclinical hypothyroidism (29.32%). We found no evidence of a relationship between TD and components of MetS, although there was significant difference in waist circumference between four groups of TD.
Conclusion
Patients with MetS had subclinical hypothyroidism greatly. Although there was no evidence of any relationship between thyroid status and all components of MetS, TD should be taken into account when evaluating and treating patients with MetS to reduce the impending risk.
Keywords: Atherosclerotic cardiovascular disease risk, Metabolic syndrome, Subclinical hypothyroidism, Thyroid dysfunction
INTRODUCTION
The metabolic syndrome (MetS) is a clustering of multiple risk factors for atherosclerotic cardiovascular disease (ASCVD) such as central obesity, impaired fasting glucose (IFG) or type 2 diabetes mellitus (T2DM), elevated triglyceride (TG), reduced high density lipoprotein cholesterol (HDL-C) and hypertension (HTN) [1,2]. Despite of the controversy on its definition it is estimated that one out of four people around the world suffers from MetS [2]. The prevalence of MetS is increasing all over the world with different regions having individual clusters of epidemic risk factors, and distinctly there is an evidence for high prevalence of MetS in India and other South Asian countries [3,4,5].
Thyroid dysfunction (TD) is defined as the altered serum thyroid stimulating hormone (TSH) level with normal or altered thyroid hormones (free triiodothyronine [fT3] and free thyroxine [fT4]) [6]. TD is risk factors for ASCVD mediated by the effects of thyroid hormones on lipid and glucose metabolism and blood pressure, hence on components of MetS [7].
MetS and TD are both characterized by a cluster of common abnormalities such as abdominal obesity, hyperglycemia, HTN, reduced HDL-C, and elevated TG and recognized independent risk factors of ASCVD [7,8,9]. It is possible that patients suffering from both disease entities might have compounded risk. There are several studies regarding the prevalence of TD among MetS patients, the association or relationship of MetS and its components with TD, but the results are controversial [8,10]. Furthermore, when exploring the relationship between TD and components of MetS, most studies have focused on the subclinical hypothyroidism (SCH). Unrecognized TD may impair metabolic control and add to cardiovascular disease risk in patients with MetS. A study on TD in MetS population may help us to know the magnitude of overlap of these two disease entities and may highlight the importance of thyroid function tests in patients with MetS. This can lead to proper planning and adequate management strategies, resulting in significant reduction in cardiovascular morbidity and mortality due to MetS with TD. Thus, this study was intended to evaluate the pattern of TD in patients with MetS and to explore the relationship between TD and components of the MetS.
METHODS
We performed a cross-sectional analysis by using data from the study on prevalence of MetS in Dhulikhel Municipality population, Dhulikhel, Nepal between August 2011 to September 2013. Trained interviewers, using a structured questionnaire, interviewed the participants at home to obtain the information on sociodemographic characteristics, physical activity, smoking, alcohol drinking habits, dietary characteristics, personal and family history of diseases and hospitalization. Patients with known diabetes or other endocrine disorders, patients receiving any medication that may alter thyroid functions or lipid levels, pregnant women, and patients with a cardiovascular disease, corticosteroid use, active liver disease, and renal dysfunction, including nephrotic syndrome were excluded.
The participants were asked to attend the mobile examination center, where they completed additional questionnaires, underwent various examinations, and provided a blood sample during an overnight fast of 8 to 12 hours. Anthropometric measurements (with measuring tape) and blood pressure measurements (digital BP monitoring device; Microlife Corp., Taipei, Taiwan) were obtained after complete physical examination by a trained nurse. The blood samples were transported to the Department of Clinical Biochemistry of Dhulikhel Hospital-Kathmandu University Hospital, Dhulikhel, Nepal for further processing and analysis. Fasting blood glucose (FBG) and lipid measurements (HDL-C and TG) were done on the same day while remaining serum sample was immediately frozen and stored at -80℃ for thyroid function panel tests-serum TSH, serum fT4, and serum fT3 until analysis.
Three hundred fifty-eight patients (232 females and 126 males) with MetS who fulfilled National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III) modified for Asians criteria (three out of five criteria positive) were included in the study group. The age of the study participant was between 30 to 70 years. Three hundred forty-one patients who had no features of MetS (0 out of 5 criteria for MetS) were included in the control group. Written informed consent was obtained from each patient after full explanation of the purpose and nature of all procedures used and the Institutional Review Committee of our tertiary care hospital approved the study.
Laboratory measurements
FBG, TG were measured using the enzymatic colorimetric method (Dialab GmbH, Neudorf, Austria), and HDL-C using elimination method (Gesan Production Srl, Campobello di Mazara TP, Italy) using a Flexor Junior autoanalyzer (Vital Scientific, Spankeren, Netherlands). Serum TSH, fT4, and fT3 measurements were made by using colorimetric enzyme-linked immunosorbent assay (Diagnostic Automation/Cortez Diagnostics Inc., Calabasas, CA, USA). The analytical sensitivity of TSH was 0.005 µIU/mL, for fT4 was 0.005 ng/dL, and for fT3 was 0.05 pg/mL. Normal range for TSH was 0.4 to 4.2 µIU/mL, for fT4 was 0.8 to 2.0 ng/dL, and for fT3 was 1.4 to 4.2 pg/mL. All of the assays were routinely monitored by participation in external quality-control programs and using assayed chemistry and assayed immunoassay plus controls (Bio-Rad Lab, Hercules, CA, USA).
Definitions
Definition of metabolic syndrome
For the purpose of this study, MetS was diagnosed based on modified Asian NCEP-ATP III panel criteria. Modified Asian ATP III criteria are the same as original ATP III except waist circumference (WC) greater than 90 cm in men and 80 cm in women [4]. Diagnosis requires having any three of five risk factors: abnormal WC, TG levels ≥150.0 mg/dL or pharmacologic treatment (Rx), HDL-C levels: <40.0 mg/dL in male and <50.0 mg/dL in female; or Rx, blood pressure: >130 mm Hg systolic and >85 mm Hg diastolic or Rx, and FBG concentration >100.0 mg/dL or Rx [11].
Definition of thyroid dysfunction and euthyroid
Subjects were stratified into one of the following five groups based on the guidelines for the use of thyroid function tests. (1) Euthyroid was defined as a normal thyroid function test. (2) Hyperthyroidism was defined as a TSH concentration of less than 0.40 µIU/mL with an elevated fT4 and fT3 level. (3) Subclinical hyperthyroidism was defined as a TSH concentration of less than 0.40 µIU/mL with a normal fT4 and fT3 concentration. (4) Hypothyroidism was defined as a TSH concentration of more than 4.20 µIU/mL with an fT4 and fT3 concentration level below normal. (5) SCH was defined as a TSH concentration of more than 4.20 µIU/mL with a normal fT4 and fT3 concentration [12].
The American Thyroid Association recommends the combined use of TSH and fT4 as the most efficient combination of blood tests for diagnosis and follow-up of both ambulatory and hospitalized patients [13].
Statistical analysis
Statistical analysis was performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). The results were expressed as percentage to determine the pattern of TD in MetS. Baseline characteristics of the study participants were expressed as mean±standard deviation. Student t-test was used to analyze differences in baseline characteristics between patients with MetS and the control group. Pearson correlation coefficients were calculated to assess any significant relationship between the components of MetS and thyroid profile parameters (TSH and fT4 levels). The comparison between four categories of TD was done by using analysis of variance (ANOVA). Statistical significance was defined at P<0.05.
RESULTS
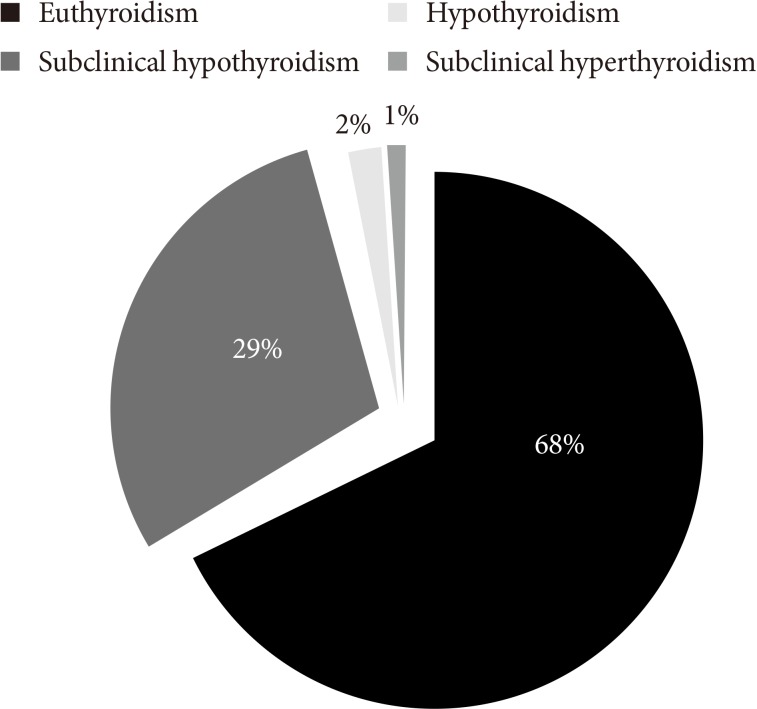
Of the 358 study subjects, 105 (29.32%) had SCH, six (1.67%) had hypothyroidism, three (0.83%) had subclinical hyperthyroidism, and 244 (68.17%) were euthyroid. Hyperthyroidism was not present in any of the subject. The pattern of TD in patients with MetS was shown in Fig. 1. Therefore, the overall prevalence of the TD was 114 (31.84%). In the healthy control group, only 15 (4.40%) had SCH, five (1.47%) had hyperthyroidism, one (0.29%) had hypothyroidism, one (0.29%) had subclinical hyperthyroidism and 319 (93.54%) were euthyroid. The overall prevalence of TD was 6.45% among healthy control.
Fig. 1. Pattern of thyroid dysfunction in patients with metabolic syndrome (n=358).
After separating the subjects by gender; 34(29.82%) male subjects and 80 (70.17%) female subjects had TD. The pattern of TD in male subject was 28 (24.56%), four (3.50%), and two (1.75%) with SCH, hypothyroidism, and subclinical hyperthyroidism respectively. Similarly, the pattern of TD in female subject was 77 (67.54%), two (1.75%), and one (0.88%) with SCH, hypothyroidism and subclinical hyperthyroidism, respectively.
Differences between anthropometric and biochemical parameters between patient with MetS and healthy control were tested by Student t-test. There was significant differences in the mean values for each of the components of MetS except fT3, and are summarized in Table 1. Thyroid function test parameters in both the MetS group and healthy control group were assessed with fT3, fT4, and TSH assay. TSH was significantly higher in the MetS group than in the control group, fT4 was lower in the control group but there was no significant difference in levels of fT3 in both groups.
Table 1. Anthropometric and biochemical parameters for patient with metabolic syndrome and healthy control subjects.
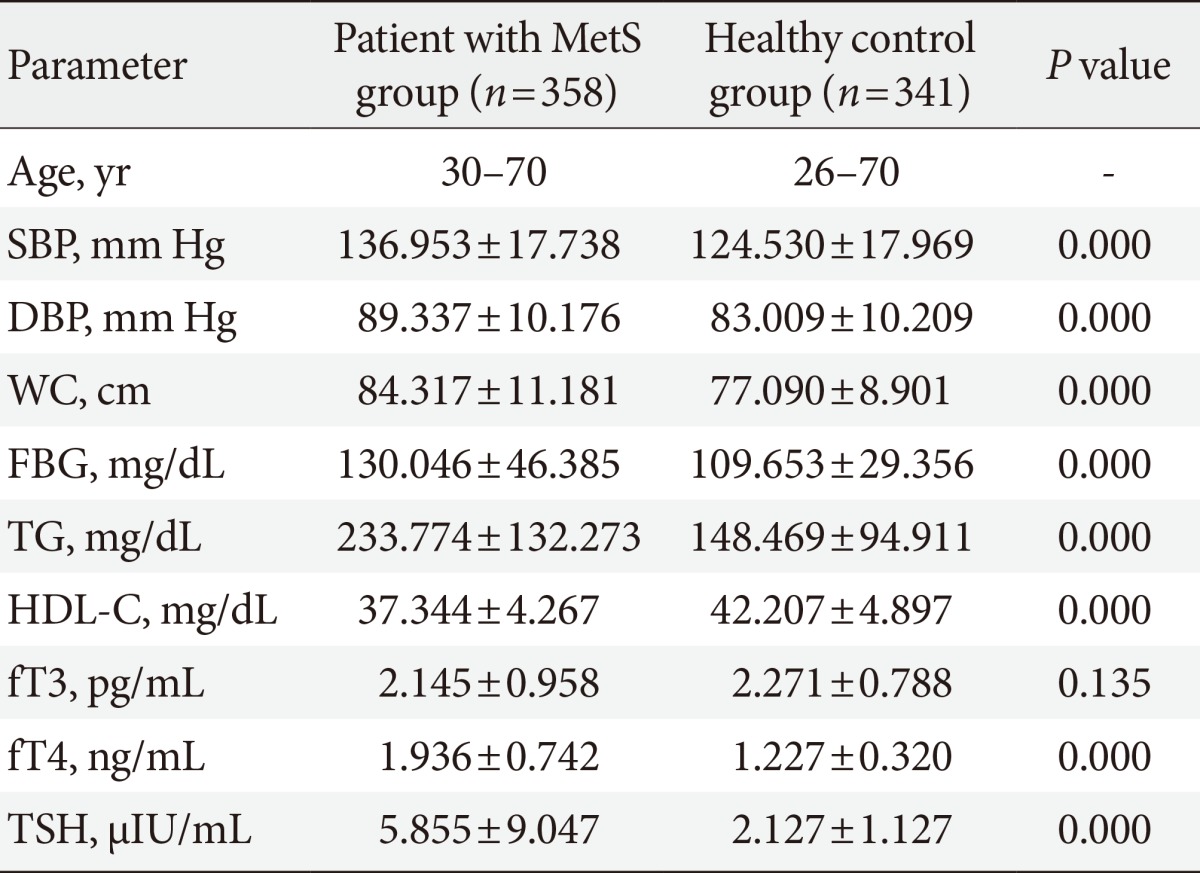
Values are presented as group range or mean±standard deviation.
P<0.05 from the student t-test of the measured variables between MetS and control group.
MetS, metabolic syndrome; SBP, systolic blood pressure; DBP, diastolic blood pressure; WC, waist circumference; FBG, fasting blood glucose; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; fT3, free triiodothyronine; fT4, free thyroxine; TSH, thyroid stimulating hormone.
The relationship of TSH and fT4 levels with the presence of MetS components was assessed using Pearson correlation coefficients analysis and is shown in Table 2. Increased FBS was positively related with high TSH levels but were not statistically significant, while increased WC, systolic and diastolic blood pressure were negatively related with higher TSH but again were not statistically significant. Similarly, increased WC, systolic and diastolic blood pressure, and FBG were positively related with high fT4 levels but were not statistically significant, while TG was negatively related with higher fT4 but again was not statistically significant.
Table 2. Correlation between components of MetS with levels of fT4 and TSH among patients with MetS (linear regression model).
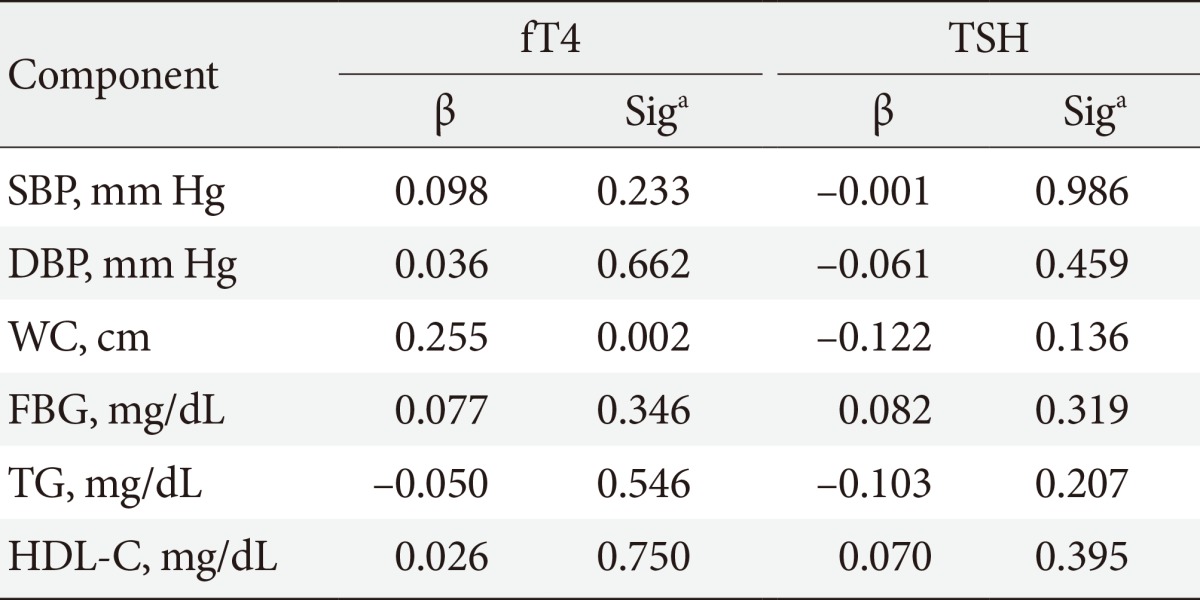
MetS, metabolic syndrome; fT4, free thyroxine; TSH, thyroid stimulating hormone; SBP, systolic blood pressure; DBP, diastolic blood pressure; WC, waist circumference; FBG, fasting blood glucose; TG, triglyceride; HDL-C, high density lipoprotein cholesterol.
aCorrelation is significant at the 0.05 level.
The results for comparison of anthropometric and biochemical parameters between the four categories of TD was done by using ANOVA but there was no significant differences in components of MetS between four groups except WC and were shown in Table 3.
Table 3. Difference in components of metabolic syndrome among thyroid dysfunction subgroups.
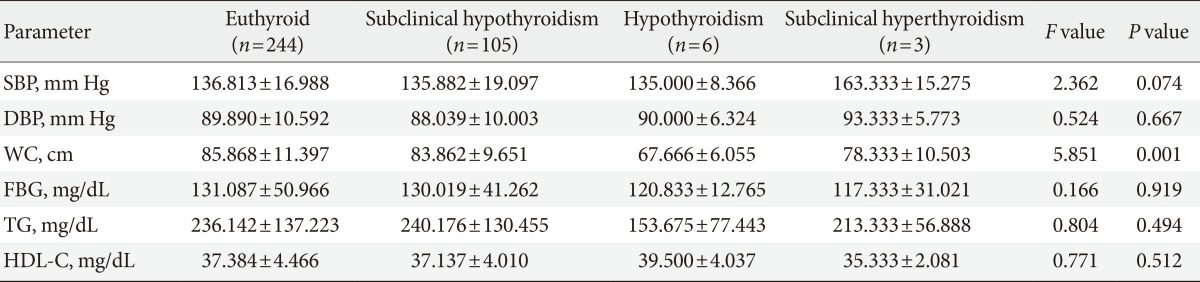
Values are presented as mean±standard deviation. F value and P value derived from one-way analysis of variance that used to evaluate in the four groups.
SBP, systolic blood pressure; DBP, diastolic blood pressure; WC, waist circumference; FBG, fasting blood glucose; TG, triglyceride; HDL-C, high density lipoprotein cholesterol.
DISCUSSION
Our study showed that the prevalence of TD in patients with MetS was 31.84% and its pattern showed high prevalence of SCH (29.32%) followed by hypothyroidism (1.67%) and subclinical hyperthyroidism (0.83%).
The above results are in agreement with previous studies showing an association between MetS and TD [9,14,15,16,17,18,19,20,21]. A study by Meher et al. [14] showed a high prevalence of SCH (22%) and overt hypothyroidism (4%) in the MetS group. A study by Shantha et al. [9] in India has shown a high prevalence of SCH (21.90%) and overt hypothyroidism (7.40%) in patients with MetS. A recent study in Taiwan by Wang et al. [20] reported that TD was present in 7.21% of Taiwan MetS patients. This study had shown 4.55% had SCH and 2.64% had subclinical hyperthyroidism [20]. Yet, another study in Taiwan by Lai et al. [21] showed that the overall prevalence of TD was 7.60% with 2.10% to be subclinical hypothyroid and 5.50% to be subclinical hyperthyroid patients. Another study by Uzunlulu et al. [22] in Turkey had shown SCH prevalence to be 16.40% in the MetS group. However this study did not address patients with overt hypothyroidism and all observations were with SCH patients only [22].
Many studies looking at the association of MetS with TD has found more SCH, rather than overt hypothyroidism. In contrast to our finding a study in India by Agarwal et al. [8] of the seventy six patients with MetS, 78.0% had TD with 53.0% to be SCH and 25.0% had overt hypothyroidism. Similarly, Jayakumar [23] found that out of 120 patients who were diagnosed to have MetS, thyroid functions were normal in 48 patients, 18 patients had hypothyroidism, 52 had SCH, and two had subclinical hyperthyroidism. Hypothyroidism and SCH were present in 60% of patients with MetS [23]. Yet another study by Ogbera et al. [24] in Nigeria showed the proportion of subjects with hypothyroidism, euthyroidism and hyperthyroidism who had MetS was 40.0%, 42.0%, and 24.0%, respectively.
Our study revealed that the prevalence of TD was more among the females with MetS than those among male subjects, which is consistent with previous reports [2,9,17,19,22,24].
There was significant difference in the mean values for each anthropometric and biochemical parameters among patients with MetS and healthy control subjects; only fT3 was not significantly altered; thus, TD was related with all the components of MetS. These findings were similar to those obtained by Kota et al. [25] who mentioned body mass index (BMI), WC, mean systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting blood glucose (FBG), total cholesterol, low density lipoprotein cholesterol (LDL-C), TG, and TSH were significantly higher in MetS group compared to control group and HDL-C was significantly lower in study group. Yet another study done by Meher et al. [14] showed that the mean SBP, DBP, FBG, total cholesterol, LDL-C, TG, WC, and BMI were significantly higher and HDL-C levels were significantly lower in the MetS group than in the control group. Similar findings were obtained in the studies by Garduno-Garcia Jde et al. [18] on Hispanic population and by Shantha et al. [9] on Chennai urban population.
In concert with those studies, Oh et al. [26] found a significant association between elevated TSH levels within normal range and the MetS. WC, systolic and diastolic blood pressure, and TG were significantly associated with TSH levels, though fasting hyperglycemia and low HDL-C levels were not significantly associated with TSH levels [26]. Similarly, a recent study in Taiwan by Lai et al. [16] explored the relationship between serum TSH levels and components of MetS, concluding that even slight increases in TSH, as in SCH, may be a MetS risk factor; in that study, TSH levels were significantly higher in the MetS group than in the non-MetS group.
Associations or relationship between TD and components of MetS have been suggested in previous studies and is well established. There are several studies that have looked into the possibility of a relationship between the MetS and TD, but the results are disputed [10]. We found no evidence of a relationship between thyroid status and all components of MetS in our study. Similar study, did not find the relationship between TSH and MetS as well as its components in people with subclinical hyperthyroidism [16]. Another study by Wang et al. [27] in a large healthy population receiving routine health examination, no statistical correlation was found between subclinical thyroid diseases and MetS.
In contrast, a significant relationship between thyroid hormones and the components of the MetS has also been shown in the study by Kim et al. [28]. A study done by Shantha et al. [9] concluded that although thyroid hormone significantly affected each component of MS, there was no relationship between TD and euthyroid subjects. When exploring the relationship between TD and components of MetS, most studies have focused on the SCH.
However, although components of MetS showed no positive correlation with TSH and fT4 levels, TSH was significantly high and fT4 was normal in patients with MetS suggesting that MetS may be associated with increased risk of SCH. The relationship or association between MetS and TD is complex and still not clear. The present study explores the relationship between four categories of TD with components of MetS, although there was higher prevalence of SCH in our study subjects. We found no significant differences in baseline characteristics between four groups of TD except WC.
In other studies, they showed a significant association of SCH with components of MetS [29]. Similarly, the results of Shantha et al. [9] study, which showed the association of MetS and primary hypothyroidism in the urban population. The study by Uzunlulu et al. [22] also had shown significant association of SCH and MetS, which do not supports our data. Therefore, pattern of TD in MetS and its relationship with components varies in different studies. The geographic location, age, gender, diet, intake of iodine and other genetic, and environmental factor might possibly account for these discrepancies in pattern and relationships [2,9,30].
Hence, the main finding of this report was the documentation of the pattern of TD in patients with MetS. The occurrence of the TD in patients with MetS may connote a state of "double jeopardy" in persons with MetS. The report extent the existing information on relationship between TD and the components of MetS. We found no evidence of a relationship between thyroid status and the prevalence of MetS. This study did not find the relationship between TSH and fT4 with MetS as well as its components. On the other hand, the relation between MetS and TD is not clearly identified yet, if it does then all observations were on Subclinical hypothyroidism patients only.
MetS, a complex of disorders including the abdominal obesity, lipid abnormalities, HTN, and impaired fasting glucose, is one of the known risk factors for ASCVD. The concept of MetS has also been widely used for the comanagement of multiple cardiovascular risk factors including diabetes, HTN, and dyslipidemia in high risk persons for optimal outcomes [31]. The thyroid gland controls our metabolism through two hormones it produces: fT3 and fT4 [9]. MetS has been linked to thyroid disease due to the pathophysiology of TD on lipid and glucose metabolism, blood pressure, and cardiovascular dysfunction [21]. Considerable overlap occurs in the pathogenic mechanisms of ASCVD by MetS and hypothyroidism. Unrecognized TD in patients MetS may impair metabolic control and add to ASCVD risk in patients with MetS. The prevalence of TD in patients with MetS was high, which indicates a possible interplay between thyroid status and MetS. Hence, a study on TD in MetS population may help us to know the magnitude of overlap of these two groups and may highlight the importance of thyroid function tests in identifying hypothyroid population from MetS. This can lead to proper planning and adequate management strategies, resulting in significant reduction in cardiovascular morbidity and mortality due to MetS with TD.
The pattern and relationship of thyroid function with such diseases or syndromes has never been studied in Nepalese population. Nepal is one of the high risk populations for TD [32]. Hence, TD should be considered in the differential diagnosis of patients with MetS to avoid misdiagnosis. The prevalence of obesity and the MetS is rapidly increasing in South Asians residing on the Indian subcontinent (e.g., India, Pakistan, Bangladesh, and Nepal), leading to increased morbidity and mortality due to T2DM and ASCVD [4,5]. Thus, it is necessary to initiate early detection of these chronic diseases in underdeveloped countries in Asia like Nepal, so that preventive action can minimize the consequences. Our findings indicate a need for a systematic approach to investigate the presence of TD when evaluating and treating patients with MetS.
There are few limitations of the present study, first is that; this is a cross-sectional study, a cause and effect of relationship could not be determined. Further cohort study is needed to evaluate the deleterious effect of TD on cardiovascular disease and metabolic functions. Second, this study did not find the association between TSH and fT4 and MetS as well as its components in people with TD and its subforms, and the reason might be there were few subjects with hypothyroidism and subclinical hyperthyroidism. Therefore, large epidemiological studies are needed to evaluate the relationship between subgroups of TD in patients with MetS.
In conclusion, the prevalence of TD in patients with MetS was high, distinctively in SCH and females were at increased risk. Although, thyroid hormone significantly affects each component of MetS, there was no relationship between TD and all components of MetS. There is an increased incidence of TD, especially elevated TSH with normal T4 and T3 in MetS. Furthermore the coexistence of the two disease entities might have substantially increase ASCVD, hence it is worthwhile to order for TSH and fT4 in all patients with MetS which can help in reducing the risk.
ACKNOWLEDGMENTS
We are indebted to each of the study participants for the substantial time and effort contributed to this study. We would like to acknowledge all technical staff at Department of Clinical Biochemistry and paramedical staff of Dhulikhel Hospital-Kathmandu University Hospital, Dhulikhel, Nepal for their technical support. We also wish to thank Ms. Niti Pant and Ms. Sudata Gurung for their invaluable assistance in collecting the data.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.International Diabetes Federation: The IDF consensus worldwide definition of the metabolic syndrome. [updated 2014 Dec 17]. Available from: http://www.idf.org/webdata/docs/MetSyndrome_FINAL.pdf.
- 2.Tehrani FR, Tohidi M, Dovom MR, Azizi F. A population based study on the association of thyroid status with components of the metabolic syndrome. J Diabetes Metab. 2011;2:156–162. [Google Scholar]
- 3.Sharma SK, Ghimire A, Radhakrishnan J, Thapa L, Shrestha NR, Paudel N, Gurung K, R M, Budathoki A, Baral N, Brodie D. Prevalence of hypertension, obesity, diabetes, and metabolic syndrome in Nepal. Int J Hypertens. 2011;2011:821971. doi: 10.4061/2011/821971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Misra A, Khurana L. The metabolic syndrome in South Asians: epidemiology, determinants, and prevention. Metab Syndr Relat Disord. 2009;7:497–514. doi: 10.1089/met.2009.0024. [DOI] [PubMed] [Google Scholar]
- 5.Misra A, Misra R, Wijesuriya M, Banerjee D. The metabolic syndrome in South Asians: continuing escalation & possible solutions. Indian J Med Res. 2007;125:345–354. [PubMed] [Google Scholar]
- 6.Diaz-Olmos R, Nogueira AC, Penalva DQ, Lotufo PA, Bensenor IM. Frequency of subclinical thyroid dysfunction and risk factors for cardiovascular disease among women at a workplace. Sao Paulo Med J. 2010;128:18–23. doi: 10.1590/S1516-31802010000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu YY, Brent GA. Thyroid hormone crosstalk with nuclear receptor signaling in metabolic regulation. Trends Endocrinol Metab. 2010;21:166–173. doi: 10.1016/j.tem.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal G, Sudhakar MK, Singh M, Senthil N, Rajendran A. The prevalence of thyroid dysfunction among south Indian women with metabolic syndrome. J Clin Diagn Res. 2011;5:213–216. [Google Scholar]
- 9.Shantha GP, Kumar AA, Jeyachandran V, Rajamanickam D, Rajkumar K, Salim S, Subramanian KK, Natesan S. Association between primary hypothyroidism and metabolic syndrome and the role of C reactive protein: a cross-sectional study from South India. Thyroid Res. 2009;2:2. doi: 10.1186/1756-6614-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SB, Choi HC, Joo NS. The relation of thyroid function to components of the metabolic syndrome in Korean men and women. J Korean Med Sci. 2011;26:540–545. doi: 10.3346/jkms.2011.26.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong ND, Sciammarella MG, Polk D, Gallagher A, Miranda-Peats L, Whitcomb B, Hachamovitch R, Friedman JD, Hayes S, Berman DS. The metabolic syndrome, diabetes, and subclinical atherosclerosis assessed by coronary calcium. J Am Coll Cardiol. 2003;41:1547–1553. doi: 10.1016/s0735-1097(03)00193-1. [DOI] [PubMed] [Google Scholar]
- 12.Ladenson PW, Singer PA, Ain KB, Bagchi N, Bigos ST, Levy EG, Smith SA, Daniels GH, Cohen HD. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med. 2000;160:1573–1575. doi: 10.1001/archinte.160.11.1573. [DOI] [PubMed] [Google Scholar]
- 13.ClinLab Navigator: Thyroid function tests. [updated 2014 Dec 17]. Available from: http://www.clinlabnavigator.com/thyroid-function-tests.html.
- 14.Meher LK, Raveendranathan SK, Kota SK, Sarangi J, Jali SN. Prevalence of hypothyroidism in patients with metabolic syndrome. Thyroid Res Pract. 2013;10:60–64. [Google Scholar]
- 15.Ayturk S, Gursoy A, Kut A, Anil C, Nar A, Tutuncu NB. Metabolic syndrome and its components are associated with increased thyroid volume and nodule prevalence in a mild-to-moderate iodine-deficient area. Eur J Endocrinol. 2009;161:599–605. doi: 10.1530/EJE-09-0410. [DOI] [PubMed] [Google Scholar]
- 16.Lai Y, Wang J, Jiang F, Wang B, Chen Y, Li M, Liu H, Li C, Xue H, Li N, Yu J, Shi L, Bai X, Hou X, Zhu L, Lu L, Wang S, Xing Q, Teng X, Teng W, Shan Z. The relationship between serum thyrotropin and components of metabolic syndrome. Endocr J. 2011;58:23–30. doi: 10.1507/endocrj.k10e-272. [DOI] [PubMed] [Google Scholar]
- 17.Tarcin O, Abanonu GB, Yazici D, Tarcin O. Association of metabolic syndrome parameters with TT3 and FT3/FT4 ratio in obese Turkish population. Metab Syndr Relat Disord. 2012;10:137–142. doi: 10.1089/met.2011.0098. [DOI] [PubMed] [Google Scholar]
- 18.Garduno-Garcia Jde J, Alvirde-Garcia U, Lopez-Carrasco G, Padilla Mendoza ME, Mehta R, Arellano-Campos O, Choza R, Sauque L, Garay-Sevilla ME, Malacara JM, Gomez-Perez FJ, Aguilar-Salinas CA. TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur J Endocrinol. 2010;163:273–278. doi: 10.1530/EJE-10-0312. [DOI] [PubMed] [Google Scholar]
- 19.Singh BM, Goswami B, Mallika V. Association between insulin resistance and hypothyroidism in females attending a tertiary care hospital. Indian J Clin Biochem. 2010;25:141–145. doi: 10.1007/s12291-010-0026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JY, Wang CY, Pei D, Lai CC, Chen YL, Wu CZ, Chang YL, Hsu CH, Pei C, Tang SH. Association between thyroid function and metabolic syndrome in elderly subjects. J Am Geriatr Soc. 2010;58:1613–1614. doi: 10.1111/j.1532-5415.2010.02998.x. [DOI] [PubMed] [Google Scholar]
- 21.Lai CC, Tang SH, Pei D, Wang CY, Chen YL, Wu CZ, Hsiao FC, Chen HS, Wang JY. The prevalence of subclinical thyroid dysfunction and its association with metabolic syndrome in Taiwanese elderly. Int J Gerontol. 2011;5:25–29. [Google Scholar]
- 22.Uzunlulu M, Yorulmaz E, Oguz A. Prevalence of subclinical hypothyroidism in patients with metabolic syndrome. Endocr J. 2007;54:71–76. doi: 10.1507/endocrj.k06-124. [DOI] [PubMed] [Google Scholar]
- 23.Jayakumar RV. Hypothyroidism and metabolic syndrome. Thyroid Res Pract. 2013;10:1–2. [Google Scholar]
- 24.Ogbera AO, Kuku S, Dada O. The metabolic syndrome in thyroid disease: a report from Nigeria. Indian J Endocrinol Metab. 2012;16:417–422. doi: 10.4103/2230-8210.95688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kota SK, Meher LK, Krishna S, Modi K. Hypothyroidism in metabolic syndrome. Indian J Endocrinol Metab. 2012;16(Suppl 2):S332–S333. doi: 10.4103/2230-8210.104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh JY, Sung YA, Lee HJ. Elevated thyroid stimulating hormone levels are associated with metabolic syndrome in euthyroid young women. Korean J Intern Med. 2013;28:180–186. doi: 10.3904/kjim.2013.28.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang CY, Chang TC, Chen MF. Associations between subclinical thyroid disease and metabolic syndrome. Endocr J. 2012;59:911–917. doi: 10.1507/endocrj.ej12-0076. [DOI] [PubMed] [Google Scholar]
- 28.Kim BJ, Kim TY, Koh JM, Kim HK, Park JY, Lee KU, Shong YK, Kim WB. Relationship between serum free T4 (FT4) levels and metabolic syndrome (MS) and its components in healthy euthyroid subjects. Clin Endocrinol (Oxf) 2009;70:152–160. doi: 10.1111/j.1365-2265.2008.03304.x. [DOI] [PubMed] [Google Scholar]
- 29.Dessein PH, Joffe BI, Stanwix AE. Subclinical hypothyroidism is associated with insulin resistance in rheumatoid arthritis. Thyroid. 2004;14:443–446. doi: 10.1089/105072504323150750. [DOI] [PubMed] [Google Scholar]
- 30.Tseng FY, Lin WY, Lin CC, Lee LT, Li TC, Sung PK, Huang KC. Subclinical hypothyroidism is associated with increased risk for all-cause and cardiovascular mortality in adults. J Am Coll Cardiol. 2012;60:730–737. doi: 10.1016/j.jacc.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 31.Kang HM, Kim DJ. Metabolic syndrome versus Framingham risk score for association of self-reported coronary heart disease: The 2005 Korean Health and Nutrition Examination Survey. Diabetes Metab J. 2012;36:237–244. doi: 10.4093/dmj.2012.36.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aryal M, Gyawali P, Rajbhandari N, Aryal P, Pandeya DR. A prevalence of thyroid dysfunction in Kathmandu University Hospital, Nepal. Biomed Res. 2010;21:411–415. [Google Scholar]