Abstract
Although GTPases of the Ras family have been implicated in many aspects of the regulation of cells, little is known about the roles of individual family members. Here, we analyzed the mechanisms of activation of H-Ras, N-Ras, K-Ras 4B, and M-Ras by two types of external stimuli, growth factors and ligation of the antigen receptors of B or T lymphocytes (BCRs and TCRs). The growth factors interleukin-3, colony-stimulating factor 1, and epidermal growth factor all preferentially activated M-Ras and K-Ras 4B over H-Ras or N-Ras. Preferential activation of M-Ras and K-Ras 4B depended on the presence of their polybasic carboxy termini, which directed them into high-buoyant-density membrane domains where the activated receptors, adapters, and mSos were also present. In contrast, ligation of the BCR or TCR resulted in activation of H-Ras, N-Ras, and K-Ras 4B, but not M-Ras. This pattern of activation was not influenced by localization of the Ras proteins to membrane domains. Activation of H-Ras, N-Ras, and K-Ras 4B instead depended on the presence of phospholipase C-γ and RasGRP. Thus, the molecular mechanisms leading to activation of Ras proteins vary with the stimulus and can be influenced by either colocalization with activated receptors or differential sensitivity to the exchange factors activated by a stimulus.
The p21 Ras proteins H-Ras, N-Ras, K-Ras 4A, and K-Ras 4B are members of a subfamily of the Ras superfamily of small GTPases, which also includes M-Ras, R-Ras, TC21, Rap1A/B, Rap2A/B, and RalA/B. The members of this subfamily exhibit remarkable structural similarities in regions involved in interactions with guanine nucleotide exchange factors (GEFs) and downstream effectors, resulting in considerable overlap in their regulation and effector functions (18). Although as a group these proteins have been implicated in many aspects of the regulation of various cell types, relatively little is known about the regulation and functions of individual family members. This stems in part from the fact that commonly used research tools fail to discriminate between many of these closely related proteins. For example, the monoclonal antibody Y13-259, which has been extensively used to measure the activation of p21 Ras proteins, is now known to cross-react with M-Ras and TC21 (reviewed in reference 18). Moreover, contrary to common assumptions, the use of a dominant-active or dominant-negative mutant of a particular Ras protein does not allow the drawing of firm conclusions about the function of that protein. This is because the dominant-negative mutants can sequester GEFs that are shared among multiple members of the Ras or even Rho GTPase families, potentially blocking activation of multiple small GTPases. Likewise, dominant-active mutants can activate effectors that are shared by other family members. It is thus conceivable that functions that have been attributed to “p21 Ras” are shared with or belong exclusively to other Ras family members. In particular, there is also no available information on whether different extracellular stimuli activate the closely related Ras proteins in parallel or differentially. The latter possibility is supported by evidence that the different Ras family members have specific functions. For example, only K-Ras is required for embryonic development in mice, whereas mice that lack functional alleles of both H-Ras and N-Ras develop normally (reviewed in reference 18).
Growth factors, such as interleukin-3 (IL-3), colony-stimulating factor 1 (CSF-1, or M-CSF), and epidermal growth factor (EGF), were reported to activate p21 Ras on the basis of assays using Y13-259 (6, 16, 21, 61). Likewise, ligation of the B- or T-cell antigen receptors (BCRs or TCRs) was also reported to result in activation of Ras proteins precipitated by Y13-259 (14, 26). However, given the cross-reactivity of this antibody, it is now evident that the activation that was measured could have reflected activation of other members of the Ras family, such as M-Ras or TC21. EGF can induce the activation of H-Ras, N-Ras, and K-Ras 4B (36, 48), but it is unclear if these proteins are activated to the same extent. It is also not known whether EGF induces the activation of M-Ras, which is expressed at much higher levels than p21 Ras proteins in fibroblasts (20) and could contribute to the essential roles of EGF in proliferation and differentiation (45, 65).
A variety of effects due to expression of dominant-negative mutants of p21 Ras in cells or in transgenic mice have been attributed to inhibition of functions of p21 Ras. These include inhibition of IL-3-dependent proliferation and inhibition of survival and terminal differentiation of macrophages (32, 52). However, the sharing of GEFs and GAPs among small GTPases means that the observed phenotype may not have been due to inhibition of activation of p21 Ras. Transgenic expression of dominant-negative H-Ras or genetic disruptions in the Ras-Erk pathway have also been shown to block the development of B and T cells (13, 28, 53, 69). Even though there is additional evidence implicating Ras signaling downstream of the BCR or TCR in lymphocyte development (17, 28), it remains unknown which members of the Ras family are the critical players.
One factor that could contribute to a possible differential activation of the closely related Ras family GTPases by extracellular stimuli is the differential usage of GEFs by the different receptors. Although there is evidence that some GEFs discriminate among members of the Ras family (11, 33), there is little information on whether this is physiologically relevant. In the case of growth factor receptors, activation of Ras proteins by the IL-3, CSF-1, and EGF receptors is thought to be mediated through mSos, which is recruited to tyrosine-phosphorylated receptor chains through complexes with Grb2, Shc, or SHP-2 (5, 37, 59, 70). Since p21 Ras proteins and M-Ras are activated by common GEFs, including mSos (50), it is conceivable that p21 Ras proteins and M-Ras would be activated in parallel by these receptors. However, factors other than sensitivity to GEFs may also contribute to the differential activation of different Ras proteins. For example, there is compelling evidence that H-Ras occurs in lipid rafts, whereas K-Ras 4B is excluded from these membrane domains (48, 57, 60). This differential localization is a result of differences in the posttranslational modifications of their carboxy termini. Thus, the carboxy terminus of H-Ras exhibits sites for palmitoylation and directs its transport via the Golgi apparatus to lipid rafts in the plasma membrane. In contrast, the carboxy terminus of K-Ras 4B lacks palmitoylation sites and instead exhibits a stretch of multiple basic residues. K-Ras 4B does not associate with the Golgi apparatus and takes a different, largely undefined route to the plasma membrane, where it localizes outside rafts (1, 57). This differential localization to membrane domains was shown to affect downstream signaling (31, 60). It is conceivable that this differential localization to membrane domains could affect susceptibility to activation following the ligation of cell surface receptors. The BCR and TCR can occur in lipid rafts (8, 77), but much less is known about the localization of growth factor receptors to membrane domains.
Here, we compared the activation of H-Ras, N-Ras, K-Ras 4B, and M-Ras following stimulation of cells by the growth factors IL-3, CSF-1, and EGF or ligation of the BCR or TCR. We found that the four Ras proteins were differentially activated by these stimuli and that distinct mechanisms controlled the pattern of activation downstream of the growth factor receptors and immunoreceptors.
MATERIALS AND METHODS
Constructs.
The cDNAs for K-Ras 4B, N-Ras (a gift from Janis Jackson, The Scripps Research Institute, La Jolla, Calif.), H-Ras, and M-Ras were cloned into pEGFP-C1 (Clontech) for expression of the Ras proteins fused at their amino termini with enhanced green fluorescent protein (EGFP) or into pcMyc (20), a vector derived from pCDNA3.1 (Stratagene), for expression of proteins fused at their amino termini with the myc epitope tag. Retroviral vectors were constructed by subcloning the myc-tagged Ras cDNAs into pMXpie (a gift from Alice Mui, Jack Bell Research Centre, Vancouver, Canada). This vector drives expression of EGFP from an internal ribosomal entry site downstream of the Ras cDNA and features a puromycin resistance gene driven by a separate promoter. Constructs encoding chimeric Ras proteins, in which the carboxy-terminal hypervariable regions following amino acid R164 (H-Ras and K-Ras 4B) or R176 (M-Ras) were exchanged, were generated using standard PCR techniques and cloned into pEGFP-C1, pcMyc, and pMXpie. The integrity of all constructs was verified by DNA sequencing. The mSos1 plasmid was a gift from Larry Feig (Tufts University School of Medicine, Boston, Mass.).
Cells, transfections, and stimulations.
Murine A20 B cells, murine WEHI-231 B cells, and human Jurkat T cells were maintained in RPMI medium with 10% fetal calf serum, 100 U of penicillin/ml, 50 U of streptomycin/ml, 1 mM sodium pyruvate, 2 mM l-glutamine, and 50 μM β-mercaptoethanol. DT40 chicken B cells, a DT40 clone lacking functional phospholipase C-γ2 (PLC-γ2), and a clone of the latter cells that had been reconstituted with exogenous PLC-γ2 (71) were grown in the above-mentioned medium supplemented with 1% chicken serum. The human wild-type CSF-1 receptor (CSF-1R) (a gift from Martine Roussel, St. Jude Children's Research Hospital, Memphis, Tenn.) with the addition of a carboxy-terminal hemagglutinin (HA) tag was expressed in IL-3-dependent Ba/F3 pro-B cells. A clone designated Ba/F3-Fms was established. Ba/F3-Fms cells were maintained in the above-mentioned medium supplemented with 3% of a 10×-concentrated conditioned medium from WEHI-3B cells as a source of IL-3. BOSC23 and NIH 3T3 cells were cultured in Dulbecco's modified Eagle's medium with 100 U of penicillin/ml, 50 U of streptomycin/ml, and 10% fetal calf serum or 10% calf serum, respectively.
Ba/F3-Fms cells were retrovirally transduced by incubation with supernatants from BOSC23 packaging cells that had been transiently transfected with pMXpie-Ras constructs in the presence of 10 μg of Polybrene/ml. Infected cells were selected in 2 μg of puromycin/ml. These polyclonal cell populations were used for stimulations within 2 weeks postinfection. NIH 3T3 cells were transiently transfected by electroporation with 20 μg of pcMyc-based constructs and used 1 to 2 days posttransfection. A20, Jurkat, and DT40 cells were electroporated with 20 μg of pEGFP constructs. After 4 to 8 (A20 or Jurkat cells) or 16 (DT40 cells) h, dead cells were removed by density centrifugation with Ficoll-Paque (Amersham Pharmacia), and live cells were used for stimulations. The cells were subjected to serum (and factor) starvation (1.5 to 2 h), followed by stimulation with either 15 μg of synthetic IL-3 peptide (a gift from the late Ian Clark-Lewis, The Biomedical Research Centre, Vancouver, Canada)/ml, 100 ng of recombinant human CSF-1 (R&D Systems)/ml, 100 ng of EGF (R&D Systems)/ml, 100 μg of anti-mouse immunoglobulin G (IgG) [F(ab′)2; Jackson Immunoresearch]/ml, 10 μg of anti-human CD3 clone OKT3 (a gift from Maya Kotturi, The Biomedical Research Centre)/ml, 5 μg of anti-chicken IgM (Southern Biotechnology Associates)/ml, 30 or 50 μg of anti-IgM [F(ab′)2; Jackson Immunoresearch]/ml, or 50 μM phorbol dibutyrate (PdBu; Sigma).
Ras activation assay and Western blots.
Cells were lysed in pull-down buffer (1% NP-40, 50 mM Tris-HCl, pH 7.5, 200 mM NaCl, 5 mM MgCl2, 15% glycerol) with protease and phosphatase inhibitors (40 μg of phenylmethylsulfonyl fluoride/ml, 0.7 μg of pepstatin/ml, 10 μg of soy bean trypsin inhibitor/ml, 1 mM sodium vanadate). Approximately 20 μg of fusion proteins of glutathione S-transferase (GST) with the Ras-binding domain (RBD) of Raf-1 or Nore1 (19) bound to glutathione-Sepharose (Amersham Pharmacia) was incubated with aliquots of lysates for 30 min at 4°C to precipitate activated H-Ras, N-Ras, or K-Ras 4B (with Raf-1) or M-Ras (with Nore1). Preliminary experiments demonstrated that these reagents were the most efficient for precipitating activated p21 Ras and M-Ras, respectively. Control experiments comparing the efficiencies of these reagents for precipitating constitutively active H-Ras or M-Ras showed that in each case ∼70 to 80% of the activated Ras was depleted. Pull-down samples were run in parallel with an aliquot of the cell lysate, derived from the unstimulated controls, on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to allow determination by densitometry of the percentage of total exogenous Ras that was activated for each stimulus (using NIH Image software), thus allowing comparison of the degrees of activation. After SDS-PAGE, proteins were transferred to nitrocellulose membranes that were immunoblotted with antibodies to the myc tag (9E10 mouse ascites; a gift from Hermann Ziltener, The Biomedical Research Centre), GFP (Clontech or Santa Cruz Biotechnology), or p21 Ras (clone Ras10; Upstate Biotechnology) to visualize myc- or GFP-tagged Ras proteins or endogenous p21 Ras. The anti-PKCδ antibody (BD Transduction Laboratories) was a gift from Michael Gold, University of British Columbia, Vancouver, Canada. Anti-EGF receptor (EGFR), anti-phospho-Erk1/2, antiactin, and anti-HA were purchased from Upstate Biotechnology, BD Transduction Laboratories, Cell Signaling Technologies, Sigma, and Covance, respectively; all other antibodies were from Santa Cruz Biotechnology. After incubation with primary antibodies, the blots were incubated with horseradish peroxidase-labeled secondary antibodies, and enhanced-chemiluminescence detection (Amersham Pharmacia) was performed. Equivalency of loading was always verified by blotting for the exogenous Ras protein.
Sucrose gradients.
To assess the segregation of Ras proteins, receptors, and receptor-associated molecules into different areas of the membrane, we used a modification of the detergent-free method for purification of caveolin-rich membranes that was first employed by Song et al. to demonstrate an association of H-Ras with these membranes (67). Ba/F3-Fms cells were starved of serum, stimulated as described above, and then lysed in 0.5 M Na2CO3 in MBS-M (25 mM morpholinoethanesulfonic acid, 150 mM NaCl, 5 mM MgCl2, pH 6.5), followed by three sonication bursts of 15 to 20 s at 6 to 7 W. The sonicate (200 μl) was mixed with 200 μl of 90% sucrose in MBS-M and placed at the bottom of a 2-ml ultracentrifuge tube. The mixture was overlaid with 1.2 ml of 35% sucrose in MBS-M and 400 μl of 5% sucrose in MBS-M. Protease and phosphatase inhibitors were present throughout the gradient. The samples were centrifuged at 250,000 × g in a Beckman TL-100 ultracentrifuge for ∼14 h at 4°C. Twelve 160-μl fractions were collected from the top of the gradient. The first two fractions were discarded, as preliminary experiments showed that they did not contain detectable amounts of protein on Western blots. Fractions 3 to 12 were each diluted in 370 μl of MBS-M, and the membranes were pelleted at 100,000 × g for 30 min. The membrane pellets were resuspended in SDS loading buffer, boiled, and subjected to SDS-PAGE and Western blotting.
RESULTS
Growth factors preferentially activate M-Ras and K-Ras 4B over H-Ras or N-Ras.
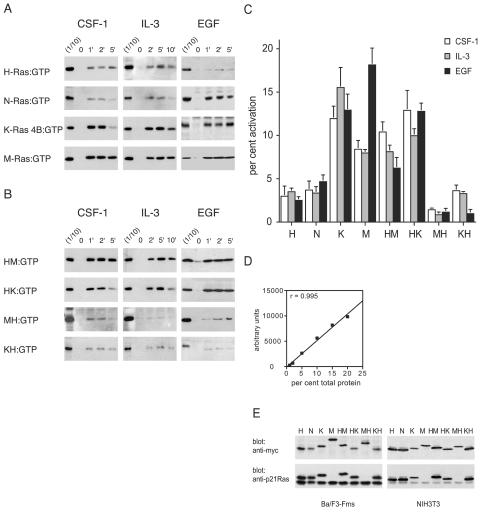
To determine whether there were differences in the activations of different members of the Ras subfamily of GTPases by different extracellular stimuli, we chose to study four members of the Ras family, H-Ras, N-Ras, K-Ras 4B, and M-Ras. We first examined the responses to three growth factors: CSF-1 and EGF, which act through classical receptor tyrosine kinases, and IL-3, which acts through a receptor of the cytokine receptor family. We expressed tagged versions of the four Ras proteins in various cell lines to allow their identification with full confidence. We observed that stimulation of Ba/F3-Fms cells with either IL-3 or CSF-1 resulted in efficient activation of both K-Ras 4B and M-Ras, with maximal levels of activation between 8 and 15%. In contrast, the levels of activation of H-Ras or N-Ras by either growth factor were consistently less, with only ∼4% of total H-Ras or N-Ras being activated (Fig. 1A). Stimulation of fibroblasts with EGF also led to more efficient activation of K-Ras 4B (∼12%) than of H-Ras or N-Ras (∼3 to 5%), and the activation of M-Ras was very strong (∼17%; Fig. 1A). Collectively, these data show that there were significant quantitative differences in the levels of activation of different Ras proteins by growth factors, with M-Ras and K-Ras 4B being preferentially activated over H-Ras or N-Ras by all three growth factors (Fig. 1C).
FIG. 1.
Growth factors preferentially activate polybasic Ras proteins. (A) Activation of H-Ras, N-Ras, K-Ras 4B, or M-Ras expressed in Ba/F3-Fms or NIH 3T3 cells was assessed after stimulation of the cells for the indicated times (in minutes) with CSF-1, IL-3, or EGF. GST-Raf-1 RBD was used to precipitate activated, GTP-bound H-Ras, N-Ras, and K-Ras 4B from cell lysates, and GST-Nore1 RBD was used to precipitate GTP-bound activated M-Ras. One-tenth of the amount of the lysate used for precipitation was run in parallel on the same gel (1/10) to allow quantitation by densitometry of the percentage of total Ras that was precipitated. (B) Activation of chimeric Ras proteins expressed in Ba/F3-Fms or NIH 3T3 cells was assessed as described for panel A. All samples of cell lysates were also analyzed for levels of phosphorylated Erk1/2, and equivalency of loading was confirmed by blotting for the exogenous Ras protein (not shown). The results shown are representative of at least three independent experiments for each stimulus and Ras construct. (C) Densitometry was performed in three experiments for each of the constructs and stimuli shown in panels A and B. The value for the first lane (1/10) of each blot was set to 10%, and the relative values of the intensities of bands from pull-down samples were expressed as percentages of the total exogenous Ras. In cases where the precipitation of Ras from the unstimulated sample (at time zero) showed detectable levels of activation, this value was subtracted from those of other samples. The graph shows means and standard errors. P values, calculated using a nonpaired, two-tailed t test, comparing each of the Ras proteins with polybasic tails with each of the palmitoylated Ras proteins were all <0.05. The one exception was the comparison of the HM chimera with N-Ras in the EGF series, where P was 0.3. (D) To validate our assay system (enhanced-chemiluminescence detection and densitometry), lysates from cells expressing tagged Ras proteins were titrated. The percentages of total protein indicate the amounts of cell lysate in relation to that usedin the pull-down assays. After Western blotting, densitometry was performed, and the values were plotted against the amount of protein loaded. The graph shows results, and a linear-regression coefficient (r) of 0.995. The linear-regression coefficient in a second experiment was 0.95. (E) Lysates from Ba/F3-Fms or NIH 3T3 cells were analyzed to allow comparison of the expression levels of exogenous and endogenous Ras proteins using an anti-p21 Ras antibody (which does not detect M-Ras). Also shown are the relative levels of expression of the exogenous proteins used, detected with an anti-myc antibody.
The nature of the carboxy terminus affects the level of activation of Ras proteins by growth factors.
The two Ras family members that were preferentially activated by growth factors, K-Ras 4B and M-Ras, both differ from H-Ras and N-Ras in having polybasic carboxy termini. To explore the possibility that the observed differences in levels of activation of Ras proteins by growth factors correlated with the presence of polybasic carboxy termini, we investigated the activation of the chimeric Ras proteins in which the carboxy termini had been exchanged. We observed that chimeras of M-Ras or K-Ras 4B with the palmitoylated carboxy terminus of H-Ras (MH and KH, respectively) were not as efficiently activated as their wild-type counterparts by all three growth factors, IL-3, CSF-1, and EGF (Fig. 1A, B, and C). Conversely, chimeras of H-Ras with the polybasic carboxy termini of either K-Ras 4B or M-Ras (HK and HM, respectively) were activated much more efficiently than was wild-type H-Ras, again by all three factors (Fig. 1A, B, and C). The levels of overexpression of exogenous Ras proteins did not appear to affect their relative levels of activation. We obtained very similar results for both Ba/F3-Fms cells, in which levels of exogenous Ras proteins were comparable to those of endogenous p21 Ras, and NIH 3T3 cells, in which exogenous Ras proteins were grossly overexpressed (Fig. 1E). These findings thus indicated that the preferential activation of K-Ras 4B and M-Ras by growth factors correlated with the presence of a polybasic carboxy terminus.
Palmitoylated Ras proteins localize to lipid rafts in hematopoietic cells.
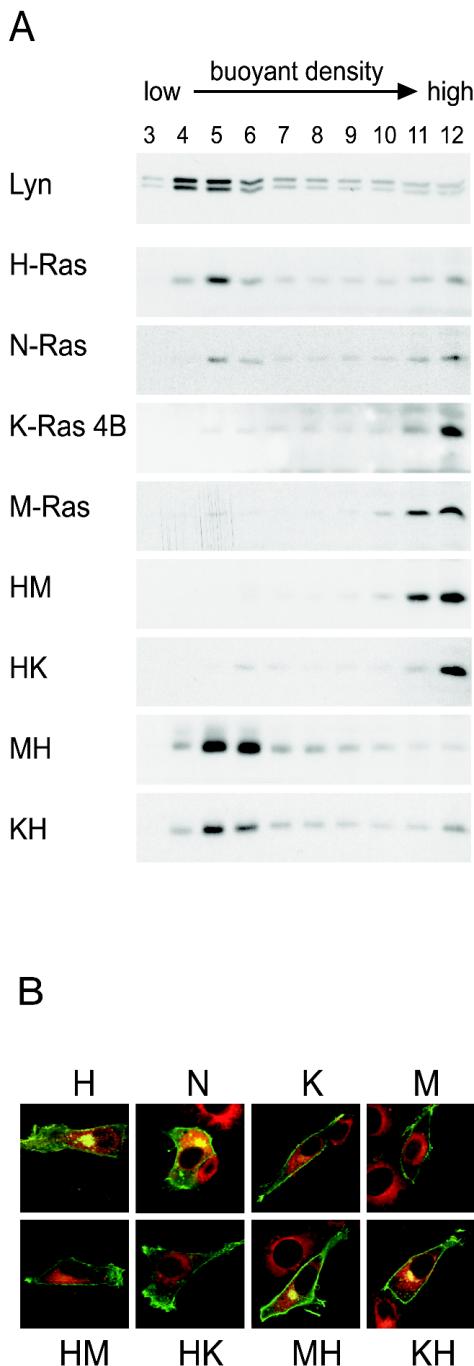
The fact that the preferential activation of Ras proteins correlated with the presence of polybasic carboxy termini suggested that this might be influencing the efficiency of Ras activation by growth factors by directing the Ras proteins to the disordered regions of the membrane. It was not known whether H-Ras and K-Ras 4B localized to the raft and nonraft regions of the membranes of hematopoietic cells, which lack the caveolae that were present on the cells used in other studies (48, 57). Thus, we investigated whether H-Ras and K-Ras 4B exhibited the patterns of membrane localization and trafficking in hematopoietic cells predicted from studies in cells with caveolae. In Ba/F3-Fms cells, H-Ras indeed accumulated in low-density fractions of sucrose gradients that also contained Lyn, a marker for lipid rafts (8) (Fig. 2A). In contrast, K-Ras 4B was excluded from the low-density fractions (Fig. 2A). We next tested the prediction that M-Ras, having a polybasic carboxy terminus, would localize in the disordered membranes and that N-Ras, having a palmitoylated carboxy terminus, would localize to rafts. This was the case (Fig. 2A). The fact that M-Ras was localized in the same regions of the membrane as K-Ras 4B extends and confirms evidence that polybasic carboxy termini of small GTPases determine localization in the disordered plasma membrane. Moreover, exchanging the palmitoylated carboxy terminus of H-Ras for the polybasic carboxy terminus of either K-Ras 4B or M-Ras resulted in targeting of these chimeras of H-Ras (HK and HM) to membranes of high buoyant density, while the palmitoylated carboxy terminus of H-Ras directed M-Ras or K-Ras 4B (MH or KH) to the low-density fractions (Fig. 2A). These data indicated that the palmitoylated carboxy terminus of H-Ras provides a necessary and sufficient signal to direct Ras proteins to membrane domains of low buoyant density in hematopoietic cells, confirming previous observations made in fibroblasts that have caveolae (48, 57).
FIG. 2.
Ras proteins with a palmitoylated carboxy terminus localize to low-density raft fractions and accumulate in the Golgi apparatus. (A) Sonicates of Ba/F3-Fms cells expressing the indicated myc-tagged Ras constructs were fractionated by buoyant density using sucrose gradients. Twelve fractions were collected from the top of the gradient. Fractions 1 and 2 were discarded. After dilution, membranes were pelleted by further ultracentrifugation. The pellets were resuspended in SDS loading buffer and subjected to SDS-PAGE and blotting with anti-myc antibodies to visualize Ras proteins. The top blot indicates the location of Lyn, a marker for rafts. Each blot is representative of at least three independent experiments. (B) NIH 3T3 cells were transiently transfected with GFP-tagged Ras constructs as indicated. The cells were stained with a red fluorescent ceramide (Molecular Probes) to mark the localization of the Golgi apparatus, fixed, and imaged by confocal microscopy. Merged images are shown. The yellow color seen in the case of Ras proteins with palmitoylated carboxy termini indicates the colocalization of green (Ras protein) and red (Golgi marker). Box size, 63 by 63 μm.
We also examined whether M-Ras, with its polybasic carboxy terminus, would resemble K-Ras 4B in its failure to associate with the Golgi apparatus (1). We expressed GFP-tagged H-Ras, N-Ras, K-Ras 4B, and M-Ras or chimeras in which we had exchanged the carboxy termini in NIH 3T3 cells. As expected, H-Ras and N-Ras, but not K-Ras 4B, accumulated in the Golgi apparatus (Fig. 2B) (1, 10). M-Ras clearly behaved like K-Ras 4B, with no evidence of association with the Golgi apparatus. Moreover, the chimera MH confirmed that the presence of a palmitoylated carboxy terminus was sufficient to dictate its accumulation in the Golgi apparatus (Fig. 2B). We saw a similar lack of association of M-Ras or K-Ras 4B in the Golgi apparatus of the B-cell line A20, whereas H-Ras and N-Ras accumulated in this region (not shown). Thus, all Ras proteins used in our study exhibited the patterns of trafficking and membrane association predicted from studies of H-Ras and K-Ras 4B in fibroblasts.
The activated IL-3R, CSF-1R, and EGFR localize to nonraft membranes.
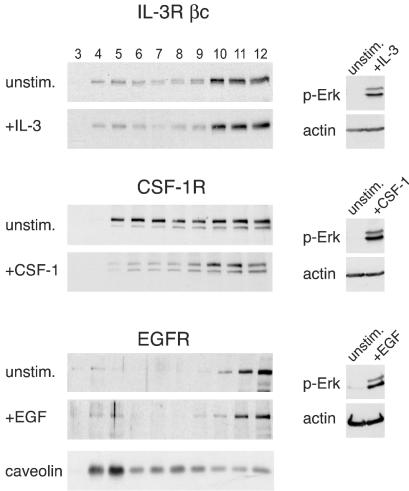
To explore the mechanisms through which stimulation of growth factor receptors preferentially activate Ras proteins with polybasic carboxy termini (M-Ras and K-Ras 4B), we investigated the possibility that the IL-3 receptor (IL-3R), CSF-1R, and EGFR colocalized in the same regions of the membrane as M-Ras and K-Ras 4B. In unstimulated Ba/F3-Fms cells, the βc chain of the IL-3R (the signal-transducing subunit of the α/β heterodimeric receptor) was detected in the high-density fractions of the sucrose gradients. This location of βc in high-density fractions was unchanged after stimulation with IL-3 (Fig. 3). In contrast, whereas in resting cells the CSF-1R occurred in fractions across the gradient, in cells treated with CSF-1 it occurred preferentially in the high-density fractions (Fig. 3), where M-Ras and K-Ras 4B were also found (Fig. 2A). We also determined the location of the EGFR in the membranes of NIH 3T3 cells. As expected, caveolin was enriched in the low-density fractions of the gradients (Fig. 3). However, the EGFR occurred in the high-density fractions, both before and after stimulation with EGF (Fig. 3). We obtained similar results concerning the locations of these receptors in experiments using gradients containing detergents (data not shown). These data indicated that, independent of their activation states, the IL-3Rβc and the EGFR were found outside of lipid rafts. In contrast, the CSF-1R was present in both rafts and disordered membranes in unstimulated cells but moved out of rafts into the high-density fractions following activation. These results indicate that in the case of all three growth factor receptors, the activated receptors cofractionated in the disordered membrane with those Ras proteins, M-Ras and K-Ras 4B, that were preferentially activated.
FIG. 3.
Locations of growth factor receptors in sucrose gradients with and without stimulation. Sonicates from unstimulated Ba/F3-Fms or NIH 3T3 cells or from cells that had been stimulated with IL-3 for 5 min, with CSF-1 for 2 min, or with EGF for 1.5 min were prepared and fractionated over sucrose gradients, and the membranes were pelleted. The locations of the receptors in fractions from the gradients were determined by blotting with anti-βc or anti-HA (for the CSF-1R) or with anti-EGFR (left). Aliquots from the same sonicates that were applied to sucrose gradients were also analyzed for phosphorylated Erk1/2 to assess the efficiency of growth factor stimulation (right). Each blot is representative of at least three independent experiments. The bottom blot indicates the location of caveolin in gradients obtained from NIH 3T3 cells.
Shc, Grb2, and mSos localize outside lipid rafts.
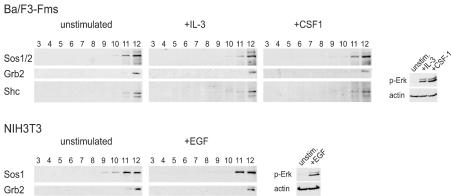
There is evidence for a critical role of the exchange factor mSos in the activation of Ras proteins induced by IL-3 and EGF and for at least a partial role in that induced by CSF-1 (5, 37, 70). All p21 Ras proteins, as well as M-Ras, can be activated by mSos (50), which is recruited to activated receptors through the interaction of its binding protein, Grb2. To analyze in more detail the underlying mechanisms of the preferential activation of M-Ras and K-Ras 4B downstream of growth factor receptors, we asked whether Grb2, Shc, and mSos colocalized with the receptors and the relevant Ras proteins. We observed that membrane-bound mSos localized exclusively to high-density nonraft fractions of gradients from either Ba/F3-Fms or NIH 3T3 cells (Fig. 4). Its localization in disordered membranes did not change upon stimulation with any of the growth factors IL-3, CSF-1, or EGF. We also noted that the adapter proteins Shc and Grb2 were present in the high-density fractions, regardless of activation of the receptors (Fig. 4). Although we could not quantify any changes in the amounts of mSos, Grb2, and Shc associated with membranes following stimulation with growth factors, as the gradients from unstimulated and stimulated cells were analyzed on different immunoblots, this question had been investigated previously, and no increase in the amounts of mSos, Grb2, or Shc associated with membranes had been found following stimulation with hematopoietic growth factors (75). Certainly, our data show that multiple components of the activated receptor complex, including Shc, Grb2, and mSos, occurred in high-density membrane fractions together with the preferentially activated K-Ras 4B and M-Ras.
FIG. 4.
mSos1/2, Grb2, and Shc occur in high-density fractions in sucrose gradients. Sonicates from Ba/F3-Fms or NIH 3T3 cells that were either unstimulated or stimulated with IL-3 for 5 min, CSF-1 for 2 min, or EGF for 1.5 min were fractionated over sucrose gradients. The locations of mSos1/2, Grb2, and Shc in membrane fractions obtained from the gradients were determined by Western blotting using the corresponding antibodies (left). Aliquots from the same sonicates that were applied to sucrose gradients were also analyzed for levels of phosphorylated Erk1/2 to assess the efficiency of growth factor stimulation (right). Each blot is representative of at least three independent experiments.
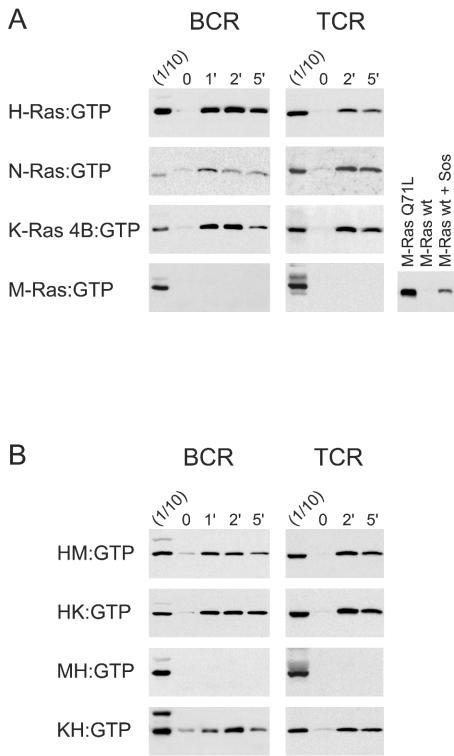
Ligation of the BCR or TCR induces activation of H-Ras, N-Ras, and K-Ras 4B, irrespective of their localization to membrane domains, but does not lead to activation of M-Ras.
We next investigated the patterns of activation of the four different Ras proteins in response to ligation of the receptors for antigens on B or T lymphocytes. These are both multisubunit receptors that lack intrinsic tyrosine kinase activity but are associated with a number of kinases of the Src, Syk, and Tec families. In contrast to our results with stimulation by growth factors, ligation of either the BCR or TCR resulted in efficient activation of both the palmitoylated H-Ras and N-Ras, as well as the nonpalmitoylated K-Ras 4B (Fig. 5A). Strikingly, M-Ras, which had been efficiently activated by stimulation of all three growth factor receptors, was not activated at all (Fig. 5A). In light of the plentiful evidence that the activated BCR and TCR localize to lipid rafts (8, 15, 47, 55, 77), where they could potentially activate signaling molecules that also preferentially localize to rafts, the efficient activation of K-Ras 4B, which does not localize to rafts, was surprising. We therefore investigated the localization to membrane domains of the Ras proteins in A20 B cells. These studies confirmed that K-Ras 4B, M-Ras, and the chimeric Ras proteins with polybasic carboxy termini localized to high-density nonraft fractions of A20 B lymphocytes, while H-Ras and the chimeras with the carboxy terminus of H-Ras still localized in low-density raft fractions, as expected (data not shown).
FIG. 5.
Ligation of BCR or TCR induces activation of H-Ras, N-Ras, and K-Ras 4B, but not M-Ras, irrespective of localization to membrane domains. A20 B cells and Jurkat T cells were transfected with the indicated wild-type (A) or chimeric (B) Ras constructs and stimulated with either anti-IgG or anti-CD3 for the indicated times (in minutes). Lysates were subjected to pull-down assays to precipitate activated, GTP-bound Ras proteins. One-tenth of the amount of lysate used for precipitation was run in parallel with the precipitates (1/10). The last blot in the bottom row of panel A shows a control experiment in which activated M-Ras was precipitated from HEK293 cells transfected with equal amounts of M-Ras constructs with or without mSos1. All cell lysates were also analyzed for levels of phosphorylated Erk1/2, and equal loading was confirmed by blotting for the exogenous Ras protein (not shown). The results shown are representative of at least three independent experiments for each stimulus and Ras construct.
We also examined the effects of ligation of the BCR or TCR on activation of chimeras of H-Ras that were targeted to nonraft membranes by virtue of the polybasic carboxy termini of either M-Ras or K-Ras 4B (HM or HK). We observed that both chimeras were strongly activated (Fig. 5B). Conversely, even when M-Ras was targeted to lipid rafts by replacement of its polybasic carboxy terminus with the palmitoylated carboxy terminus of H-Ras (MH), it was still not activated (Fig. 5B). Thus, ligation of the BCR or TCR resulted in activation of H-Ras, N-Ras, or K-Ras 4B, regardless of whether they were targeted to rafts or nonraft domains. In contrast to stimulation by growth factors, where the preferential activation of the four different Ras proteins correlated with whether they were localized in the disordered membrane, localization in membrane subdomains was not a factor in determining the degree of activation of Ras family members following ligation of the BCR or TCR.
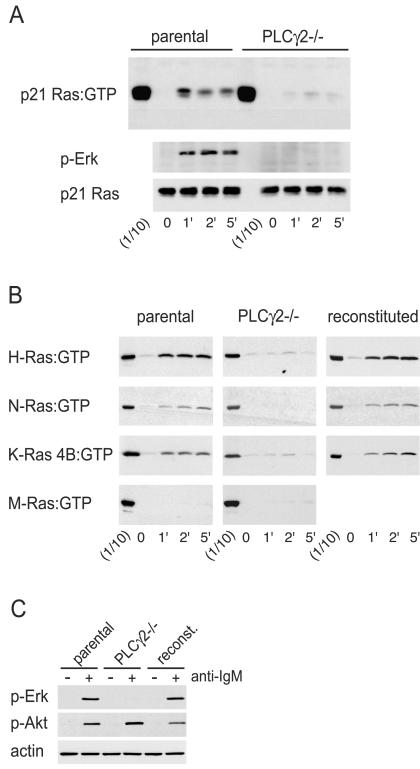
Activation of H-Ras, N-Ras, and K-Ras 4B by the BCR is dependent on PLC-γ2.
Since the ability of Ras proteins to become activated by the BCR and TCR was not determined by the localization of the former to specific membrane domains, we asked whether their differential activation resulted from differences in susceptibility to activation by the relevant GEFs. Recent data suggested a critical role for members of the RasGRP family in TCR-mediated p21 Ras activation (17), although the roles of these DAG-responsive GEFs in BCR-induced Ras activation were unclear. DAG can be generated through cleavage of PI(4,5)P2 by PLC-γ enzymes, and the PLC-γ2 isoform accounts for all increases in PLC-γ activity by ligation of the BCR (71). Therefore, we examined the activation of Ras proteins induced by ligation of the BCR in a clone of the chicken B-cell line DT40 that lacks PLC-γ2 (71). As shown in Fig. 6A, the absence of PLC-γ2 resulted in a dramatic reduction in activation of endogenous p21 Ras following ligation of the BCR. This result is in agreement with data obtained by pharmacological inhibition of PLC-γ (7). Phosphorylation of Erk was also severely reduced (Fig. 6A and C), as had been reported before (27), while the phosphorylation of Akt was unaffected (Fig. 6C). We next investigated whether BCR-mediated activation of specific Ras proteins was dependent on the presence of PLC-γ2. In the parental DT40 cells, ligation of the BCR resulted in efficient activation of exogenous H-Ras, N-Ras, or K-Ras 4B, but the activation of M-Ras was undetectable (Fig. 6B), consistent with the results from the A20 mouse B cells (Fig. 5A). However, in the DT40 cells lacking PLC-γ2, the activation of H-Ras, N-Ras, and K-Ras 4B was dramatically reduced (Fig. 6B). To confirm that the lack of activation of H-Ras, N-Ras, and K-Ras 4B in the DT40 cells lacking PLC-γ2 was indeed caused by a lack of PLC-γ2, we investigated the responsiveness of a clone of these cells into which PLC-γ2 cDNA had been reintroduced (71). These cells responded to ligation of the BCR by induction of phosphorylation of Erk (Fig. 6C). We observed that expression of exogenous PLC-γ2 in DT40 cells lacking endogenous PLC-γ2 restored their ability to activate H-Ras, N-Ras, or K-Ras 4B after ligation of the BCR. These results indicated that the activation of H-Ras, N-Ras, or K-Ras 4B induced by ligation of the BCR all occurred through pathways that depended on PLC-γ2.
FIG. 6.
Reduced Ras activation in the absence of PLC-γ2. (A) Parental or PLC-γ2-deficient DT40 B cells were stimulated with anti-IgM for the indicated times (in minutes), and activated endogenous p21 Ras was precipitated by using the Raf-1 RBD. One-tenth of the amount of lysate used for a precipitation was run next to the precipitates (1/10). The same lysates used for the precipitations probed with anti-phospho-Erk (p-Erk) and anti-p21 Ras to monitor stimulation and loading, respectively. (B) Activated exogenous Ras proteins expressed in parental and PLC-γ2−/−cells and PLC-γ2−/− cells that had been reconstituted with exogenous PLC-γ2 were precipitated with the Raf-1 RBD (H-Ras, N-Ras, and K-Ras 4B) or Nore1 RBD (M-Ras). The same lysates used for the pull-down assays were also probed with anti-phospho-Erk and anti-p21 Ras to ensure, respectively, the effi-ciency of BCR ligation and the equivalency of loading (not shown). The experiments shown were repeated two more times with similar results. (C) Parental, PLC-γ2−/−, and reconstituted DT40 cells were subjected to stimulation (+) with anti-IgM antibodies for 5 min. The cell lysates were probed with anti-phospho-Erk and anti-phospho-Akt (S473) to demonstrate stimulation and with antiactin to demonstrate equivalency of loading.
The phorbol ester PdBu stimulates activation of H-Ras, N-Ras, and K-Ras 4B, but not of M-Ras.
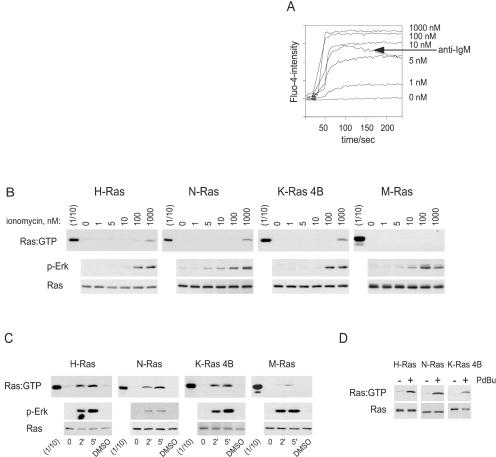
The requirement for PLC-γ2 for activation of H-Ras and K-Ras 4B in response to ligation of the BCR suggested that the GEFs involved were activated by increases in intracellular calcium or DAG. To investigate whether calcium fluxes alone could stimulate Ras activation in B cells, we first determined the concentration of the calcium ionophore ionomycin that stimulated a calcium flux comparable to that induced by anti-IgM stimulation of DT40 cells. This was between 5 and 10 nM (Fig. 7A). However, even when cells were stimulated with concentrations of ionomycin of up to 100 nM, there was no detectable activation of any of the Ras proteins (Fig. 7B). At very high concentrations of ionomycin (1 μM), H-Ras, N-Ras, and K-Ras 4B were weakly activated, perhaps due to activation of PLCs by a massive influx of calcium. However, our results indicate that calcium fluxes equivalent to those evoked by BCR ligation were insufficient for the activation of H-Ras, N-Ras, and K-Ras 4B in B cells. Thus, the calcium-activated GEFs RasGRF1 and RasGRF2 are unlikely to be involved in BCR-induced activation of Ras.
FIG. 7.
Stimulation with PdBu, unlike increases in intracellular calcium, is sufficient to activate H-Ras, N-Ras, and K-Ras 4B, but not M-Ras. (A) Calcium fluxes induced by different concentrations of ionomycin or by 5 μg of anti-IgM/ml in DT40 cells were assessed by flow cytometry using the calcium-activated dye Fluo-4 (Molecular Probes). The baseline was measured for 30 s before addition of ionomycin or anti-IgM. (B and C) DT40 cells were transiently transfected with the indicated Ras constructs, and the cells were stimulated with the indicated concentrations of ionomycin (B) or with PdBu for the indicated times (in minutes) or with dimethyl sulfoxide (DMSO) carrier for 5 min (C). Precipitations of activated, GTP-bound Ras proteins were performed using the Raf-1 RBD (for H-Ras, N-Ras, and K-Ras 4B) or the Nore1 RBD (for M-Ras) (top). One-tenth of the amount of lysate used for precipitation was run next to the precipitates (1/10). The middle and lower blots are of the same lysates probed with anti-phospho-Erk (p-Erk) and anti-GFP. The results are representative of three similar experiments. (D) PLC-γ2−/− DT40 cells expressing exogenous H-Ras, N-Ras, or K-Ras 4B were stimulated with PdBu (+) or DMSO (−) for 5 min. Activated Ras proteins were precipitated using the Raf-1 RBD (top). (Bottom) Expression of the Ras protein in cell lysates.
To assess the role of the second product of PLC-γ2 activity, DAG, we used a functional analog, the phorbol ester PdBu. We observed that stimulation of DT40 cells with PdBu alone was sufficient for activation of H-Ras, N-Ras, and K-Ras 4B, but not of M-Ras (Fig. 7C), paralleling the results obtained by ligation of the BCR or TCR. Moreover, H-Ras, N-Ras, and K-Ras 4B were also activated in response to PdBu in DT40 cells lacking PLC-γ2, indicating that PdBu bypassed the defect in Ras activation in these cells (Fig. 7D). Collectively, our results indicate that the production of DAG by PLC-γ2 is both necessary and sufficient for activation of H-Ras, N-Ras, and K-Ras 4B, but not M-Ras, following ligation of the BCR.
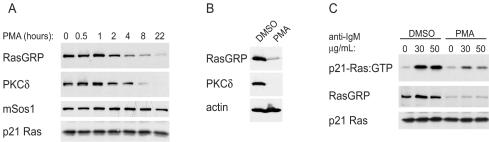
Down-regulation of RasGRP by prolonged exposure to PMA results in reduced activation of p21 Ras by ligation of the BCR.
To gain more direct evidence for a possible role for DAG-responsive RasGRP proteins in BCR-mediated activation of Ras proteins, we tested the effects on p21 Ras activation of reductions in the levels of endogenous RasGRPs. Since the levels of the classical and novel isoforms of protein kinase C (PKC) that exhibit DAG-binding C1 domains are known to be down-regulated by chronic exposure to phorbol esters, such as phorbol myristate acetate (PMA) or PdBu, we speculated that this might also be the case for RasGRP proteins, which also exhibit a C1 domain. As shown in Fig. 8A, exposure of WEHI-231 B cells to PMA did indeed result in a time-dependent down-regulation of RasGRP. The kinetics of reduction in RasGRP levels were similar to those of the reduction in levels of PKCδ (Fig. 8A). The loss of RasGRP in solubilized cell extracts was not due to sequestration in detergent-insoluble compartments, as solubilization of whole cells in SDS loading buffer gave similar results (Fig. 8B). The levels of mSos1 were unchanged by exposure to PMA, as were the levels of p21 Ras (Fig. 8A). A similar down-regulation of RasGRP by exposure to PdBu was also seen in WEHI-231 and A20 cells (not shown). When WEHI-231 cells that had been treated with PMA to reduce levels of RasGRP were stimulated with anti-IgM antibodies, the activation of endogenous p21 Ras was significantly reduced (Fig. 8C). These results strongly implicate RasGRP proteins as the critical RasGEFs that are activated by ligation of the BCR.
FIG. 8.
Reduced levels of RasGRP correlate with reduced activation of p21 Ras following ligation of BCR. (A) WEHI-231 B cells were exposed to 100 nM PMA and lysed after the indicated times, and the lysates were subjected to Western blotting with the indicated antibodies. (B) WEHI-231 cells were treated with 100 nM PMA overnight. The cells were lysed directly in SDS loading buffer, and the lysates were sonicated before analysis of the total expression of RasGRP, PKCδ, and actin by immunoblotting. (C) WEHI-231 cells were exposed to either dimethyl sulfoxide (DMSO) or PMA overnight and subsequently stimulated with 30 or 50 μg of anti-IgM/ml. Levels of activated endogenous p21 Ras were determined by pull-down assays with Raf-1 RBD. Cell lysates were also analyzed for RasGRP and p21 Ras. Similar results were obtained in two additional experiments.
DISCUSSION
Here, we demonstrate that different extracellular stimuli trigger quantitative or absolute differences in the activation of different members of the Ras family. Moreover, the mechanisms underlying selective or preferential activation of a subset of Ras proteins differ with the type of stimulus and the GEFs that are activated. We also provide the first direct evidence that members of the p21 Ras family of proteins, H-Ras, N-Ras, and K-Ras 4B, are activated differentially by growth factors and show that M-Ras is more efficiently activated by IL-3, CSF-1, and EGF than H-Ras or N-Ras. In that M-Ras is also activated by nerve growth factor and basic fibroblast growth factor (34), it is likely that activation of M-Ras is an important aspect of growth factor action. In this respect, the fact that M-Ras is expressed in all tissues, including myeloid progenitor cells and macrophages, and that in fibroblasts it occurs at much higher levels than all forms of p21 Ras together (20) is particularly intriguing. We show that the preferential activation of M-Ras and K-Ras 4B was determined by their polybasic carboxy termini and correlated with their localization in high-density membrane fractions, where the activated receptors were found. Finally, we show that in the case of BCRs and TCRs, localization of different Ras proteins in different membrane domains does not influence the degree of activation and that instead sensitivity to catalytic action of the major GEF was the key factor determining which Ras family members were activated.
The polybasic carboxy termini of K-Ras 4B and, as shown here, M-Ras determine not only their preferential activation by growth factors but also two features of their cellular localization where the activation takes place: their presence in nonraft areas of the membrane and a lack of association with the Golgi apparatus. It has been reported recently that after stimulation with EGF, H-Ras that is activated at the plasma membrane is rapidly inactivated by CAPRI, a calcium-activated GAP. Consequently, at later times, activation of H-Ras is observed mainly on the Golgi apparatus (3, 9). The action of CAPRI is unlikely to account for the differences we observed, as there is no evidence that it acts differently on H-Ras and N-Ras as opposed to K-Ras 4B and M-Ras (41), and IL-3 does not stimulate calcium fluxes. These considerations suggest that growth factor stimulation will result in efficient activation of M-Ras and K-Ras 4B in disordered regions of the plasma membrane and lesser increases in activated H-Ras or N-Ras, which may occur mainly on the Golgi apparatus. The functional significance of this compartmentalization of the different forms of activated Ras following growth factor stimulation is of great interest and requires further investigation.
Our data reveal a clear correlation between the localization in high-buoyant-density membrane domains of those Ras proteins with polybasic carboxy termini, activated growth factor receptors, the adaptors Shc and Grb2, and the exchange factor mSos, potentially explaining their preferential activation. The finding that stimulation with CSF-1 resulted in the CSF-1R moving out of low-density membranes was unexpected, in that the closely related receptor for platelet-derived growth factor has been reported to be localized in rafts and/or to caveolae in low-density membranes (40, 44). Our finding that the EGFR occurred in high-density membrane fractions is in agreement with some observations (S. Robbins, personal communication), although others have reported that the EGFR was present in caveolae and/or rafts (44, 46, 74). There are very few reports indicating whether cytokine receptors, like IL-3R, that lack intrinsic tyrosine kinase activity are associated with raft- or nonraft membranes. The related receptor for gamma interferon and the gp130 component of multiple cytokine receptors were found in raft fractions, as was tumor necrosis factor receptor 1 (39, 64). A number of groups have investigated the possible association of the IL-2R with rafts, but the conclusions have varied (22, 42, 43, 73). Differences in the conclusions of these studies and our own could be due to differences in the cells used or the choice of the technique that was used to study rafts (66). In addition, there appear to be various classes of rafts (15, 54, 63, 76). The extent of heterogeneity of native rafts and details of their sizes and lipid and protein compositions are still largely unknown. However, we believe that it is possible to conclude from our data that Ras proteins with polybasic carboxy termini occur together with the activated growth factor receptors, mSos, and adapters in membrane domains that share a high buoyant density.
In dramatic contrast to the activation of Ras proteins by growth factors, localization to high-density membrane domains was not a determining factor in their activation following ligation of antigen receptors. That H-Ras, N-Ras, and K-Ras 4B, as well as chimeras of H-Ras with the carboxy termini of either K-Ras 4B or M-Ras, were strongly activated by ligation of the BCR or TCR indicates that localization of Ras proteins in either high- or low-density membrane fractions had no effect on the efficiency of their activation and implies that the relevant BCR- and TCR-generated signals occurred both inside and outside rafts. Only a fraction of the BCR or TCR is present in rafts after ligation of the receptors, and proteins that become tyrosine phosphorylated following antigen receptor ligation were found in both raft and nonraft parts of the membrane (8, 15, 55, 77). Thus, activated receptors are likely to be present in both rafts and high-density regions of the membrane. Ligation of the BCR on the immature B-cell line WEHI-231, in which the activated BCR fails to translocate into rafts (68), also resulted in the activation of H-Ras and K-Ras 4B, but not M-Ras (data not shown). This further supports the idea that there is no absolute requirement for localization of the activated antigen receptor to particular membrane domains in order to transmit signals that lead to activation of Ras proteins and is in agreement with other evidence suggesting that signaling events downstream of immunoreceptors are not restricted to rafts (2, 12, 25, 35, 38).
One important factor that may account for the lack of influence of localization of Ras proteins in specific parts of the membrane is the likely dominance of GEFs of the RasGRP family in activation of Ras downstream of antigen receptors (discussed in detail below). Thus, recent evidence shows that following stimulation of cells with EGF or DAG analogs, RasGRP localizes to the Golgi apparatus, where it activates H-Ras (3). Conceivably, once activated, RasGRP could also activate Ras proteins at the plasma membrane, irrespective of whether they were colocalized with the activated antigen receptors in membrane domains. One could envisage long- and short-range classes of GEFs. The first, represented by mSos, would activate only those Ras species in the immediate vicinity of the activated receptors, which, in the case of the growth factor receptors we looked at, was in the disordered membrane. The second class of GEFs, represented by the RasGRP proteins, would activate susceptible species of Ras, e.g., H-Ras, N-Ras, and K-Ras 4B, irrespective of whether they were localized in the same membrane domains as the activated receptors.
The activation of H-Ras, N-Ras, and K-Ras 4B following BCR or TCR ligation correlated with their sensitivity to activation by RasGRP and the phorbol ester PdBu. Several lines of evidence support the notion that RasGRP family members are critical in activation of Ras downstream of antigen receptors. The fact that reduced levels of RasGRP expression correlated with reduced levels of activation of p21 Ras points to a critical role for RasGRPs (Fig. 8). They are the only GEFs known to be regulated by DAG, and RasGRP1 has been clearly implicated in the activation of p21 Ras induced by ligation of the TCR (17). Given that physiological increases in cytosolic calcium alone failed to activate H-Ras or K-Ras 4B, a major role for the calcium-activated GEFs RasGRF1/2 in BCR-mediated activation of Ras proteins can be excluded. Likewise, since the activation of H-Ras, N-Ras, and K-Ras 4B required the presence of PLC-γ2, and mSos1/2 are not known to function downstream of this enzyme, our results also preclude a major role for mSos1/2 in BCR-induced activation of Ras proteins. The fact that M-Ras is readily activated by mSos1 and RasGRF both in vitro and in vivo (50, 58) but was not activated by ligation of the BCR and TCR also supports the notion that mSos and RasGRF were not involved in the latter events. Finally, during the revision of this report, it was shown that DT40 cells in which RasGRP3 had been ablated exhibited gross deficiencies in p21 Ras activation (51).
Although mSos1/Grb2/Shc complexes are recruited to the activated BCR and complexes of mSos1 with p36LAT and Grb2 are rapidly formed after TCR ligation (49, 62), the functional significance of these complexes is unclear. In favor of a role for mSos in Ras activation by the BCR is evidence that the activation of Erk was reduced by overexpression of dominant-negative Shc or dominant-negative Grb2 (30). Moreover, p21 Ras was activated normally after ligation of the BCR in DT40 cells lacking expression of BLNK, despite the fact that PLC-γ was not activated (29). Thymocytes of mice with haploinsufficiency for Grb2 show reduced, but not absent, activation of p21 Ras after TCR ligation (23). This discrepancy remains to be resolved. The strengths of signals may be one important factor. Thus, RasGRP1 is critical for Erk activation by weak, but not strong, TCR signals (56). It is conceivable that a similar mechanism could apply to the BCR, although the stimuli that were used in our study—ligation by polyclonal antibodies—probably represent a strong signal.
Our data imply that RasGRPs do not efficiently activate M-Ras. While overexpression of RasGRP1 can lead to activation of M-Ras (references 34 and 51 and our own observations), in vitro RasGRP1 has only weak exchange activity on M-Ras, and RasGRP2 and RasGRP3 are inactive (50). Consistent with our observations, stimulation of PC12 neuronal cells with the phorbol ester PMA did not result in activation of M-Ras, despite the fact that these cells express endogenous RasGRP1, although M-Ras was activated in cells in which RasGRP1 was overexpressed (34). We conclude that RasGRPs are much weaker activators of M-Ras than of p21 Ras proteins. The molecular basis of the lack of sensitivity of M-Ras to the catalytic activity of RasGRP is unclear. We noted that mutants of M-Ras made more similar to H-Ras (P40D, E79D, truncation of the N terminus, or exchange of the entire helix 3-loop 7 region) still failed to be activated following ligation of the BCR (data not shown), suggesting that the structural determinants of sensitivity to activation by RasGRP are not restricted to single small parts of the structure. Our data showing that M-Ras is not activated by ligation of antigen receptors suggest that M-Ras is unlikely to be involved in those aspects of T-cell differentiation affected by the absence of RasGRP (13). This does not exclude a role in those aspects of T-cell differentiation in which Grb2/mSos act downstream of the TCR (23, 56).
Our observation that RasGRP was down-regulated by chronic exposure to PMA is intriguing, as to date, this treatment has only been reported to lead to the down-regulation of isoforms of PKC. It is possible that RasGRP binds to PKC and is passively degraded with it or that downregulation of RasGRP depends on phosphorylation by PKC (72). Alternatively, RasGRP may be depleted as a direct result of the binding of phorbol ester to its C1 domain, raising the possibility that other proteins containing phorbol ester binding C1 domains, such as chimaerins, the Munc-13 family of proteins, DAG kinases, and PKD1/PKCμ, may also be down-regulated. Thus, we cannot rule out the possibility that the reduction in Ras activation by BCR ligation in cells pretreated with PMA was solely due to depletion of RasGRP, although the tissue distribution and functions of other C1 domain-containing proteins (4) make it unlikely that they were important in regulation of the activation of p21 Ras. Prolonged exposure of cells to PMA has been extensively used to gain insight into the functions of PKC. However, careful reevaluation of data obtained from such experiments is required to exclude the possibility that the observed effect resulted from the loss of proteins other than PKC.
By showing that individual members of the Ras family are activated differentially, and that the patterns of activation vary with different classes of stimuli, we have provided one answer to the question of why there are so many members of the Ras family. We have identified two mechanisms that account for these differences in activation, the degree of colocalization of an individual Ras protein with activated receptors in membrane domains and its relative sensitivity to the GEFs activated by the receptor. Finally, the differences among members of the Ras family in their localizations to the plasma membranes, endosomes, and the Golgi apparatus (24) are also likely to have functional implications, for example, in the patterns of effector pathways they engage.
Acknowledgments
We thank Michael Gold for many valuable reagents and advice; Tomohiro Kurosaki, Maya Kotturi, Janis Jackson, Larry Feig, and Martine Roussel for their gifts of the DT40 cells lacking PLC-γ2, the OKT3 antibody, and cDNAs; Steven Robbins, Rob Gerl, and Kelly McNagny for helpful discussions; and John Hamilton for critical reading of the manuscript.
This work was supported by the Canadian Institutes of Health Research.
REFERENCES
- 1.Apolloni, A., I. A. Prior, M. Lindsay, R. G. Parton, and J. F. Hancock. 2000. H-ras but not K-ras traffics to the plasma membrane through the exocytic pathway. Mol. Cell. Biol. 20:2475-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balamuth, F., D. Leitenberg, J. Unternaehrer, I. Mellman, and K. Bottomly. 2001. Distinct patterns of membrane microdomain partitioning in Th1 and Th2 cells. Immunity 15:729-738. [DOI] [PubMed] [Google Scholar]
- 3.Bivona, T. G., I. Perez De Castro, I. M. Ahearn, T. M. Grana, V. K. Chiu, P. J. Lockyer, P. J. Cullen, A. Pellicer, A. D. Cox, and M. R. Philips. 2003. Phospholipase Cγ activates Ras on the Golgi apparatus by means of RasGRP1. Nature 424:694-698. [DOI] [PubMed] [Google Scholar]
- 4.Brose, N., and C. Rosenmund. 2002. Move over protein kinase C, you've got company: alternative cellular effectors of diacylglycerol and phorbol esters. J. Cell Sci. 115:4399-4411. [DOI] [PubMed] [Google Scholar]
- 5.Buday, L., and J. Downward. 1993. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell 73:611-620. [DOI] [PubMed] [Google Scholar]
- 6.Buday, L., and J. Downward. 1993. Epidermal growth factor regulates the exchange rate of guanine nucleotides on p21ras in fibroblasts. Mol. Cell. Biol. 13:1903-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caloca, M. J., J. L. Zugaza, D. Matallanas, P. Crespo, and X. R. Bustelo. 2003. Vav mediates Ras stimulation by direct activation of the GDP/GTP exchange factor Ras GRP1. EMBO J. 22:3326-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, P. C., M. L. Dykstra, R. N. Mitchell, and S. K. Pierce. 1999. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J. Exp. Med. 190:1549-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu, V. K., T. Bivona, A. Hach, J. B. Sajous, J. Silletti, H. Wiener, R. L. Johnson II, A. D. Cox, and M. R. Philips. 2002. Ras signalling on the endoplasmic reticulum and the Golgi. Nat. Cell Biol. 4:343-350. [DOI] [PubMed] [Google Scholar]
- 10.Choy, E., V. K. Chiu, J. Silletti, M. Feoktistov, T. Morimoto, D. Michaelson, I. E. Ivanov, and M. R. Philips. 1999. Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell 98:69-80. [DOI] [PubMed] [Google Scholar]
- 11.Clyde-Smith, J., G. Silins, M. Gartside, S. Grimmond, M. Etheridge, A. Apolloni, N. Hayward, and J. F. Hancock. 2000. Characterization of RasGRP2, a plasma membrane-targeted, dual specificity Ras/Rap exchange factor. J. Biol. Chem. 275:32260-32267. [DOI] [PubMed] [Google Scholar]
- 12.Costello, P. S., M. Gallagher, and D. A. Cantrell. 2002. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nat. Immunol. 3:1082-1089. [DOI] [PubMed] [Google Scholar]
- 13.Dower, N. A., S. L. Stang, D. A. Bottorff, J. O. Ebinu, P. Dickie, H. L. Ostergaard, and J. C. Stone. 2000. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat. Immunol. 1:317-321. [DOI] [PubMed] [Google Scholar]
- 14.Downward, J., J. D. Graves, P. H. Warne, S. Rayter, and D. A. Cantrell. 1990. Stimulation of p21ras upon T-cell activation. Nature 346:719-723. [DOI] [PubMed] [Google Scholar]
- 15.Drevot, P., C. Langlet, X. J. Guo, A. M. Bernard, O. Colard, J. P. Chauvin, R. Lasserre, and H. T. He. 2002. TCR signal initiation machinery is pre-assembled and activated in a subset of membrane rafts. EMBO J. 21:1899-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duronio, V., M. J. Welham, S. Abraham, P. Dryden, and J. W. Schrader. 1992. p21ras activation via hemopoietin receptors and c-kit requires tyrosine kinase activity but not tyrosine phosphorylation of p21ras GTPase-activating protein. Proc. Natl. Acad. Sci. USA 89:1587-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebinu, J. O., S. L. Stang, C. Teixeira, D. A. Bottorff, J. Hooton, P. M. Blumberg, M. Barry, R. C. Bleakley, H. L. Ostergaard, and J. C. Stone. 2000. RasGRP links T-cell receptor signaling to Ras. Blood 95:3199-3203. [PubMed] [Google Scholar]
- 18.Ehrhardt, A., G. Ehrhardt, X. Guo, and J. Schrader. 2002. Ras and relatives—job sharing and networking keep an old family together. Exp. Hematol. 30:1089-1106. [DOI] [PubMed] [Google Scholar]
- 19.Ehrhardt, G. R., C. Korherr, J. S. Wieler, M. Knaus, and J. W. Schrader. 2001. A novel potential effector of M-Ras and p21 Ras negatively regulates p21 Ras-mediated gene induction and cell growth. Oncogene 20:188-197. [DOI] [PubMed] [Google Scholar]
- 20.Ehrhardt, G. R., K. B. Leslie, F. Lee, J. S. Wieler, and J. W. Schrader. 1999. M-Ras, a widely expressed 29-kD homologue of p21 Ras: expression of a constitutively active mutant results in factor-independent growth of an interleukin-3-dependent cell line. Blood 94:2433-2444. [PubMed] [Google Scholar]
- 21.Gibbs, J. B., M. S. Marshall, E. M. Scolnick, R. A. Dixon, and U. S. Vogel. 1990. Modulation of guanine nucleotides bound to Ras in NIH3T3 cells by oncogenes, growth factors, and the GTPase activating protein (GAP). J. Biol. Chem. 265:20437-20442. [PubMed] [Google Scholar]
- 22.Goebel, J., K. Forrest, L. Morford, and T. L. Roszman. 2002. Differential localization of IL-2- and -15 receptor chains in membrane rafts of human T cells. J. Leukoc. Biol. 72:199-206. [PubMed] [Google Scholar]
- 23.Gong, Q., A. M. Cheng, A. M. Akk, J. Alberola-Ila, G. Gong, T. Pawson, and A. C. Chan. 2001. Disruption of T cell signaling networks and development by Grb2 haploid insufficiency. Nat. Immunol. 2:29-36. [DOI] [PubMed] [Google Scholar]
- 24.Hancock, J. F. 2003. Ras proteins: different signals from different locations. Nat. Rev. Mol. Cell Biol. 4:373-384. [DOI] [PubMed] [Google Scholar]
- 25.Harriague, J., and G. Bismuth. 2002. Imaging antigen-induced PI3K activation in T cells. Nat. Immunol. 3:1090-1096. [DOI] [PubMed] [Google Scholar]
- 26.Harwood, A. E., and J. C. Cambier. 1993. B cell antigen receptor cross-linking triggers rapid protein kinase C independent activation of p21ras1. J. Immunol. 151:4513-4522. [PubMed] [Google Scholar]
- 27.Hashimoto, A., H. Okada, A. Jiang, M. Kurosaki, S. Greenberg, E. A. Clark, and T. Kurosaki. 1998. Involvement of guanosine triphosphatases and phospholipase C-γ2 in extracellular signal-regulated kinase, c-Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase activation by the B cell antigen receptor. J. Exp. Med. 188:1287-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iritani, B. M., K. A. Forbush, M. A. Farrar, and R. M. Perlmutter. 1997. Control of B cell development by Ras-mediated activation of Raf. EMBO J. 16:7019-7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishiai, M., M. Kurosaki, R. Pappu, K. Okawa, I. Ronko, C. Fu, M. Shibata, A. Iwamatsu, A. C. Chan, and T. Kurosaki. 1999. BLNK required for coupling Syk to PLC gamma 2 and Rac1-JNK in B cells. Immunity 10:117-125. [DOI] [PubMed] [Google Scholar]
- 30.Jacob, A., D. Cooney, M. Pradhan, and K. M. Coggeshall. 2002. Convergence of signaling pathways on the activation of ERK in B Cells. J. Biol. Chem. 277:23420-23426. [DOI] [PubMed] [Google Scholar]
- 31.Jaumot, M., J. Yan, J. Clyde-Smith, J. Sluimer, and J. F. Hancock. 2002. The linker domain of the H-Ras hypervariable region regulates interactions with exchange factors, Raf-1 and phosphoinositide 3-kinase. J. Biol. Chem. 277:272-278. [DOI] [PubMed] [Google Scholar]
- 32.Jin, D. I., S. B. Jameson, M. A. Reddy, D. Schenkman, and M. C. Ostrowski. 1995. Alterations in differentiation and behavior of monocytic phagocytes in transgenic mice that express dominant suppressors of ras signaling. Mol. Cell. Biol. 15:693-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones, M. K., and J. H. Jackson. 1998. Ras-GRF activates Ha-Ras, but not N-Ras or K-Ras 4B, protein in vivo. J. Biol. Chem. 273:1782-1787. [DOI] [PubMed] [Google Scholar]
- 34.Kimmelman, A. C., N. N. Rodriguez, and A. M. Chan. 2002. R-Ras3/M-Ras induces neuronal differentiation of PC12 cells through cell-type-specific activation of the mitogen-activated protein kinase cascade. Mol. Cell. Biol. 22:5946-5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovarova, M., P. Tolar, R. Arudchandran, L. Draberova, J. Rivera, and P. Draber. 2001. Structure-function analysis of Lyn kinase association with lipid rafts and initiation of early signaling events after Fcɛ receptor I aggregation. Mol. Cell. Biol. 21:8318-8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kranenburg, O., I. Verlaan, and W. H. Moolenaar. 2001. Regulating c-Ras function. Cholesterol depletion affects caveolin association, GTP loading, and signaling. Curr. Biol. 11:1880-1884. [DOI] [PubMed] [Google Scholar]
- 37.Lee, A. W., and D. J. States. 2000. Both src-dependent and -independent mechanisms mediate phosphatidylinositol 3-kinase regulation of colony-stimulating factor 1-activated mitogen-activated protein kinases in myeloid progenitors. Mol. Cell. Biol. 20:6779-6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee, K. H., A. D. Holdorf, M. L. Dustin, A. C. Chan, P. M. Allen, and A. S. Shaw. 2002. T cell receptor signaling precedes immunological synapse formation. Science 295:1539-1542. [DOI] [PubMed] [Google Scholar]
- 39.Legler, D. F., O. Micheau, M. A. Doucey, J. Tschopp, and C. Bron. 2003. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFα-mediated NF-κB activation. Immunity 18:655-664. [DOI] [PubMed] [Google Scholar]
- 40.Liu, P., Y. Ying, Y. G. Ko, and R. G. Anderson. 1996. Localization of platelet-derived growth factor-stimulated phosphorylation cascade to caveolae. J. Biol. Chem. 271:10299-10303. [DOI] [PubMed] [Google Scholar]
- 41.Lockyer, P. J., S. Kupzig, and P. J. Cullen. 2001. CAPRI regulates Ca2+-dependent inactivation of the Ras-MAPK pathway. Curr. Biol. 11:981-986. [DOI] [PubMed] [Google Scholar]
- 42.Marmor, M. D., and M. Julius. 2001. Role for lipid rafts in regulating interleukin-2 receptor signaling. Blood 98:1489-1497. [DOI] [PubMed] [Google Scholar]
- 43.Matko, J., A. Bodnar, G. Vereb, L. Bene, G. Vamosi, G. Szentesi, J. Szollosi, R. Gaspar, V. Horejsi, T. A. Waldmann, and S. Damjanovich. 2002. GPI-microdomains (membrane rafts) and signaling of the multi-chain interleukin-2 receptor in human lymphoma/leukemia T cell lines. Eur. J. Biochem. 269:1199-1208. [DOI] [PubMed] [Google Scholar]
- 44.Matveev, S. V., and E. J. Smart. 2002. Heterologous desensitization of EGF receptors and PDGF receptors by sequestration in caveolae. Am. J. Physiol. Cell Physiol. 282:C935-C946. [DOI] [PubMed] [Google Scholar]
- 45.Miettinen, P. J., J. E. Berger, J. Meneses, Y. Phung, R. A. Pedersen, Z. Werb, and R. Derynck. 1995. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature 376:337-341. [DOI] [PubMed] [Google Scholar]
- 46.Mineo, C., G. L. James, E. J. Smart, and R. G. Anderson. 1996. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J. Biol. Chem. 271:11930-11935. [DOI] [PubMed] [Google Scholar]
- 47.Montixi, C., C. Langlet, A. M. Bernard, J. Thimonier, C. Dubois, M. A. Wurbel, J. P. Chauvin, M. Pierres, and H. T. He. 1998. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 17:5334-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niv, H., O. Gutman, Y. Kloog, and Y. I. Henis. 2002. Activated K-Ras and H-Ras display different interactions with saturable nonraft sites at the surface of live cells. J. Cell Biol. 157:865-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nunes, J. A., Y. Collette, A. Truneh, D. Olive, and D. A. Cantrell. 1994. The role of p21ras in CD28 signal transduction: triggering of CD28 with antibodies, but not the ligand B7-1, activates p21ras. J. Exp. Med. 180:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohba, Y., N. Mochizuki, S. Yamashita, A. M. Chan, J. W. Schrader, S. Hattori, K. Nagashima, and M. Matsuda. 2000. Regulatory proteins of R-Ras, TC21/R-Ras2, and M-Ras/R-Ras3. J. Biol. Chem. 275:20020-20026. [DOI] [PubMed] [Google Scholar]
- 51.Oh-hora, M., S. Johmura, A. Hashimoto, M. Hikida, and T. Kurosaki. 2003. Requirement for Ras guanine nucleotide releasing protein 3 in coupling phospholipase C-γ2 to Ras in B cell receptor signaling. J. Exp Med. 198:1841-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okuda, K., T. J. Ernst, and J. D. Griffin. 1994. Inhibition of p21ras activation blocks proliferation but not differentiation of interleukin-3-dependent myeloid cells. J. Biol. Chem. 269:24602-24607. [PubMed] [Google Scholar]
- 53.Pages, G., S. Guerin, D. Grall, F. Bonino, A. Smith, F. Anjuere, P. Auberger, and J. Pouyssegur. 1999. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science 286:1374-1377. [DOI] [PubMed] [Google Scholar]
- 54.Petrie, R. J., and J. P. Deans. 2002. Colocalization of the B cell receptor and CD20 followed by activation-dependent dissociation in distinct lipid rafts. J. Immunol. 169:2886-2891. [DOI] [PubMed] [Google Scholar]
- 55.Petrie, R. J., P. P. Schnetkamp, K. D. Patel, M. Awasthi-Kalia, and J. P. Deans. 2000. Transient translocation of the B cell receptor and Src homology 2 domain-containing inositol phosphatase to lipid rafts: evidence toward a role in calcium regulation. J. Immunol. 165:1220-1227. [DOI] [PubMed] [Google Scholar]
- 56.Priatel, J. J., S. J. Teh, N. A. Dower, J. C. Stone, and H. S. Teh. 2002. RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity 17:617-627. [DOI] [PubMed] [Google Scholar]
- 57.Prior, I. A., A. Harding, J. Yan, J. Sluimer, R. G. Parton, and J. F. Hancock. 2001. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat. Cell Biol. 3:368-375. [DOI] [PubMed] [Google Scholar]
- 58.Quilliam, L. A., A. F. Castro, K. S. Rogers-Graham, C. B. Martin, C. J. Der, and C. Bi. 1999. M-Ras/R-Ras3, a transforming ras protein regulated by Sos1, GRF1, and p120 Ras GTPase-activating protein, interacts with the putative Ras effector AF6. J. Biol. Chem. 274:23850-23857. [DOI] [PubMed] [Google Scholar]
- 59.Rogge, R. D., C. A. Karlovich, and U. Banerjee. 1991. Genetic dissection of a neurodevelopmental pathway: son of sevenless functions downstream of the sevenless and EGF receptor tyrosine kinases. Cell 64:39-48. [DOI] [PubMed] [Google Scholar]
- 60.Roy, S., R. Luetterforst, A. Harding, A. Apolloni, M. Etheridge, E. Stang, B. Rolls, J. F. Hancock, and R. G. Parton. 1999. Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat. Cell Biol. 1:98-105. [DOI] [PubMed] [Google Scholar]
- 61.Satoh, T., M. Nakafuku, A. Miyajima, and Y. Kaziro. 1991. Involvement of ras p21 protein in signal-transduction pathways from interleukin 2, interleukin 3, and granulocyte/macrophage colony-stimulating factor, but not from interleukin 4. Proc. Natl. Acad. Sci. USA 88:3314-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saxton, T. M., I. van Oostveen, D. Bowtell, R. Aebersold, and M. R. Gold. 1994. B cell antigen receptor cross-linking induces phosphorylation of the p21ras oncoprotein activators SHC and mSOS1 as well as assembly of complexes containing SHC, GRB-2, mSOS1, and a 145-kDa tyrosine-phosphorylated protein. J. Immunol. 153:623-636. [PubMed] [Google Scholar]
- 63.Schade, A. E., and A. D. Levine. 2002. Lipid raft heterogeneity in human peripheral blood T lymphoblasts: a mechanism for regulating the initiation of TCR signal transduction. J. Immunol. 168:2233-2239. [DOI] [PubMed] [Google Scholar]
- 64.Sehgal, P. B., G. G. Guo, M. Shah, V. Kumar, and K. Patel. 2002. Cytokine signaling: STATS in plasma membrane rafts. J. Biol. Chem. 277:12067-12074. [DOI] [PubMed] [Google Scholar]
- 65.Sibilia, M., and E. F. Wagner. 1995. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science 269:234-238. [DOI] [PubMed] [Google Scholar]
- 66.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 67.Song, K. S., S. Li, T. Okamoto, L. A. Quilliam, M. Sargiacomo, and M. P. Lisanti. 1996. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J. Biol. Chem. 271:9690-9697. [DOI] [PubMed] [Google Scholar]
- 68.Sproul, T. W., S. Malapati, J. Kim, and S. K. Pierce. 2000. Cutting edge: B cell antigen receptor signaling occurs outside lipid rafts in immature B cells. J. Immunol. 165:6020-6023. [DOI] [PubMed] [Google Scholar]
- 69.Swan, K. A., J. Alberola-Ila, J. A. Gross, M. W. Appleby, K. A. Forbush, J. F. Thomas, and R. M. Perlmutter. 1995. Involvement of p21ras distinguishes positive and negative selection in thymocytes. EMBO J. 14:276-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tago, K., Y. Kaziro, and T. Satoh. 1998. Functional involvement of mSos in interleukin-3 and thrombin stimulation of the Ras, mitogen-activated protein kinase pathway in BaF3 murine hematopoietic cells. J. Biochem. (Tokyo) 123:659-667. [DOI] [PubMed] [Google Scholar]
- 71.Takata, M., Y. Homma, and T. Kurosaki. 1995. Requirement of phospholipase C-gamma 2 activation in surface immunoglobulin M-induced B cell apoptosis. J. Exp. Med. 182:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teixeira, C., S. L. Stang, Y. Zheng, N. S. Beswick, and J. C. Stone. 2003. Integration of DAG signaling systems mediated by PKC-dependent phosphorylation of RasGRP3. Blood 102:1414-1420. [DOI] [PubMed] [Google Scholar]
- 73.Vereb, G., J. Matko, G. Vamosi, S. M. Ibrahim, E. Magyar, S. Varga, J. Szollosi, A. Jenei, R. Gaspar, Jr., T. A. Waldmann, and S. Damjanovich. 2000. Cholesterol-dependent clustering of IL-2Rα and its colocalization with HLA and CD48 on T lymphoma cells suggest their functional association with lipid rafts. Proc. Natl. Acad. Sci. USA 97:6013-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Waugh, M. G., S. Minogue, J. S. Anderson, M. dos Santos, and J. J. Hsuan. 2001. Signalling and non-caveolar rafts. Biochem. Soc. Trans. 29:509-511. [DOI] [PubMed] [Google Scholar]
- 75.Welham, M. J., V. Duronio, K. B. Leslie, D. Bowtell, and J. W. Schrader. 1994. Multiple hemopoietins, with the exception of interleukin-4, induce modification of Shc and mSos1, but not their translocation. J. Biol. Chem. 269:21165-21176. [PubMed] [Google Scholar]
- 76.Wilson, B., J. Pfeiffer, and J. Oliver. 2002. Fc varɛ RI signaling observed from the inside of the mast cell membrane. Mol. Immunol. 38:1259-1268. [DOI] [PubMed] [Google Scholar]
- 77.Xavier, R., T. Brennan, Q. Li, C. McCormack, and B. Seed. 1998. Membrane compartmentation is required for efficient T cell activation. Immunity 8:723-732. [DOI] [PubMed] [Google Scholar]