ABSTRACT
Activation of sphingosine-1-phosphate receptor 1 (S1PR1) plays a key role in repairing endothelial barrier function. We addressed the role of phosphorylation of the three intracellular tyrosine residues of S1PR1 in endothelial cells in regulating the receptor responsiveness and endothelial barrier function regulated by sphingosine 1-phosphate (S1P)-mediated activation of S1PR1. We demonstrated that phosphorylation of only Y143 site was required for S1PR1 internalization in response to S1P. Maximal S1PR1 internalization was seen in 20 min but S1PR1 returned to the cell surface within 1 h accompanied by Y143-dephosphorylation. Cell surface S1PR1 loss paralleled defective endothelial barrier enhancement induced by S1P. Expression of phospho-defective (Y143F) or phospho-mimicking (Y143D) mutants, respectively, failed to internalize or showed unusually high receptor internalization, consistent with the requirement of Y143 in regulating cell surface S1PR1 expression. Phosphorylation of the five S1PR1 C-terminal serine residues did not affect the role of Y143 phosphorylation in signaling S1PR1 internalization. Thus, rapid reduction of endothelial cell surface expression of S1PR1 subsequent to Y143 phosphorylation is a crucial mechanism of modulating S1PR1 signaling, and hence the endothelial barrier repair function of S1P.
KEY WORDS: Endothelium, Sphingosine-1-phosphate receptor-1, Tyrosine phosphorylation, Serine phosphorylation, Endothelial barrier function, Adherens junctions
INTRODUCTION
Sphingosine-1-phosphate receptor-1 (S1PR1) is a high affinity G-protein-coupled receptor (GPCR) ligated by the lipid mediator S1P (Hla, 2004; Spiegel and Milstien, 2003; Wang and Dudek, 2009). S1PR1 expressed in endothelial cells regulates angiogenesis (Argraves et al., 2010), contributes to the mechanism of vascular inflammation (McVerry et al., 2004; Peng et al., 2004) and reseals the endothelial cell barrier by mediating the assembly of adherens junctions (Lee et al., 2009; McVerry and Garcia, 2004; Tauseef et al., 2008). S1PR1 functions in endothelial cells through interaction with heterotrimeric Gi proteins and downstream activation of the monomeric Rho GTPase Rac1 (Garcia et al., 2001; Lee et al., 2000; Mehta et al., 2005; Thennes and Mehta, 2012).
As the normal plasma concentration of S1P is high, from 500 nM–1 µM (Graeler et al., 2002; Ruwisch et al., 2001), we surmised mechanisms must exist to downregulate the responsiveness of the receptor to S1P through modulating the expression of S1PR1 in endothelial cells. Such a mechanism might involve internalization of S1PR1 from the endothelial cell plasmalemma (Kohno et al., 2002; Liu et al., 1999) and could be regulated by phosphorylation of S1PR1 at serine and tyrosine residues (Garris et al., 2013; Huang et al., 2009; Oo et al., 2011; Watterson et al., 2002). Phosphorylation of S1PR1 at the five C-terminus serine residues, S351, S353, S355, S358 and S359, induced by the stable S1P analog FTY720P has been shown to promote interaction of S1PR1 with the ubiquitin ligase WWP2 leading to S1PR1 polyubiquitylation and receptor degradation (Oo et al., 2011; Oo et al., 2007). Loss of S1PR1 in this manner reduced the responsiveness of the receptor to S1P (Oo et al., 2011). Here, we demonstrate that tyrosine phosphorylation of S1PRI in endothelial cells also regulates receptor expression at the cell surface and hence the responsiveness to S1P. We observed that phosphorylation of S1PR1 at Y143 was required for rapid (within 20 min) internalization of the receptor and thereby dampened S1P-mediated responses until the receptor reappeared at the cell surface within 60 min following dephosphorylation of Y143. Thus, Y143 phosphorylation of S1PR1 is a key mechanism in endothelial cells regulating surface S1PR1 expression, and might modulate S1PR1 signaling in the face of high plasma S1P concentrations.
RESULTS
S1P induces Src-dependent S1PR1 tyrosine phosphorylation
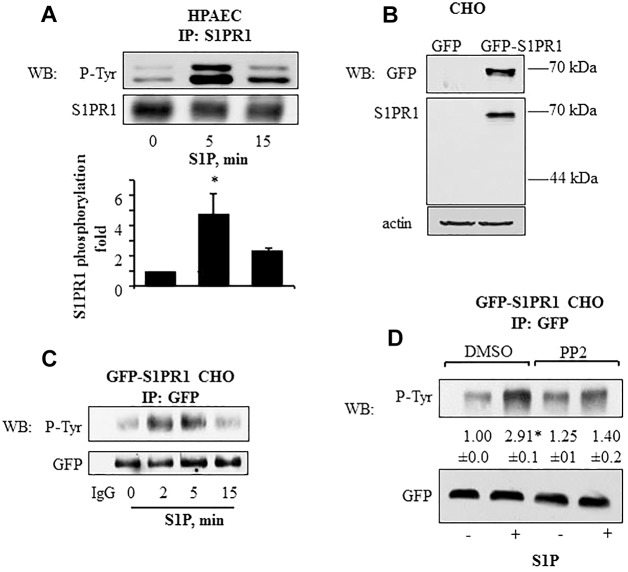
We first addressed whether S1P concentrations equivalent to plasma levels (Graeler et al., 2002; Ruwisch et al., 2001) induced phosphorylation of S1PR1 at the three intracellular tyrosine residues. Using lysates obtained from S1P-stimulated human pulmonary arterial endothelial cells (HPAECs), we observed S1PR1 phosphorylation increased fivefold over the control within 5 min of S1P exposure and remained elevated above baseline for up to 15 min (Fig. 1A). We next transfected GFP-tagged S1PR1 cDNA (Liu et al., 1999) into Chinese hamster ovary (CHO) cells (Fig. 1B), which lack S1P receptors (Okamoto et al., 2000; Paik et al., 2001), and observed, as in endothelial cells, that S1P exposure induced S1PR1 tyrosine phosphorylation in a similar time frame as that in endothelial cells (Fig. 1C). As S1P activates Src family kinases in endothelial cells (Shikata et al., 2003a; Shikata et al., 2003b; Zhao et al., 2009), we determined the effects on receptor phosphorylation of treating the CHO cells expressing GFP–S1PR1 with PP2, an inhibitor of Src kinases (Hanke et al., 1996; Zhu et al., 1999). S1PR1 phosphorylation was significantly reduced in PP2-treated cells (Fig. 1D) indicating that the S1P-mediated S1PR1 phosphorylation occurs, at least in part, through a Src-dependent mechanism.
Fig. 1.
Tyrosine phosphorylation kinetics of S1PR1 in endothelial and CHO cells. (A) S1P induces S1PR1 tyrosine phosphorylation in HPAECs. HPAECs were serum starved for 1 h after which they were stimulated with 1 µM S1P for the indicated times. Cell lysates were immunoprecipitated (IP) with anti-S1PR1 antibody and immunoblotted (WB) with anti-phosphotyrosine (P-Tyr) or anti-S1PR1 antibodies. The bar graph shows mean±s.d. (n = 4) of the fold increase in S1PR1 tyrosine phosphorylation. *P<0.05 compared with unstimulated cells. (B,C) S1P induces tyrosine phosphorylation of GFP–S1PR1 in CHO cells. (B) CHO cells were stably transfected with GFP–S1PR1 as indicated in the Materials and Methods. Untransfected CHO cells served as control. Cell lysates were immunoblotted with anti-GFP and anti-S1PR1 to confirm expression. Immunoblotting with anti-β-actin antibody was used as a loading control. (C) CHO cells stably expressing GFP–S1PR1 were serum starved for 1 h and challenged with 1 µM S1P for indicated times. Lysates were immunoprecipitated with anti-GFP antibody, and immunoblotted for phosphotyrosine or GFP (loading control). immunoprecipitation with rabbit IgG served as negative control. Representative immunoblots from three independent experiments are shown. (D) GFP–S1PR1-expressing CHO cells were serum starved for 30 min followed by exposure to 5 µM PP2 for 45 min in serum-free medium. Cells were challenged with 1 µM S1P for 5 min and lysates were immunoprecipitated with anti-GFP antibody, and immunoblotted for phosphotyrosine or GFP (loading control) to determine phosphorylation. A representative immunoblot is shown. Numbers indicate the densitometric analysis of the fold change (±s.d.) in phosphorylation over that at 0 min in control cells from three individual experiments. *P<0.05 compared with unstimulated cells.
Phosphorylation of S1PR1 at Y143 regulates cell surface receptor expression
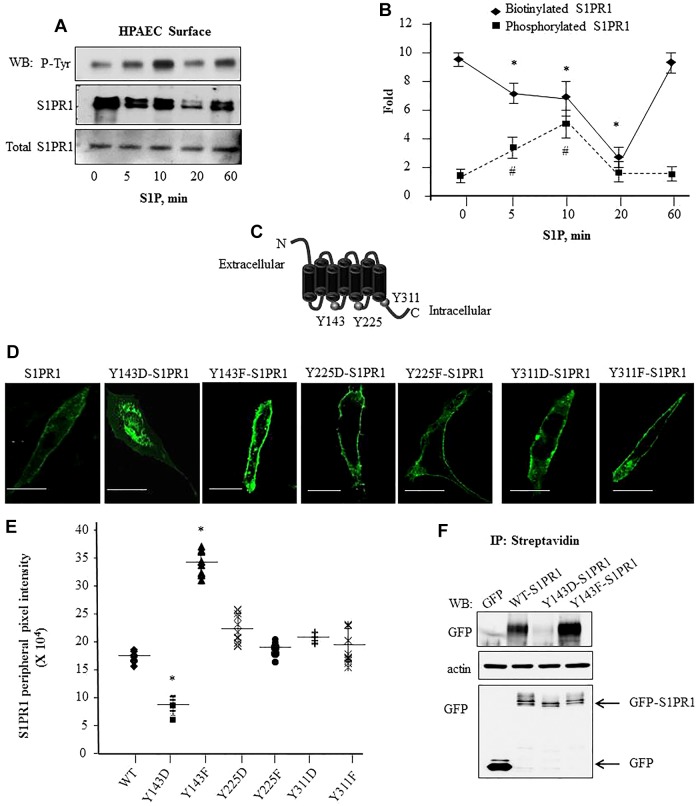
We next addressed the role of each of these intracellular S1PR1 tyrosine residues in regulating cell surface expression of S1PR1. Endothelial cells stimulated with S1P were biotinylated and equal amounts of lysate were immunoprecipitated with anti-S1PR1 antibody followed by a second immunoprecipitation of released S1PR1 complex with streptavidin A/G beads. Immunocomplexes were immunoblotted with anti-phosphotyrosine or anti-S1PR1 antibody to determine the cell surface S1PR1 amount and S1PR1 tyrosine phosphorylation. S1P induced S1PR1 tyrosine phosphorylation reaching a maximum of ∼fivefold above baseline within 5–10 min, but thereafter phosphorylation returned towards baseline levels in the next 60 min (Fig. 2A,B). S1PR1 was localized on the cell surface under basal conditions, but S1PR1 expression decreased at the surface, with levels reaching 20% of baseline after 20 min of S1P stimulation in association with increased S1PR1 tyrosine phosphorylation (Fig. 2A,B). However, restoration of cell surface S1PR1 expression to baseline at 60 min post-S1P-stimulation was coupled to S1PR1 dephosphorylation (Fig. 2A,B). Thus, tyrosine phosphorylation of S1PR1 was negatively correlated with cell surface S1PR1 expression and maximal S1PR1 tyrosine phosphorylation preceded the loss of cell surface S1PR1.
Fig. 2.
Phosphorylation of S1PR1 at Y143 is associated with reduced cell surface S1PR1 localization. (A,B) S1P-induced tyrosine phosphorylation is coupled to reduced cell surface retention of S1PR1. HPAECs were serum starved and stimulated with 1 µM S1P for the indicated times. Cell surface proteins were biotinylated followed by immunoprecipitation (IP) with anti-S1PR1 antibody; biotin-bound S1PR1 was isolated using streptavidin–agarose beads as indicated in the Materials and Methods. Protein lysates were separated by SDS-PAGE and immunoblotted (WB) with anti-phosphotyrosine (P-Tyr) or anti-S1PR1 antibodies. Total cell lysates were assessed for S1PR1 to confirm equal protein loading. A shows a representative immunoblot blot and B shows the mean±s.d. fold change in phosphorylation or biotinylated S1PR1 versus total S1PR1 expression at each time. Fold increase was calculated taking values at ‘0’ time as 1. from multiple experiments. *P<0.05 for a reduction compared to at 0 min; #P<0.05 for an increase in compared to at 0 min. (C) Schematic showing potential tyrosine residues in S1PR1 that can be phosphorylated. (D,E) Phosphorylation at Y143 reduces GFP–S1PR1 cell surface localization. (D) CHO cells were transfected with the indicated mutants and after 24 h cells were visualized using confocal microscopy. Scale bars: 10 µm. (E) The mean pixel intensity (line) at the cell periphery from various cells was quantified as described in the Materials and Methods. Plot shows pixel intensity at the cell periphery in cells expressing the mutants from three independent experiments. In each experiment at least ten cells were counted. *P<0.05 compared with cells expressing WT-S1PR1. (F) Cell surface proteins in CHO cells transiently transfected with indicated GFP-tagged constructs were biotinylated as described in the Materials and Methods. Biotinylated proteins were immunoprecipitated with anti-GFP antibody to detect receptor cell surface expression. Lysates were immunoblotted with anti-GFP antibody to assess S1PR1 expression. Immunoblotting with anti-β-actin antibody was performed to assess equal protein loading control. A representative blot from three experiments performed independently is shown.
To identify the S1PR1 tyrosine phosphorylation residue regulating cell surface S1PR1 dynamics, we mutated each of the Y143, Y225 and Y311 residues to either a phenylalanine residue, which mimics unphosphorylated tyrosine, or to an aspartate residue, which mimics phosphotyrosine (Fig. 2C). Wild-type (WT)-S1PR1 was primarily localized on CHO cell surface and expression of mutated Y225F-S1PR1 and Y311F-S1PR1 or Y225D-S1PR1 and Y311D-S1PR1 resulted in similar cell surface localization (Fig. 2D,E). However, the cell surface expression of mutated Y143F-S1PR1 was increased, whereas that of Y143D-S1PR1 was decreased compared to WT-S1PR1 (Fig. 2D,E) indicating the crucial role of phosphorylation at Y143 in regulating cell surface S1PR1 expression. To corroborate these findings, we identified by using the biotinylation assay, the essential role of phosphorylation of Y143 in regulating S1PR1 cell surface expression based on increased cell surface localization of the Y143F-S1PR1 mutant and markedly reduced cell surface expression of Y143D-S1PR1 mutant as compared to WT-S1PR1 (Fig. 2F). Y143F-S1PR1 and Y143D-S1PR1 mutants were expressed at similar levels in CHO cells as WT-S1PR1 (Fig. 2F), ruling out degradation of phosphorylated S1PR1 as being responsible for the impaired cell surface expression (Oo et al., 2007).
S1P differentially effects cell surface expression of WT S1PR1 and phospho-specific S1PR1 mutants
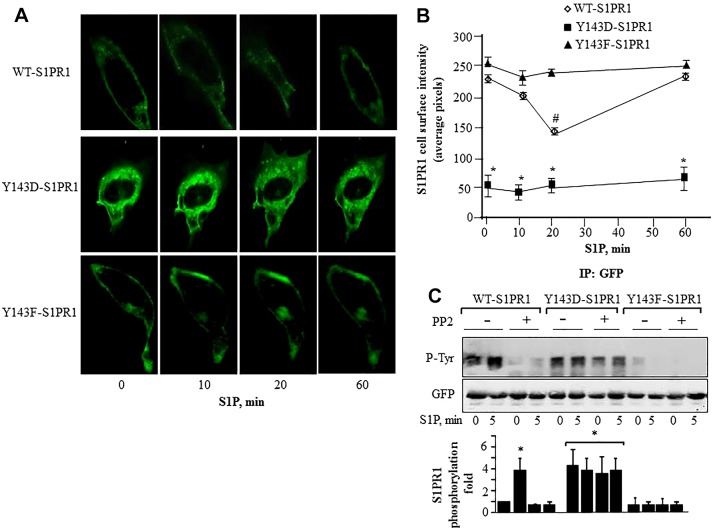
We next determined the fate of WT-S1PR1 phosphorylated at Y143 in returning to the cell surface. S1P treatment reduced CHO cell surface expression of WT-S1PR1 within 20 min which was followed by complete reappearance of the receptor at the surface in 60 min (Fig. 3A,B). In contrast, the Y143F-S1PR1 mutant remained on the cell surface following S1P stimulation (Fig. 3A,B). The Y143D-S1PR1 mutant, as well as having reduced baseline cell surface expression compared to WT-S1PR1 and Y143F-S1PR1 mutants, remained internalized after S1P stimulation (Fig. 3A,B).
Fig. 3.
Relationship between S1PR1 phosphorylation at Tyr143 and cell surface S1PR1 localization. (A) CHO cells transfected with GFP-tagged WT-S1PR1, Y143D-S1PR1 or Y143F-S1PR1 for 24 h were stimulated with 1 µM S1P and images were acquired at the indicated time points. Pixel intensity was quantified at the cell membrane using linescan in MetaMorph software. (B) Numerical values indicate mean±s.d. of pixel intensity at the cell periphery from multiple cells in each experiments which were conducted more than three times (n = 3–4). *P<0.05 for a reduction compared with cells expressing GFP–WT-S1PR1 or GFP–Y143F-S1PR1; #P<0.05 for a reduction compared with cells expressing GFP–Y143F-S1PR1. (C) CHO cells expressing the indicated mutants were serum starved for 30 min followed by exposure to 5 µM PP2 for 45 min in serum-free medium. Cells were challenged with 1 µM S1P for 5 min and lysates were immunoprecipitated with anti-GFP antibody followed by immunoblotting with anti-phosphotyrosine or anti-GFP antibodies to determine phosphorylation. Top, a representative immunoblot is shown. Bottom, bar graph of densitometric analysis of fold change in phosphorylation over that at 0 min in control cells from three individual experiments. *P<0.05.
Cells expressing the phospho-mimicking S1PR1 mutant (Y143D-S1PR1) showed similar levels of S1PR1 tyrosine phosphorylation to that in WT-S1PR1-expressing cells after S1P stimulation (Fig. 3C). Although pretreatment with PP2 significantly reduced S1PR1 phosphorylation in the cells transducing WT-S1PR1 the phosphorylation remain unaltered in cells expressing the Y143D-S1PR1 mutant (Fig. 3C) confirming Src family kinases regulated receptor phosphorylation. However, cells expressing phospho-defective Y143F-S1PR1 mutant showed, as expected, no phosphorylation in response to S1P (Fig. 3C).
Y143 phosphorylation of S1PR1 regulates cell surface receptor expression independently of phosphorylation of S1PR1 serine residues
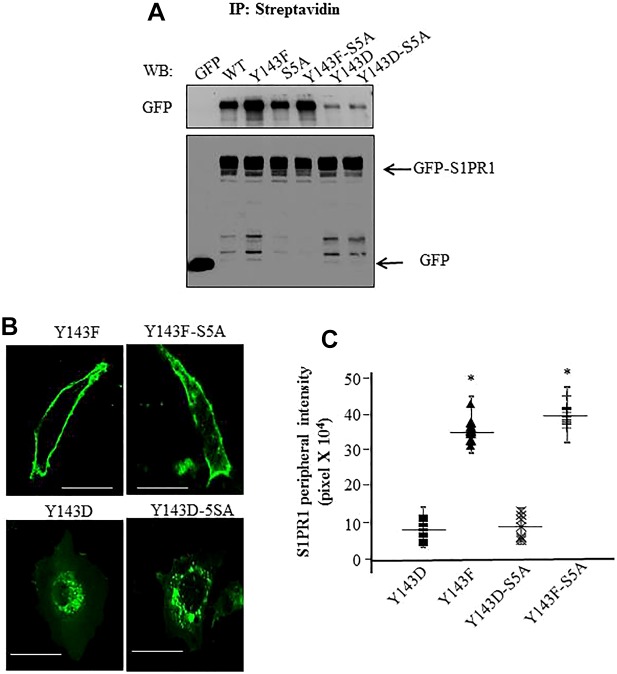
We next examined the role of serine phosphorylation of S1PR1 at the five serine residues located at C-terminus (S351, S353, S355, S358 and S359) (Oo et al., 2011; Oo et al., 2007) in regulating tyrosine-mediated cell surface expression of S1PR1. Here, we generated phospho-defective S1PR1 mutants by mutating all serine to alanine (S351A, S353A, S355A, S358A and S359A; referred to as 5SA-S1PR1) alone or together with Y143F (Y143-5SA-S1PR1) or Y143D (Y143D-5SA-S1PR1) mutations. Upon transfection in CHO cells, we found that the S5A-S1PR1 mutant was localized on the cell surface (Fig. 4A) consistent with the role of serine phosphorylation of S1PR1 in contributing to receptor internalization (Oo et al., 2007; Thangada et al., 2010). Expression of the Y143F-S1PR1 or Y143F-5SA-S1PR1 mutants resulted, in both cases, in localization of receptor on the cell surface (Fig. 4A). Importantly, expression of the Y143D-5SA-S1PR1 mutant did not rescue S1PR1 cell surface expression; that is, surface expression of Y143D-5SA-S1PR1 and Y143D mutants remained lower than that for either WT-S1PR1 or Y143F-S1PR1 (Fig. 4A), a finding consistent with the dominant role of Y143 phosphorylation in promoting the internalization of S1PR1. Immunoblotting with anti-GFP antibody showed that all mutants were expressed at similar levels (Fig. 4A), thus the results could not be ascribed to differential expression of the mutants. We also performed confocal imaging of CHO cells expressing these mutants to corroborate the above western blotting findings. We found markedly greater cell surface localization of Y143F-S1PR1 and Y143F-5SA-S1PR1 mutants than either Y143D-S1PR1 or Y143D-5SA-S1PR1 mutants (Fig. 4B,C). Thus, serine phosphorylation of S1PR1 at C-terminus sites did not influence the cell surface S1PR1 localization regulated by tyrosine phosphorylation at Y143.
Fig. 4.
Y143-phosphorylation-dependent regulation of S1PR1 localization functions independently of serine phosphorylation of S1PR1. (A) CHO cells transiently transfected with the indicated GFP-tagged constructs were biotinylated. Cell surface proteins were immunoprecipitated (IP) with anti-streptavidin antibody followed by immunoblotting (WB) with anti-GFP antibody to detect receptor cell surface expression. Cell lysates were immunoblotted with anti-GFP antibody to assess total S1PR1 expression. A representative blot from three experiments performed independently is shown. (B,C) CHO cells were transfected with the indicated mutants and after 24 h cells were visualized using confocal microscopy. Average pixel intensity at the cell periphery from various cells was quantified as described in Methods. Scale bars: 10 µm. C shows a plot of mean±s.d. of pixel intensity at the cell periphery in cells expressing the mutants from three independent experiments. In each experiment at least ten cells were counted. *P<0.05 compared with cells expressing WT-S1PR1.
Phosphorylation of S1PR1 at Y143 impairs endothelial barrier annealing induced by S1P
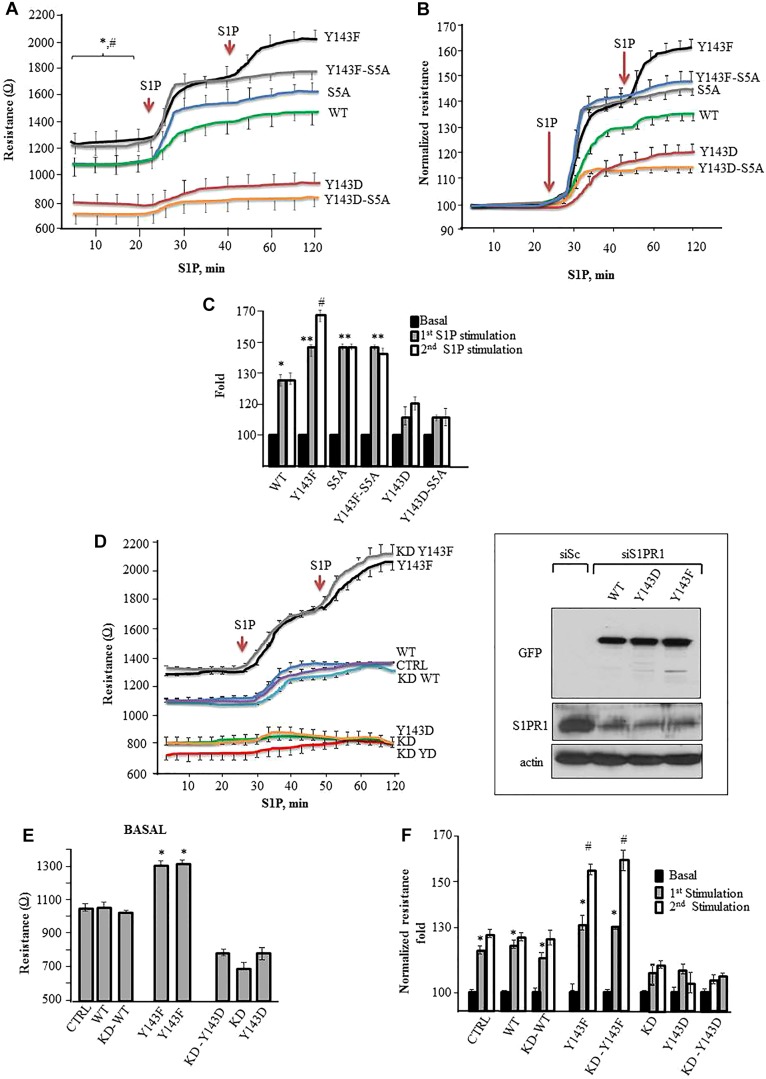
We transfected endothelial cells with WT-S1PR1, 5SA-S1PR1, Y143F-S1PR1, Y143D-S1PR1, Y143F-5SA-S1PR1 or Y143D-5SA- S1PR1 mutants to assess how expression of these S1PR1 mutants affected endothelial barrier function in response to S1P. Endothelial barrier function was determined by performing a transendothelial electrical resistance (TEER) assay. We determined whether (1) the tyrosine phosphorylation regulating S1PR1 cell surface retention also prevents enhancement of endothelial cell barrier function induced by S1P and 2) inhibition of serine phosphorylation of S1PR1 alters S1PR1 responsiveness to S1P following tyrosine phosphorylation. We found that, compared to the WT-S1PR1-transducing cells, basal TEER values were significantly greater in HPAECs expressing the Y143F-S1PR1 mutant, whereas TEER values were lower in cells expressing the Y143D-S1PR1 mutant (Fig. 5A), indicating that inhibition of tyrosine phosphorylation at Y143 enhanced basal endothelial barrier function. We next exposed these cells to S1P to determine the effects of Y143 phosphorylation in modulating receptor enhancement of the endothelial cell barrier. S1P significantly increased barrier function in cells expressing WT-S1PR1 and Y143F-S1PR1 mutants but the increase was greater in cells expressing the Y143F-S1PR1 mutant (Fig. 5A–C). S1P had a minimal effect in increasing the barrier function in cells expressing the Y143D-S1PR1 mutant (Fig. 5A–C).
Fig. 5.
S1PR1 phosphorylation at Y143 inhibits S1P-mediated enhancement of endothelial barrier annealing. (A) HPAECs seeded on gold-plated electrodes were transfected with the indicated mutants for 24 h. Cells were serum starved for 2 h after which endothelial barrier function was determined by measuring transendothelial electrical resistance (TEER) in real time in naive monolayers and following addition of 1 µM S1P. (B,C) TEER values obtained in Fig. 5A were normalized against basal values in each setting and re-plotted. *P<0.05 compared with basal; **P<0.05 compared with WT; #P<0.05 compared with initial S1P stimulation. (D–F) HPAE cells were transfected with control (CTRL, siSc) or S1PR1 siRNA (KD, siS1PR1). After 48 h, cells were transduced with indicated S1PR1 mutants and changes in TEER was assessed under basal conditions (E) and after S1P exposure (F). The inset in D is an immunoblot showing re-expression of indicated S1PR1 mutants following knockdown of S1PR1. Lysates from endothelial cells transfected with indicated mutants and S1PR1 siRNA were immunoblotted with anti-S1PR1, anti-GFP or anti-actin antibody to assess S1PR1 depletion and the re-expression of the mutants. Immunoblotting with anti-actin antibody was used as a loading control. In F, TEER values were normalized against basal values in each setting and re-plotted as fold increase over basal following S1P exposure. *P<0.05 compared with control, **P<0.05 compared with WT; #P<0.05 compared with initial S1P stimulation. Results are mean±s.d.
To address whether endothelial cells expressing the Y143F-S1PR1 mutant had differences in S1P responsiveness due to decreased S1PR1 internalization as compared to WT-S1PR1, we re-challenged the cells with S1P after a period of 20 min, at a time when WT-S1PR1 mutant was found to be internalized, and determined the TEER changes (Fig. 5A–C). We observed that repeat stimulation of WT-S1PR1-expressing cells with S1P failed to further increase TEER. However, repeat S1P stimulation increased TEER further in cells expressing the Y143F-S1PR1 mutant. Cells expressing the Y143D-S1PR1 mutant did not show an additional response to repeat S1P stimulation and the TEER response remained significantly lower than the responses in WT-S1PR1- or Y143F-S1PR1-expressing cells (Fig. 5A–C), consistent with the lower cell surface expression of the Y143D-S1PR1 mutant compared to cells expressing WT-S1PR1 and the Y143F-S1PR1 mutant.
We next determined the relationship between phosphorylation of Y143 with that of five serines (5S) of S1PR1, also known to regulate S1PR1 internalization (Oo et al., 2011; Oo et al., 2007). As above, we compared the effects of insertion of S5A mutation in S1PR1 alone or in combination with Y143F or Y143D mutation on endothelial cell barrier function. We found that basal TEER values were similar in S5A-S1PR1-S1PR1 and WT-S1PR1-expressing cells (Fig. 5A) indicating that inhibiting serine phosphorylation per se did not alter basal barrier function. We found S1P produced a significantly greater increase in TEER in endothelial cells expressing either the Y143F-5SA-S1PR1 or 5SA-S1PR1 mutant than WT-S1PR1-expressing cells (Fig. 5A–C). Insertion of 5SA into Y143D failed to rescue TEER in endothelial cells (Fig. 5A–C). In addition, endothelial cells expressing Y143F-5SA were refractory to a second S1P stimulation. Thus, phosphorylation of both Y143 and the five serine residues has the potential to downregulate S1PR1 surface expression and responsiveness to S1P, although the tyrosine and serine residues appear to operate independently of one or another.
We next transduced WT-S1PR1 and the Y143F-S1PR1 and Y143D-S1PR1 mutants in endothelial cells depleted of S1PR1 to rule out the role of endogenous S1PR1 in modulating the responsiveness of S1PR1 to S1P. S1PR1 depletion predictably reduced basal TEER, which failed to increase significantly after S1P stimulation (Fig. 5D,E). Rescuing S1PR1-depleted endothelial cells with WT-S1PR1 or Y143F-S1PR1 restored basal as well as S1P-induced TEER to control levels (Fig. 5D–F). In addition, Y143F-S1PR1 rescued TEER in S1PR1-depleted endothelial cells and these cells retained their responsiveness to repeat S1P stimuli leading to further enhancement of barrier function over the control levels (Fig. 5D,E). These changes were not seen in S1PR1-depleted endothelial cells on restoring Y143D-S1PR1 expression (Fig. 5D,E). These results demonstrate the central role of Y143 phosphorylation of S1PR1 in regulating the responsiveness to S1P stimulation and endothelial barrier enhancement.
DISCUSSION
S1PR1 in endothelial cells is a high-affinity S1P receptor with a Kd value in the nanomolar range whereas the plasma S1P concentrations are in the micromolar range (Lee et al., 1998). An important mechanism of dampening S1PR1 signaling in face of high S1P concentrations might be through post-translational modifications of S1PR1 (Oo et al., 2011; Oo et al., 2007; Watterson et al., 2002). The S1PR1 C-terminus is rich in serine residues that are phosphorylated (Gräler and Goetzl, 2004; Oo et al., 2007). Serine phosphorylation of S1PR1 leads to internalization, ubiquitylation and degradation of the receptor (Oo et al., 2011; Oo et al., 2007; Watterson et al., 2002), suggesting that phosphorylation of serine residues modulates S1PR1 signaling. In the present study, we uncovered an important role of tyrosine phosphorylation of S1PR1, specifically at Y143, in modulating endothelial cell surface expression of S1PR1 without the loss of the receptor expression through a degradation pathway. S1PR1 was internalized within 20 min following Y143 phosphorylation induced by S1P, but in contrast to serine-phosphorylated S1PR, which is shunted to a degradation pathway (Oo et al.), the tyrosine-phosphorylated receptor recycled back to the cell surface within 1 h.
A complicating factor in the present studies is the observation that the role of Y143 phosphorylation in promoting S1PR1 internalization might be influenced by S1PR1 phosphorylation at the five serine residues located in the C-terminus (Garris et al., 2013; Liu et al., 1999; Oo et al., 2011; Oo et al., 2007; Thangada et al., 2010). However, we showed that S1PR1 localization at the plasmalemma and S1PR1 responsiveness to S1P remained unaltered even when all of these residues were mutated (5SA) mutation as was also the case for the Y143F-S1PR1 (phospho-defective) or Y143D-S1PR1 (phospho-mimicking) mutants. These findings suggest that phosphorylation of Y143 on S1PR1 functions independently of serine phosphorylation in regulating endothelial cell surface expression of S1PR1, and that tyrosine phosphorylation of the receptor, unlike serine phosphorylation of S1PR1, provides a means for the receptor to be rapidly mobilized, through S1PR1 Y143 dephosphorylation, to the cell surface as needed.
The Y143 residue identified by us as the key site regulating endothelial cell S1PR1 internalization belongs to the conserved DRY/ERY motif, which is also found in other GPCRs (Hanson et al., 2012; O'Sullivan and Dev, 2013) This motif is required for transformation of the receptor from an inactive to G-protein-coupled conformation. We showed that Y143F-S1PR1, in contrast to Y143D-S1PR1, remained at the endothelial cell surface and thereby responded to repeat S1P stimulation of cells, resulting in enhanced endothelial barrier function. This finding demonstrates the central role of Y143 phosphorylation in regulating cell surface S1PR1 expression, and thereby the ability of endothelial cells to respond to S1P.
S1PR1 functions through the heterotrimeric Gi protein pathway and downstream activation of the monomeric Rho GTPase Rac1 (Garcia et al., 2001; Lee et al., 2000; Mehta et al., 2005; Thennes and Mehta, 2012). The mechanisms by which Y143 phosphorylation of S1PR1 downregulates cell surface expression of the receptor remain unknown. A tenable possibility is that Y143 phosphorylation of S1PR1 facilitates binding of S1PR1 to β-arrestin, and thereby induces receptor internalization (Ferguson et al., 1996; Luttrell and Lefkowitz, 2002; Waters et al., 2005). Arrestins function by turning off GPCR-activated responses and are important in enabling adaptation to a persistent stimulus, and hence allow active receptors to be desensitized (Ferguson et al., 1996; Reiter and Lefkowitz, 2006). Phosphorylation by a class of serine/threonine kinases, the GPCR kinases (GRKs), is essential for engagement of the arrestin machinery (Ferguson et al., 1996; Reiter and Lefkowitz, 2006). Although we did not study the function of GRKs and arrestins, we cannot rule out the possibility that internalization of S1PR1 phosphorylated on Y143 might also involve activation of specific GRKs and arrestins.
We also addressed the question as to whether phosphorylation of S1PR1 Y143 in endothelial cells has functional relevance. Previous studies have shown that internalization of S1PR1 from plasma membrane in lymphocytes is crucial to sustain S1PR1 signaling and mediate lymphocyte egress from the nodes (Matloubian et al., 2004; Willinger et al., 2014). In contrast, in endothelial cells, we observed that internalization of S1PR1 abrogated S1PR1 signaling, whereas sustained cell surface S1PR1 expression enhanced S1P signaling. Expression of the phospho-defective S1PR1 mutant Y143F-S1PR, not only induced persistent cell surface expression of the mutant but endothelial cells expressing this mutant also had a stronger S1P-mediated enhancement of endothelial barrier function than endothelial cells expressing WT-S1PR1. In contrast, the phospho-mimicking Y143D-S1PR1 mutant failed to respond to S1P and had little effect on endothelial barrier function because it was internalized. The reasons for differences in the function of S1PR1 in lymphocytes and endothelial cells are unclear but it might be that S1PR1 signaling functions in a cell-specific manner and requires activation of distinct S1PR1 signaling pathway in different cells.
Our results showed that S1P-induced phosphorylation of S1PR1 at Y143 required Src family kinases. S1P is known to activate Src kinases (Belvitch and Dudek, 2012; Bergelin et al., 2010; Waters et al., 2005), and thus it is possible that S1P ligation of S1PR1 and activation of Src is itself a signal for internalization of the receptor mediated by Src-induced phosphorylation of Y143. The mechanisms of dephosphorylation of S1PR1 that restores expression of S1PR1 are unclear. The protein tyrosine phosphatase SHP2, VE-PTP and PTP-1B all associate with adherens junctions in endothelial cells, and are known to regulate dephosphorylation of adherens-junction-associated proteins (Nakamura et al., 2008; Nottebaum et al., 2008; Timmerman et al., 2012; Ukropec et al., 2000). It is possible that a phosphatase localized with adherens junctions and dephosphorylating S1PR1 is responsible for restoring endothelial cell surface S1PR1 expression for the next round of S1P stimulation.
In conclusion, we demonstrate that phosphorylation of S1PR1 at Y143 has an important role in mediating internalization of the receptor and thereby effectively dampens S1PR1 signaling. Downregulation of S1PR1 signaling in endothelial cells subsequent to Y143 phosphorylation severely limited the barrier repair function of S1P. Thus, from the perspective of developing a S1PR1 agonist to reduce vascular inflammation and permeability, it would be necessary to prevent Y143 phosphorylation of S1PR1 in order to maintain a functional endothelial cell surface pool of S1PR1 that can be ligated by the agonist.
MATERIALS AND METHODS
Materials
Primary antibodies against phospho-tyrosine (PY20, PY99,and PY350), GFP, S1PR1 and β-actin, as well as normal rabbit IgG and protein A/G agarose beads were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). DAPI and ProLong Gold antifade and Alexa-Fluor-labeled secondary antibody were obtained from Invitrogen (Grand Island, NY). S1P was purchased from Enzo Life Sciences (Farmingdale, NY). PP2 inhibitor [4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo(3,4-d)pyrimidine] was purchased through EMD Millipore, Corp (Billerica, MA).
Generation of GFP-tagged S1PR1 constructs
Human S1PR1 cDNA was purchased from Origene (Rockville, MD) and amplified using the primers 5′-AGATCTCGAGCCACCATGGGGCCCACCAGCGTCCCG-3′ and 5′-ACCGGTGGATCCCCGGAAGAAGAGTTGACGTTTCC-3′ as described previously (Liu et al., 1999). Briefly, S1PR1 was subcloned into pEGFP-N1 plasmid using the BamHI and XhoI cloning sites, which resulted in the fusion of the GFP to the extreme C-terminus of the S1PR1 (Liu et al., 1999). cDNA sequencing confirmed that GFP–S1PR1 was free of mutations.
Phosphorylation-specific S1PR1 mutant constructs were generated through a two-step process. Overlapping DNA fragments containing base pair changes were generated in separate PCR processes during the first round of PCR with the cDNA clone GFP–S1PR1 as the template. The fragments were combined and used to amplify the entire S1PR1 cDNA containing the amino acid changes. Agarose gel-purified PCR fragments were digested with BamHI and XhoI restriction enzymes and cloned back into the pEGFP-N1 plasmid. Subsequent clones were sequenced to ensure desired identities. First round PCR primers were: forward, 5′-AGATCTCGAGCCACCATGGGGCCCACCAGCGTCCCG−3′; and reverse, 5′-ACCGGTGGATCCCCGGAAGAAGAGTTGACGTTTCC-3′. The clone-specific primer pairs used, with amino acid changes underlined, were as follows: Y143F: forward, 5′-CTCCTCGCCATCGCCATTGAGCGCTTTATCACAATG-3′; reverse, 5′-TGTGATAAAGCGCTCAATGGCGATGGCGAGGAG-3′; Y143D: forward, 5′-GCCATTGAGCGCGATATCACAATGCTGAAAATG-3′; reverse, 5′-TTCAGCATTGTGATATCGCGCTCAATGGCGAT-3′; Y225F: forward, 5′ TACTGCAGAATCTTTTCCTTGGTCAG-3′; reverse, 5′-CCTGACCAAGGAAAAGATTCTGCAGTA-3′; Y225D: forward, 5′-TACTGCAGAATCGACTCCTTGGTCAG-3′; reverse: 5′-CTGACCAAGCAGTCGATTCTGCAGTA-3′; Y311F: forward, 5′-AACCCCATCATTTTTACTCTGACCAAC-3′; reverse: 5′- GTTGGTCAGAGTAAAAATGATGGGGTT-3′; Y311D: forward: 5′AACCCCATCATTGACACTCTGACCAAC-3′; and reverse: 5′GTTGGTCAGAGTGTCAATGATGGGGTT-3′. To generate the S351, S353, S355, S358 and S359 mutants, we used GFP–S1PR1, and GFP–S1PR1-Y143D and -Y143F constructs as templates and took the following steps: step 1, 351-F which is a forward primer from vector inwards, 5′- TCGCTATTACCATGGTGATGCGGTTT-3′ and 351/3/A-R, which is a reverse primer from mutation 351, 353 and 355 towards the start, 5′-ATTGTCCGCTTTGGCGCGGGCGAATTCCAT-3′ (in forward orientation the mutations are: 351 = AGC to GCC, 353 = AGC to GCC and 355 TCG to GCG); step 2, 494-F which is a forward primer from vector inwards, 5′-ATGTCGTAACAACTCCGCCCCATTGA-3′ and 358/9A-R which is a reverse primer from mutation 358 and 359 towards the start 5′-TGGGCGGCATTGTCCGCTTTGGCGCG-3′; step 3, 358/9A-F which is a forwards primer from the mutation 358 and 359 toward the end, 5′-ACAATGCCGCCCACCCCCCAGAAAGACGAA-3′ (358 = TTC to GCC and 359 = TCC to GCC); step 4, flanking gene using primers within the vector 494-F, 5′-ATGTCGTAACAACTCCGCCCCATTGA-3′ and R820, 5′-TGAACTTCAGGGTCAGCTTCAGCTTGC-3′; step 5, primers that have correct subcloning restriction sites (Nhe1 and Xho1) were used to insert these mutants into the WT-, Y143D- and Y143F-S1PR1 vectors. Subsequent clones were sequenced to ensure sequence integrity.
Cell culture
HPAECs were cultured as described previously (Knezevic et al., 2009). Briefly, endothelial cells were plated on a T-75 flask coated with 0.1% gelatin in EBM-2 medium supplemented with 10% fetal bovine serum (FBS) and maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air until they formed a confluent monolayer. Cells from the primary flask were detached using 0.05% trypsin containing 0.02% EDTA. HPAECs were transfected with cDNA using the FUGENE HD transfection reagent (Promega, Madison, WI) or Amaxa electroporation (Lonza, San Diego, CA) as described previously (Singh et al., 2007). Depletion of S1PR1 was performed using Santa Cruz Biotechnology transfection reagent and custom-designed S1PR1 small interfering RNA (siRNA) against the 3′UTR region of receptor, sense, 5′-GGGAAGAUGAAGAUGGUUUUU-3′ and antisense, 5′-AAACCAUCUUCAUCUUCCCUU-3′. Cells were first transfected with S1PR1 siRNA or control siRNA for 48 h after which various S1PR1 cDNA constructs were transduced using the FUGENE transfection reagent. All experiments with 3′UTR S1PR1 siRNA were performed after confirming that S1PR1 siRNA failed to deplete transduced constructs. In experiments requiring S1P stimulation, a confluent monolayer of cells was first incubated in serum-free medium for 1 h before treatment. HPAECs were used between passages 6 and 8.
Chinese hamster ovary (CHO) cells were cultured in F12 medium supplemented with 10% FBS. To generate CHO cells stably expressing GFP–S1PR1, CHO cells were transfected using Lipofectamine (Invitrogen, Grand Island, NY) following the manufacturer's protocol. Transfected cells were selected using G418 (0.5 mg/ml) as described previously (Liu et al., 1999). In some studies, CHO cells were transiently transfected with S1PR1 constructs for 48 h using Lipofectamine.
Phosphorylation analysis
HPAECs or S1PR1 CHO cells grown on 100-mm dishes were serum starved with MCDB medium plus 0.1% FBS for 1 h and stimulated with 1 µM S1P. Equal concentration of cell lysates were immunoprecipitated with anti-S1PR1 or anti-GFP antibody overnight at 4°C, followed by addition of protein-A/G–agarose beads for 4 h at 4°C as previously described (Holinstat et al., 2006; Knezevic et al., 2009).
Biotinylation experiments
Serum-starved cells were washed twice with ice-cold PBS containing CaCl2 and labeled with 0.5 mg/ml of sulfo-NHS-SS Biotin (Pierce, Rockford, IL) in Ca2+- and Mg2+-containing PBS for 30 min at 4°C (Klessner et al., 2009). Cells were rinsed twice with ice-cold PBS and unbound biotin was quenched with 100 mM glycine for 20 min at 4°C, and then rinsed twice with PBS. Cells were harvested in 1% Triton-X-100-containing RIPA buffer with protease and phosphatase inhibitors. The lysate was clarified by centrifugation at 10,000 g for 10 min. Equal amounts of protein was incubated with 40 µl streptavidin–agarose resin beads at 4°C for 2 h. Beads were washed three times in RIPA by centrifugation at 2400 g for 1 min at 4°C. Proteins were eluted from the beads by boiling the samples in Laemmli buffer containing 5% β-mercaptoethanol and separated by SDS-PAGE (10% gels) and transferred onto nitrocellulose for western blot analysis using appropriate primary antibodies. For assessing phosphorylation of cell surface S1PR1 we performed a two-step immunoprecipitation as described previously (Chen and Derynck, 1994). Cells stimulated with S1P were first biotinylated as described above and equal amounts of lysate was immunoprecipitated with anti-S1PR1 antibody previously conjugated to streptavidin A/G beads. Following incubation for 2 h at 4°C, the beads were washed three times in RIPA buffer by centrifugation at 900 g for 3 min rotating at 4°C. S1PR1 from S1PR1–IgG beads was released by heating the complexes for 3 min at 90°C in immunoprecipitation buffer containing 100 µl HEPES buffered saline, 1% SDS and 1 mM phenyl-methylsulfonyl fluoride. The supernatant was isolated and the volume was brought up to 1 ml with immunoprecipitation buffer before being incubated with streptavidin–agarose beads for 1 h at 4°C with constant agitation. The streptavidin beads were then washed three times with immunoprecipitation buffer and the biotinylated S1PR1 was eluted by boiling in Laemmli buffer. These complexes were resolved by SDS-PAGE and transferred onto nitrocellulose and probed with anti-S1PR1 or anti-phosphotyrosine antibodies (Santa Cruz Biotechnology, Dallas, TX).
Immunofluorescence
Cells expressing GFP-tagged cDNA were fixed with 2% paraformaldehyde, permeabilized and stained with DAPI as described previously (Singh et al., 2007). Cells were visualized using a 63× 1.2 NA objective and appropriate filters using a LSM510 confocal microscope (Carl Zeiss, Inc.). Image analysis was achieved using the MetaMorph software. Three linescans on different cell areas were analyzed and this procedure was repeated on multiple cells at the indicated time points in each experiments. Pixel intensity at the cell periphery from several cells was averaged. Data are representative of at least three independent experiments. Live-cell imaging was performed on GFP–S1PR1-expressing CHO cells on a temperature controlled stand with a 63× 1.2 NA objective on an LSM510 confocal microscope (Carl Zeiss, Inc., Jena, Germany). After stimulation with S1P, pictures were captured at the indicated time points and the data was analyzed as described above. Images are representative of at least three separate experiments.
TEER measurement
HPAECs seeded on eight-well gold-plated electrodes (Applied Biosciences, Carlsbad, CA) were transfected with the indicated cDNA for 24 h. Cells were serum-deprived for 1 h, basal resistances were recorded, and then the cells were stimulated with 1 µM S1P as described previously (Mehta et al., 2001; Tauseef et al., 2008).
Statistical analysis
Statistical differences in mean values were assessed using ANOVA followed by two-tailed Student's t-test.
Acknowledgments
We thank Dr Debra Salvi for her help in generating S1PR1 constructs. We greatly appreciate Ms V. Kini for providing technical assistance.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
A.C., T.T.S. and D.M. designed the experiments and analyzed the data. A.C., T.T.S., P.Y., B.D., S.S., K.G.A., C.R. and N.K. performed experiments. A.C., T.T.S., A.B.M. and D.M. wrote the manuscript.
Funding
This work was supported by National Institute of Health [grant numbers HL71794, HL84153, HL060678 to D.M.; HL060678 and HL007829 to A.B.M.]; and the American Heart Association [grant number 10PRE2610268 to T.T.S.]. Deposited in PMC for release after 12 months.
References
- Argraves K. M., Wilkerson B. A., Argraves W. S. (2010). Sphingosine-1-phosphate signaling in vasculogenesis and angiogenesis. World J. Biol. Chem 1, 291–297 10.4331/wjbc.v1.i10.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvitch P., Dudek S. M. (2012). Role of FAK in S1P-regulated endothelial permeability. Microvasc. Res. 83, 22–30 10.1016/j.mvr.2011.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergelin N., Löf C., Balthasar S., Kalhori V., Törnquist K. (2010). S1P1 and VEGFR-2 form a signaling complex with extracellularly regulated kinase 1/2 and protein kinase C-alpha regulating ML-1 thyroid carcinoma cell migration. Endocrinology 151, 2994–3005. [DOI] [PubMed] [Google Scholar]
- Chen R. H., Derynck R. (1994). Homomeric interactions between type II transforming growth factor-beta receptors. J. Biol. Chem. 269, 22868–22874. [PubMed] [Google Scholar]
- Ferguson S. S., Downey W. E., 3rd, Colapietro A. M., Barak L. S., Ménard L., Caron M. G. (1996). Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271, 363–366 10.1126/science.271.5247.363 [DOI] [PubMed] [Google Scholar]
- Garcia J. G., Liu F., Verin A. D., Birukova A., Dechert M. A., Gerthoffer W. T., Bamberg J. R., English D. (2001). Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest. 108, 689–701 10.1172/JCI12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris C. S., Wu L., Acharya S., Arac A., Blaho V. A., Huang Y., Moon B. S., Axtell R. C., Ho P. P., Steinberg G. K. et al. (2013). Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation. Nat. Immunol. 14, 1166–1172 10.1038/ni.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeler M., Shankar G., Goetzl E. J. (2002). Cutting edge: suppression of T cell chemotaxis by sphingosine 1-phosphate. J. Immunol. 169, 4084–4087 10.4049/jimmunol.169.8.4084 [DOI] [PubMed] [Google Scholar]
- Gräler M. H., Goetzl E. J. (2004). The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 18, 551–553. [DOI] [PubMed] [Google Scholar]
- Hanke J. H., Gardner J. P., Dow R. L., Changelian P. S., Brissette W. H., Weringer E. J., Pollok B. A., Connelly P. A. (1996). Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271, 695–701 10.1074/jbc.271.2.695 [DOI] [PubMed] [Google Scholar]
- Hanson M. A., Roth C. B., Jo E., Griffith M. T., Scott F. L., Reinhart G., Desale H., Clemons B., Cahalan S. M., Schuerer S. C. et al. (2012). Crystal structure of a lipid G protein-coupled receptor. Science 335, 851–855 10.1126/science.1215904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T. (2004). Physiological and pathological actions of sphingosine 1-phosphate. Semin. Cell Dev. Biol. 15, 513–520 10.1016/j.semcdb.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Holinstat M., Knezevic N., Broman M., Samarel A. M., Malik A. B., Mehta D. (2006). Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. J. Biol. Chem. 281, 2296–2305 10.1074/jbc.M511248200 [DOI] [PubMed] [Google Scholar]
- Huang Y. L., Lin H. S., Chen S. U., Lee H. (2009). Tyrosine sulphation of sphingosine 1-phosphate 1 (S1P1) is required for S1P-mediated cell migration in primary cultures of human umbilical vein endothelial cells. J. Biochem. 146, 815–820 10.1093/jb/mvp131 [DOI] [PubMed] [Google Scholar]
- Klessner J. L., Desai B. V., Amargo E. V., Getsios S., Green K. J. (2009). EGFR and ADAMs cooperate to regulate shedding and endocytic trafficking of the desmosomal cadherin desmoglein 2. Mol. Biol. Cell 20, 328–337 10.1091/mbc.E08-04-0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezevic N., Tauseef M., Thennes T., Mehta D. (2009). The G protein betagamma subunit mediates reannealing of adherens junctions to reverse endothelial permeability increase by thrombin. J. Exp. Med. 206, 2761–2777 10.1084/jem.20090652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T., Wada A., Igarashi Y. (2002). N-glycans of sphingosine 1-phosphate receptor Edg-1 regulate ligand-induced receptor internalization. FASEB J. 16, 983–992 10.1096/fj.01-0809com [DOI] [PubMed] [Google Scholar]
- Lee M. J., Van Brocklyn J. R., Thangada S., Liu C. H., Hand A. R., Menzeleev R., Spiegel S., Hla T. (1998). Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 279, 1552–1555 10.1126/science.279.5356.1552 [DOI] [PubMed] [Google Scholar]
- Lee O. H., Lee D. J., Kim Y. M., Kim Y. S., Kwon H. J., Kim K. W., Kwon Y. G. (2000). Sphingosine 1-phosphate stimulates tyrosine phosphorylation of focal adhesion kinase and chemotactic motility of endothelial cells via the G(i) protein-linked phospholipase C pathway. Biochem. Biophys. Res. Commun. 268, 47–53 10.1006/bbrc.2000.2087 [DOI] [PubMed] [Google Scholar]
- Lee J. F., Gordon S., Estrada R., Wang L., Siow D. L., Wattenberg B. W., Lominadze D., Lee M. J. (2009). Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature. Am. J. Physiol. 296, H33–H42 10.1152/ajpheart.00097.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. H., Thangada S., Lee M. J., Van Brocklyn J. R., Spiegel S., Hla T. (1999). Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol. Biol. Cell 10, 1179–1190 10.1091/mbc.10.4.1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell L. M., Lefkowitz R. J. (2002). The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 115, 455–465. [DOI] [PubMed] [Google Scholar]
- Matloubian M., Lo C. G., Cinamon G., Lesneski M. J., Xu Y., Brinkmann V., Allende M. L., Proia R. L., Cyster J. G. (2004). Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 10.1038/nature02284 [DOI] [PubMed] [Google Scholar]
- McVerry B. J., Garcia J. G. (2004). Endothelial cell barrier regulation by sphingosine 1-phosphate. J. Cell. Biochem. 92, 1075–1085 10.1002/jcb.20088 [DOI] [PubMed] [Google Scholar]
- McVerry B. J., Peng X., Hassoun P. M., Sammani S., Simon B. A., Garcia J. G. (2004). Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am. J. Respir. Crit. Care Med. 170, 987–993 10.1164/rccm.200405-684OC [DOI] [PubMed] [Google Scholar]
- Mehta D., Rahman A., Malik A. B. (2001). Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J. Biol. Chem. 276, 22614–22620 10.1074/jbc.M101927200 [DOI] [PubMed] [Google Scholar]
- Mehta D., Konstantoulaki M., Ahmmed G. U., Malik A. B. (2005). Sphingosine 1-phosphate-induced mobilization of intracellular Ca2+ mediates rac activation and adherens junction assembly in endothelial cells. J. Biol. Chem. 280, 17320–17328 10.1074/jbc.M411674200 [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Patrushev N., Inomata H., Mehta D., Urao N., Kim H. W., Razvi M., Kini V., Mahadev K., Goldstein B. J. et al. (2008). Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ. Res. 102, 1182–1191 10.1161/CIRCRESAHA.107.167080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebaum A. F., Cagna G., Winderlich M., Gamp A. C., Linnepe R., Polaschegg C., Filippova K., Lyck R., Engelhardt B., Kamenyeva O. et al. (2008). VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J. Exp. Med. 205, 2929–2945 10.1084/jem.20080406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan C., Dev K. K. (2013). The structure and function of the S1P1 receptor. Trends Pharmacol. Sci. 34, 401–412 10.1016/j.tips.2013.05.002 [DOI] [PubMed] [Google Scholar]
- Okamoto H., Takuwa N., Yokomizo T., Sugimoto N., Sakurada S., Shigematsu H., Takuwa Y. (2000). Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol. Cell. Biol. 20, 9247–9261 10.1128/MCB.20.24.9247-9261.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo M. L., Thangada S., Wu M. T., Liu C. H., Macdonald T. L., Lynch K. R., Lin C. Y., Hla T. (2007). Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J. Biol. Chem. 282, 9082–9089 10.1074/jbc.M610318200 [DOI] [PubMed] [Google Scholar]
- Oo M. L., Chang S. H., Thangada S., Wu M. T., Rezaul K., Blaho V., Hwang S. I., Han D. K., Hla T. (2011). Engagement of S1P1-degradative mechanisms leads to vascular leak in mice. J. Clin. Invest. 121, 2290–2300 10.1172/JCI45403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik J. H., Chae Ss., Lee M. J., Thangada S., Hla T. (2001). Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of alpha vbeta3- and beta1-containing integrins. J. Biol. Chem. 276, 11830–11837 10.1074/jbc.M009422200 [DOI] [PubMed] [Google Scholar]
- Peng X., Hassoun P. M., Sammani S., McVerry B. J., Burne M. J., Rabb H., Pearse D., Tuder R. M., Garcia J. G. (2004). Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am. J. Respir. Crit. Care Med. 169, 1245–1251 10.1164/rccm.200309-1258OC [DOI] [PubMed] [Google Scholar]
- Reiter E., Lefkowitz R. J. (2006). GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol. Metab. 17, 159–165 10.1016/j.tem.2006.03.008 [DOI] [PubMed] [Google Scholar]
- Ruwisch L., Schäfer-Korting M., Kleuser B. (2001). An improved high-performance liquid chromatographic method for the determination of sphingosine-1-phosphate in complex biological materials. Naunyn Schmiedebergs Arch. Pharmacol. 363, 358–363 10.1007/s002100000365 [DOI] [PubMed] [Google Scholar]
- Shikata Y., Birukov K. G., Birukova A. A., Verin A., Garcia J. G. (2003a). Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling: role of Src and GIT. FASEB J. 17, 2240–2249 10.1096/fj.03-0198com [DOI] [PubMed] [Google Scholar]
- Shikata Y., Birukov K. G., Garcia J. G. (2003b). S1P induces FA remodeling in human pulmonary endothelial cells: role of Rac, GIT1, FAK, and paxillin. J. Appl. Physiol. 94, 1193–1203. [DOI] [PubMed] [Google Scholar]
- Singh I., Knezevic N., Ahmmed G. U., Kini V., Malik A. B., Mehta D. (2007). Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J. Biol. Chem. 282, 7833–7843 10.1074/jbc.M608288200 [DOI] [PubMed] [Google Scholar]
- Spiegel S., Milstien S. (2003). Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4, 397–407 10.1038/nrm1103 [DOI] [PubMed] [Google Scholar]
- Tauseef M., Kini V., Knezevic N., Brannan M., Ramchandaran R., Fyrst H., Saba J., Vogel S. M., Malik A. B., Mehta D. (2008). Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ. Res. 103, 1164–1172 10.1161/01.RES.0000338501.84810.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangada S., Khanna K. M., Blaho V. A., Oo M. L., Im D. S., Guo C., Lefrancois L., Hla T. (2010). Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J. Exp. Med. 207, 1475–1483 10.1084/jem.20091343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thennes T., Mehta D. (2012). Heterotrimeric G proteins, focal adhesion kinase, and endothelial barrier function. Microvasc. Res. 83, 31–44 10.1016/j.mvr.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman I., Hoogenboezem M., Bennett A. M., Geerts D., Hordijk P. L., van Buul J. D. (2012). The tyrosine phosphatase SHP2 regulates recovery of endothelial adherens junctions through control of β-catenin phosphorylation. Mol. Biol. Cell 23, 4212–4225 10.1091/mbc.E12-01-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukropec J. A., Hollinger M. K., Salva S. M., Woolkalis M. J. (2000). SHP2 association with VE-cadherin complexes in human endothelial cells is regulated by thrombin. J. Biol. Chem. 275, 5983–5986 10.1074/jbc.275.8.5983 [DOI] [PubMed] [Google Scholar]
- Wang L., Dudek S. M. (2009). Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc. Res. 77, 39–45 10.1016/j.mvr.2008.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters C. M., Connell M. C., Pyne S., Pyne N. J. (2005). c-Src is involved in regulating signal transmission from PDGFbeta receptor-GPCR(s) complexes in mammalian cells. Cell. Signal. 17, 263–277 10.1016/j.cellsig.2004.07.011 [DOI] [PubMed] [Google Scholar]
- Watterson K. R., Johnston E., Chalmers C., Pronin A., Cook S. J., Benovic J. L., Palmer T. M. (2002). Dual regulation of EDG1/S1P(1) receptor phosphorylation and internalization by protein kinase C and G-protein-coupled receptor kinase 2. J. Biol. Chem. 277, 5767–5777 10.1074/jbc.M110647200 [DOI] [PubMed] [Google Scholar]
- Willinger T., Ferguson S. M., Pereira J. P., De Camilli P., Flavell R. A. (2014). Dynamin 2-dependent endocytosis is required for sustained S1PR1 signaling. J. Exp. Med. 211, 685–700 10.1084/jem.20131343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Singleton P. A., Brown M. E., Dudek S. M., Garcia J. G. (2009). Phosphotyrosine protein dynamics in cell membrane rafts of sphingosine-1-phosphate-stimulated human endothelium: role in barrier enhancement. Cell. Signal. 21, 1945–1960 10.1016/j.cellsig.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Kim J. L., Newcomb J. R., Rose P. E., Stover D. R., Toledo L. M., Zhao H., Morgenstern K. A. (1999). Structural analysis of the lymphocyte-specific kinase Lck in complex with non-selective and Src family selective kinase inhibitors. Structure 7, 651–661 10.1016/S0969-2126(99)80086-0 [DOI] [PubMed] [Google Scholar]