Abstract
Positive cofactor 4 (PC4) is a coactivator that strongly augments transcription by various activators, presumably by facilitating the assembly of the preinitiation complex (PIC). However, our previous observation of stimulation of promoter escape in GAL4-VP16-dependent transcription in the presence of PC4 suggested a possible role for PC4 in this step. Here, we performed quantitative analyses of the stimulatory effects of PC4 on initiation, promoter escape, and elongation in GAL4-VP16-dependent transcription and found that PC4 possesses the ability to stimulate promoter escape in response to GAL4-VP16 in addition to its previously demonstrated effect on PIC assembly. This stimulatory effect of PC4 on promoter escape required TFIIA and the TATA box binding protein-associated factor subunits of TFIID. Furthermore, PC4 displayed physical interactions with both TFIIH and GAL4-VP16 through its coactivator domain, and these interactions were regulated distinctly by PC4 phosphorylation. Finally, GAL4-VP16 and PC4 stimulated both initiation and promoter escape to similar extents on the promoters with three and five GAL4 sites; however, they stimulated promoter escape preferentially on the promoter with a single GAL4 site. These results provide insight into the mechanism by which PC4 permits multiply bound GAL4-VP16 to attain synergy to achieve robust transcriptional activation.
Transcription of mRNA-coding genes involves RNA polymerase II and six general transcription factors (TFIIA, TFIIB, TFIID, TFIIE, TFIIF, and TFIIH) which comprise the basal transcription machinery that recognizes the core promoter elements and elicits the basal level of transcription (50). Activated transcription requires the binding of activators to the regulatory DNA sequences typically present upstream of the core promoter and their interactions with the general transcription machinery (32, 49). Despite the well-documented direct interactions of activators with the general transcription factors and RNA polymerase II, activated transcription requires yet another group of transcription factors, termed mediators or coactivators, that confer on the general transcription machinery a markedly enhanced responsiveness to activators (2, 18, 20, 36, 41).
A wide array of coactivators may be grouped into two broad categories according to the requirement of chromatin for their action in biochemical assays. The coactivators which function on the templates without chromatin include the TATA box binding protein-associated factors (TAFs) present in TFIID (58), positive cofactors (PCs) (PC1, PC2, PC3, and PC4) derived from the upstream factor stimulatory activity (USA) cofactor fraction (20), and metazoan multiprotein complexes that are structurally related to the yeast mediator (40) (TRAP/SMCC, ARC, DRIP, NAT, murine mediator, human mediator, CRSP, and PC2) (36, 41). The coactivators which require chromatin templates for their functions include CBP/p300, PCAF and its related GCN5 proteins, and p160 family proteins that display histone acetyltransferase activities (4, 65). Given their structural complexity and diversity, these coactivators are expected to show not only redundancy and cooperativity but activator and promoter selectivity as well, posing significant challenges for complete understanding of the various mechanisms by which coactivators facilitate transcription.
One way to approach the mechanisms of coactivator functions is to employ a well-defined transcription system that supports activated transcription in response to the smallest possible numbers of activators and coactivators and to identify the steps of transcription that are targeted physically and functionally by the activators and coactivators. A system well suited for this minimalist approach would be the transcription system that allows activated transcription in response to GAL4-VP16 or other GAL4-derivatives in the presence of coactivator PC4. PC4 is a coactivator that was initially identified in the USA fraction that enhances transcription by various transcriptional activators in vitro (13, 27, 38) and turned out to be identical to the 15-kDa single-stranded DNA (ssDNA)-binding protein. Although PC4 possesses both ssDNA- and double-stranded DNA (dsDNA)-binding activities, which are important for transcriptional repression, only its dsDNA-binding activity appears to correlate with the coactivator activity (62, 63). The coactivator activity of PC4 and its interaction with activators, but not the ssDNA-binding activity, are lost upon phosphorylation of the serine residues within its N-terminal region by casein kinase II (14, 27).
Since PC4 interacts with both transcriptional activators and TFIIA (13, 16) and also with TFIIB in the case of its yeast homolog, SUB1/TSP1 (16, 23), PC4 is proposed to promote the assembly of the preinitiation complex (PIC) (13, 21, 27) in activated transcription. However, given that transcription is a multistep process consisting of PIC assembly, promoter opening, initiation, promoter escape, elongation, and reinitiation, steps other than PIC assembly are potential targets for regulation as well. Indeed, despite predominant effects of activators—presumably in conjunction with coactivators—on PIC assembly, the effects on the subsequent steps (1, 28, 31, 37) have also been demonstrated in various systems, including promoter opening (60), promoter escape (10, 29), elongation (66), and reinitiation (26, 67). However, the relative contributions of activators and coactivators in stimulating individual steps of transcription remain to be more clearly defined.
In this study, we have systematically analyzed the stimulatory effects of GAL4-VP16 and PC4 and assigned their quantitative contributions to the stimulation of individual steps of transcription. Our results show that PC4 contributed to the stimulation of promoter escape as well as initiation in the presence of GAL4-VP16 and that these effects were contingent upon the presence of TFIIA and TAFs in TFIID. Consistent with the previously demonstrated requirement of the ERCC3 helicase activity of TFIIH in stimulating promoter escape (10), PC4 was found to interact specifically with TFIIH through its coactivator domain, a region that also interacted with GAL4-VP16. Furthermore, the number of GAL4 sites (and thus the number of bound GAL4-VP16 dimers) on the promoter influenced the degree of stimulation of each step by PC4, revealing possible links between the physical interactions involving PC4 and their functional consequences on the steps of transcription. Together, these results provide important clues as to the mechanism by which PC4 assists GAL4-VP16 in transcriptional activation.
MATERIALS AND METHODS
DNA templates for in vitro transcription.
DNA templates pG1HMC2AT and pG3HMC2AT were created by replacement of the five GAL4-binding sites of pG5HMC2AT with the annealed oligonucleotides. The following synthetic oligonucleotides were used for one and three GAL4-binding sites: 5′-AATTCGAGCTCGGTACCAGGGGACTAGAGTCTCCGCTCGGAGGACAGTACTCCGACCTGCA-3′ and 5′-GGTCGGAGTACTGTCCTCCGAGCGGAGACTCTAGTCCCCTGGTACCGAGCTCG-3′, respectively, for pG1HMC2AT and 5′-AATTCGAGCTCGGTACCAGGGGACTAGAGTCTCCGCTCGGAGGACAGTACTCCGCTCGGAGGACAGTACTCCGCTCGGAGGACAGTACTCCGACCTGCA-3′ and 5′-GGTCGGAGTACTGTCCTCCGAGCGGAGTACTGTCCTCCGAGCGGAGTACTGTCCTCCGAGCGGAGACTCTAGTCCCCTGGTACCGAGCTCG-3′, respectively, for pG3HMC2AT. The mixtures of the complementary oligonucleotides were denatured at 90°C for 5min and were then cooled slowly to room temperature in 10 mM Tris-HCl (pH 8.0)-1 mM EDTA-50 mM NaCl. Then, the annealed oligonucleotides were used to replace the EcoRI-PstI fragment encompassing the five GAL4-binding sites of pG5HMC2AT. The obtained DNA templates were sequenced completely on both strands to rule out the possibility of spurious mutations.
Purification of transcription factors and in vitro transcription assays.
Purification of recombinant factors (TFIIA, TFIIB, TFIIE, TFIIF, TFIIH, and GAL4-VP16), epitope-tagged TFIID, and RNA polymerase II were performed as described previously (10, 12). Recombinant PC4 was purified as described previously (11). For 390- and 20-nucleotide (nt) transcripts, in vitro transcription reaction mixtures (25 μl) contained 50 ng of negatively supercoiled pG5HMC2AT or its derivative, 12 mM HEPES-KOH (pH 7.9), 6% glycerol, 60 mM KCl, 0.6 mM EDTA, 8 mM MgCl2, 5 mM dithiothreitol (DTT), 20 U of RNase inhibitor (TaKaRa), 0.2 mM ATP, 0.2 mM UTP, 0.1 mM 3′-o-methyl GTP, 12.5 μM CTP, 10 μCi of [α-32P]CTP, 20 ng of TFIIA, 10 ng of TFIIB, 1 μl of FLAG-tagged TFIID (which corresponds to ∼0.1 ng of TATA binding protein [TBP]), 10 ng of TFIIE, 20 ng of TFIIF, 20 ng of recombinant TFIIH, and 100 ng of RNAPII. Where indicated, the reaction mixtures contained 25 ng of GAL4-VP16 and 200 ng of PC4 as well. Transcription reactions for the initiation product were performed as described previously (10), and the derived pppApC was treated with calf intestinal phosphatase to form ApC before electrophoresis (29). After electrophoresis, the transcripts were quantified by using a Fujix Bas2000 bioimaging analyzer. Stimulation of promoter escape (n-fold) was calculated by dividing the stimulation of the 20G transcript (n-fold) by that of ApC. Similarly, stimulation of elongation (n-fold) was calculated by dividing the stimulation of the 390-nt transcript (n-fold) by that of the 20G transcript.
Preparation of PC4-GST.
The coding region of PC4 was amplified by PCR with the primers 5′-GGCCTCTAGACATATGCCTAAATCAAAGG-3′ and 5′-GGCCGGATCCCAGCTTTCTTACTGCGTC-3′, which both incorporated XbaI and NdeI sites at the 5′ end and a BamHI site in place of the stop codon at the 3′ end. After digestion with XbaI and BamHI, the XbaI-BamHI fragment was subcloned into pBluescript II KS(−) (Stratagene) that was digested with XbaI and BamHI to create pBKS(−)-PC4. Then, the coding region of Schistosoma japonicum glutathione S-transferase (GST) was amplified by PCR from expression vector pGEX2TL(+) by using the primers 5′-GGCCGGATCCCCTATACTAGGTTATTG-3′ and 5′-GGCCGGATCCAGATCTCAGTCAGTCATTTTGGAGGATGGTCGCC-3′, which both incorporated a BamHI site at the 5′ end and BglII and BamHI sites at the 3′ end. The amplified PCR product was digested with BamHI, and the derived BamHI-BamHI fragment was inserted into the BamHI site of pBKS(−)-PC4 to create pBKS(−)-PC4-GST. After confirmation of its DNA sequence, the entire PC4-GST coding region was cut out with NdeI and BglII digestion and inserted into the NdeI and BamHI sites of pET3a (TaKaRa). PC4 deletion mutants, either as GST-PC4 or as PC4-GST, were created by using the same strategy and were inserted into the same expression vectors in place of wild-type PC4.
Recombinant GST-PC4 and PC4-GST and their derivatives were expressed in Escherichia coli BL21(DE3) pLysS at 30°C for 3 h, and the extract was prepared by sonication in buffer C (20 mM HEPES-KOH [pH 7.9], 10% glycerol, 1 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 1 mM DTT) containing 100 mM KCl. After removal of insoluble material by centrifugation, the soluble fraction was used for GST pull-down assays.
GST pull-down assays.
Ten microliters of glutathione-Sepharose 4B (Amersham Pharmacia Biotech) was equilibrated with buffer C containing 100 mM KCl and 0.1% Triton X-100 and was incubated with E. coli extract containing a GST-fusion protein at 4°C for 1 h. The quantity of each E. coli extract was adjusted so that an equal amount of GST fusion proteins could be retained on the resin. In all assays, each GST fusion protein immobilized on glutathione-Sepharose 4B was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to ensure that essentially the same amount of GST fusion proteins was immobilized on each resin. After extensive washing of the resin with the same buffer, either 200 ng of recombinant TFIIH or 240 ng of FLAG-tagged GAL4-VP16 was added to the resin suspended in 100 μl of buffer C, and the mixture was incubated at 4°C for 1 h with constant rotation. After the resin had been washed extensively with the equilibration buffer, the bound proteins were eluted with 10 μl of buffer C containing 1 M KCl and 0.1% Triton X-100. Two microliters of each eluate was separated by SDS-12% PAGE and detected by Western blotting with anti-FLAG M2 antibody (Sigma).
Phosphorylation of PC4-GST.
Fifty micrograms of PC4-GST immobilized on 10 μl of glutathione-Sepharose 4B was phosphorylated in reaction mixtures (200 μl) containing 20 mM Tris-HCl (pH 7.5), 50 mM KCl, 10 mM MgCl2, 0.2 mM ATP, and 50 U of casein kinase II (New England Biolabs)/μl at 30°C for 1 h. Phosphorylation of PC4-GST was confirmed by both radiolabeling with [γ-32P]ATP and the mobility change on a SDS-15% polyacrylamide gel.
DNase I footprint assays.
The promoter regions that contained GAL4 sites were PCR amplified from pG1HMC2AT, pG3HMC2AT, and pG5HMC2AT by using the primers 5′-GTAAAACGACGGCCAGT-3′ and 5′-CCGGGGATCCGGGGATGAGAGTGAATGATGATAGATTTG-3′. After digestion with EcoRI and BamHI, the PCR fragments were subcloned between the EcoRI and BamHI sites of pUC19 to create pUC19-G1, pUC19-G3, and pUC19-G5, which were then entirely sequenced to rule out the possibility of spurious mutations. The plasmids were digested with PvuI and XbaI, and the DNA fragments containing GAL4 sites were isolated after separation on a 4% polyacrylamide gel. Four picomoles of the DNA fragments were labeled by Klenow fragment (New England Biolabs) by using 50 μCi [α-32P]dCTP. Each labeled DNA fragment was then diluted with the same unlabeled fragment to obtain ∼2 × 104 cpm per 24 femtomoles of each fragment.
DNA binding reaction mixtures (25 μl) contained 12 mM HEPES-KOH (pH 7.9), 6% glycerol, 60 mM KCl, 0.6 mM EDTA, 8 mM MgCl2, 5 mM DTT, the indicated amount of GAL4-VP16, and 24 fmol of the labeled fragment and pUC19, which corresponds to approximately 50 ng of pG5HMC2AT used for in vitro transcription reactions. The reaction mixtures were incubated at 30°C for 60 min, and the DNA fragment was digested with DNase I for 2 min at room temperature by adding 25 μl of 5 mM CaCl2-10 mM MgCl2 containing 0.002 U of DNase I (TaKaRa). The DNase I digestion was stopped by adding 150 μl of stop solution (0.2% SDS, 20 mM EDTA), 20 μg of glycogen, and 5 μg of proteinase K, and the reaction mixtures were further incubated at 37°C for 60 min. After extraction with phenol and chloroform, the DNA fragment was precipitated with ethanol and rinsed twice with 70% ethanol. The dried pellet was redissolved in 2 μl of 90% formamide-0.025% (wt/vol) xylene cyanol and separated on a 4% denaturing polyacrylamide gel.
RESULTS
Promoter escape is a target for the coactivator activity of PC4.
To gain a mechanistic insight into the coactivator function in transcriptional activation, we utilized a model in vitro transcription system that included GAL4-VP16 as an activator and PC4 as a coactivator. This in vitro transcription system contained well-defined components, including recombinant factors (TFIIA, TFIIB, TFIIE, TFIIF, TFIIH, PC4, and GAL4-VP16) as well as highly purified HeLa cell-derived FLAG-tagged TFIID and RNA polymerase II (10-12), and exhibited marked transcriptional activation in response to GAL4-VP16 in a highly PC4-dependent manner. These features of this system provided an excellent opportunity to analyze mechanistic aspects of the coactivator function of PC4 in a quantitative manner.
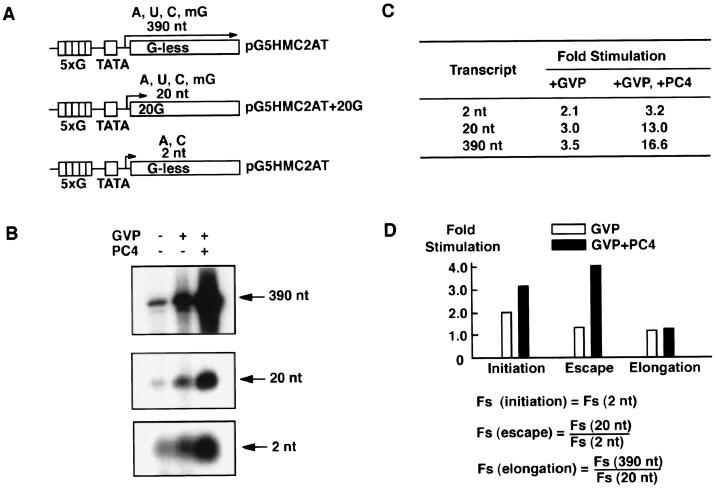
To accurately quantify the effects of PC4, we focused exclusively on measuring the amounts of the 2-, 20-, and 390-nt transcripts (Fig. 1A). We took this approach because measuring the amount of PIC (either by immunoblotting or by gel-mobility shift) and the degree of promoter opening (by potassium permanganate footprinting) did not give sufficiently accurate values compared to measuring the amounts of transcripts and therefore did not provide useful information for detailed quantitative analyses. For this reason, the effects of PC4 on PIC assembly and promoter opening are subsumed in the effects on the 2-nt ApC formation, which corresponds to the initiation step on the templates used in this study (Fig. 1A). Accordingly, unless otherwise stated, the term “initiation,” used hereafter for brevity, includes all three steps: PIC assembly, promoter opening, and ApC formation.
FIG. 1.
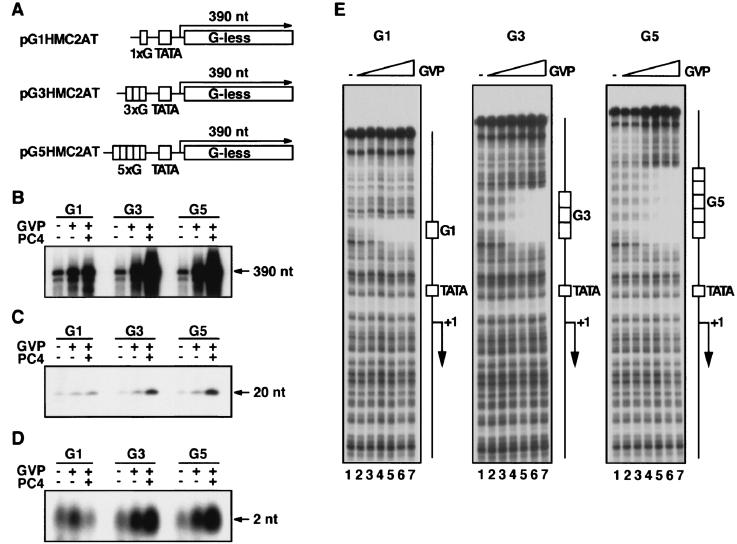
Effect of PC4 on promoter escape. (A) DNA templates used for in vitro transcription analyses. The template pG5HMC2AT contains five GAL4-binding sites upstream of the human immunodeficiency virus TATA box and the initiator from the Ad2 ML promoter fused to a 380-bp G-less cassette. This template produces the 390-nt transcript in the presence of ATP (A), CTP (C), UTP (U), and 3′-o-methyl GTP (mG) and the 2-nt transcript (initiation product) in the presence of ATP and CTP. The template pG5HMC2AT+20G, which is identical to pG5HMC2AT except that it contains a guanine residue at the +20 position, produces the 20-nt transcript in the presence of ATP, CTP, UTP, and 3′-o-methyl GTP. (B) Effect of GAL4-VP16 and PC4 upon the 390-, 20-, and 2-nt transcripts. All transcription reactions contained general transcription factors (TFIIA, TFIIB, TFIID, TFIIE, TFIIF, and TFIIH) and RNAPII in the presence or absence of GAL4-VP16 and PC4. The transcripts were separated on a denaturing polyacrylamide gel and autographed. (C) The levels of stimulation (n-fold) for the 2-, 20-, and 390-nt transcripts. The transcripts were quantified by using Fujix Bas 2000, and stimulation (n-fold) was determined for transcripts in the presence of GAL4-VP16 or GAL4-VP16 and PC4 by using the level for the transcript in the absence of GAL4-VP16 and PC4 as the basal level of transcription. (D) Fold stimulation (Fs) for each step of transcription was determined as indicated by using the values of Fs for the 2-, 20-, and 390-nt transcripts shown in panel C. Open bars indicate Fs in the presence of GAL4-VP16, whereas closed bars indicate Fs in the presence of GAL4-VP16 and PC4. For example, a value of 4.1 for Fs (escape) in the presence of GAL4-VP16 and PC4, indicated by a closed bar above “Escape” in the bar graph, was obtained by dividing of Fs (20 nt) (13.0) by Fs (2 nt) (3.2) in the presence of GAL4-VP16 and PC4.
Using this reconstituted in vitro transcription system, we measured the levels of the 2-, 20-, and 390-nt transcripts produced from the template pG5HMC2AT or its derivatives (Fig. 1A). The 2-nt initiation transcript, which is ApC on the template pG5HMC2AT, was produced in the presence of ATP and CTP. The 20-nt transcript was produced in the presence of ATP, CTP, UTP, and 3′-o-methyl GTP from template pG5HMC2AT+20G, which contained a G residue at the +20 position, at which transcription terminates through incorporation of 3′-o-methyl GTP (Fig. 1A). The 390-nt transcript was produced from template pG5HMC2AT in the presence of ATP, CTP, UTP, and 3′-o-methyl GTP. After the relative amounts of these transcripts had been determined, the effects of GAL4-VP16 or GAL4-VP16 and PC4 on initiation, promoter escape, and elongation were estimated (Fig. 1B and C). The effect on initiation was estimated directly from the stimulation (n-fold) of the 2-nt transcript. Also, the effect on promoter escape was estimated by dividing the stimulation (n-fold) of the 20-nt transcript by that of the 2-nt transcript, and likewise, the effect on elongation was estimated by dividing the stimulation (n-fold) of the 390-nt transcript by that of the 20-nt transcript.
As shown in Fig. 1B and C, GAL4-VP16 alone stimulated the production of the 2-, 20-, and 390-nt transcripts 2.1-, 3.0-, and 3.5-fold, respectively. Thus, the effects of GAL4-VP16 on initiation, promoter escape, and elongation were calculated as 2.1-, 1.4-, and 1.2-fold, respectively, indicating that GAL4-VP16 stimulated mostly initiation and had lesser effects on promoter escape and elongation (Fig. 1D). Further inclusion of PC4 in these reactions stimulated the production of the 2-, 20-, and 390-nt transcripts 3.2-, 13.0-, and 16.6-fold, respectively (Fig. 1C). Thus, the combined stimulatory effects of GAL4-VP16 and PC4 on initiation, promoter escape, and elongation were 3.2-, 4.1-, and 1.3-fold, respectively, indicating that PC4 augments the ability of GAL4-VP16 to stimulate initiation, promoter escape, and, to a lesser degree, elongation (Fig. 1D). It is notable that the effects of PC4 on the coactivation of GAL4-VP16 are more pronounced at the promoter escape step than at the other steps (Fig. 1D). Taken together, these results not only corroborate a previous demonstration that PC4 acts through the facilitated PIC assembly (13, 21) but also highlight promoter escape as yet another step facilitated by the coactivator activity of PC4.
PC4 requires TFIIA and TAFs for stimulating promoter escape in response to GAL4-VP16.
Previous biochemical studies demonstrated that TFIIA and the TAF subunits of TFIID greatly enhance transcriptional activation in vitro. Despite the well-documented roles of TFIIA and TFIID during the assembly of PIC (7, 8, 24, 33), their effects on promoter escape in activated transcription remain undefined. Moreover, observations that TBP alone supports transcriptional activation by various activators, including GAL4-VP16 (39, 43, 59, 64), suggest that some steps may be stimulated without TAFs.
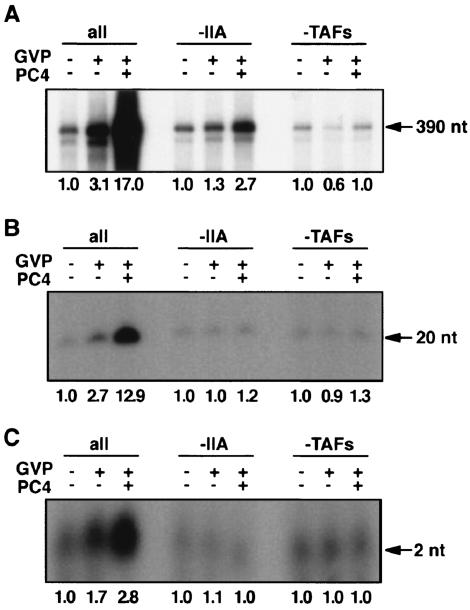
To determine whether TFIIA and TAFs are required for mediating the stimulation of promoter escape by GAL4-VP16 and PC4, we performed in vitro transcription assays in the presence or absence of TFIIA and TAFs. When TFIIA was removed from the reactions, the 2-, 20-, and 390-nt transcripts were stimulated 1.1-, 1.0-, and 1.3-fold, respectively, in the presence of GAL4-VP16 alone and 1.0-, 1.2-, and 2.7-fold, respectively, in the presence of GAL4-VP16 and PC4 (Fig. 2). Thus, in the absence of TFIIA, there was little stimulation of initiation and promoter escape by PC4 in response to GAL4-VP16. Interestingly, an approximately twofold stimulatory effect on elongation by GAL4-VP16 and PC4 remained intact even in the absence of TFIIA, albeit in a TAF-dependent manner (Fig. 2A and B), although this effect was not pursued further in this study. When TFIID was replaced by TBP, the 2-, 20-, and 390-nt transcripts were stimulated 0.6-, 0.9-, and 1.0-fold, respectively, in the presence of GAL4-VP16 and 1.0-, 1.3-, and 1.0-fold, respectively, in the presence of GAL4-VP16 and PC4 (Fig. 2), demonstrating that TAFs are essential for stimulating all of the steps, including initiation, promoter escape, and elongation, at least under the present assay conditions. Together, these observations suggest that both TFIIA and TAFs are indispensable for PC4 to effect noticeable stimulation of promoter escape as well as initiation (probably via facilitated PIC assembly) in response to GAL4-VP16.
FIG. 2.
Requirement of TFIIA and TAFs for stimulation of promoter escape by PC4. Stimulation of the 390-nt (A), 20-nt (B), and 2-nt (C) transcripts in the absence of TFIIA or TAFs. Transcription reactions were done in the absence of TFIIA (−IIA) or the TAF subunits of TFIID by using pG5HMC2AT or pG5HMC2AT+20G. The levels of stimulation of each transcript (n-fold) are indicated below the autoradiograms of the gels. In the absence of TFIIA or TAF subunits of TFIID, little stimulatory effect was observed on initiation, promoter escape, and elongation in the presence of GAL4-VP16 or in the presence of both GAL4-VP16 and PC4 except for a small stimulatory effect (i.e., ∼2.3-fold) on elongation by PC4 in the absence of TFIIA, an effect that was dependent upon the presence of TFIID.
PC4 interacts with TFIIH and GAL4-VP16 via its coactivator domain.
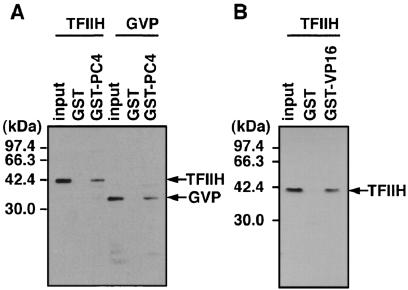
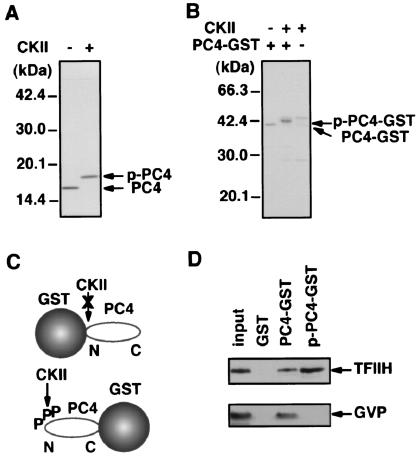
A previous study showed that TFIIH is required for the stimulation of promoter escape because of its ERCC3 helicase activity (10) in activated transcription by GAL4-VP16 and PC4. The fact that both PC4 and TFIIH are required simultaneously to facilitate promoter escape prompted us to examine a possible physical interaction between PC4 and TFIIH. To do this, we performed GST pull-down assays by using PC4 fused to the C terminus of GST, which was expressed in E. coli and retained on the glutathione-Sepharose resin. Since recombinant TFIIH has a FLAG tag at the C terminus of its MO15 subunit and GAL4-VP16 has an N-terminal FLAG tag, both proteins were detected by Western blotting with anti-FLAG M2 antibody after separation by SDS-PAGE. As shown in Fig. 3A, TFIIH was found to bind to GST-PC4 but not to GST alone, indicating that PC4 interacts with TFIIH specifically. The interaction between PC4 and TFIIH seemed as strong as the well-characterized interaction between PC4 and VP16 (Fig. 3A) and that between TFIIH and the VP16 activation domain (Fig. 3B), since similar proportions (∼10%) of input TFIIH and GAL4-VP16 were bound to GST-PC4 under the same conditions.
FIG. 3.
PC4 interacts directly with TFIIH. (A) Interaction of PC4 with TFIIH. PC4 was fused to the C terminus of GST and expressed in E. coli as GST-PC4. TFIIH and GAL4-VP16 were allowed to interact with GST or GST-PC4 prebound to glutathione-Sepharose, and, after extensive washing, bound proteins were eluted, separated by SDS-PAGE along with ∼20% of the amount of the input protein, and detected by immunoblotting. TFIIH and GAL4-VP16 were detected with anti-FLAG M2 antibody, since the MO15 subunit of TFIIH and GAL4-VP16 were tagged with a FLAG epitope. The positions of molecular mass markers are indicated on the left. The positions of MO15 (TFIIH) and GAL4-VP16 (GVP) are also indicated on the right. (B) Interaction of TFIIH and GAL4-VP16. GST pull-down assays were done under the same conditions as used for the tests presented in panel A, with GST-VP16 being used in place of GST-PC4.
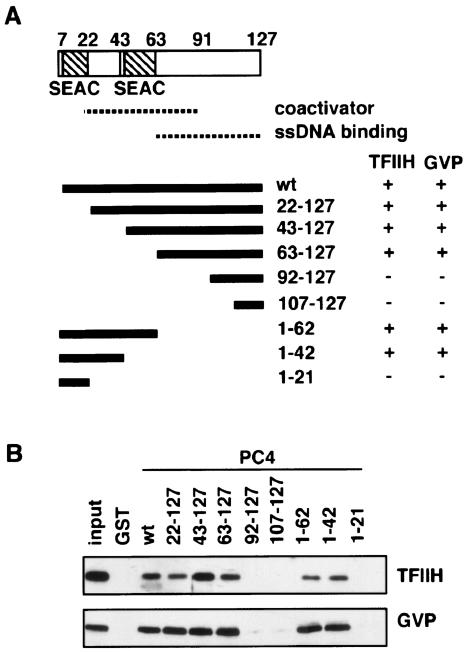
To explore the relevance of the interactions of PC4 with TFIIH and with GAL4-VP16 for the coactivator activity of PC4, we localized the region of PC4 that interacted with TFIIH and GAL4-VP16. We created N-terminal and C-terminal deletion mutants of PC4, as shown in Fig. 4A, and tested their interactions with TFIIH and GAL4-VP16. As shown in Fig. 4B and also summarized in Fig. 4A, the mutants PC4(22-127), PC4(43-127), PC4(63-127), PC4(1-62), and PC4(1-42) interacted with both TFIIH and GAL4-VP16, whereas PC4(92-127), PC4(107-127), and PC4(1-21) did not interact with either TFIIH or GAL4-VP16 (Fig. 4A and B), showing that PC4 interacts with TFIIH and GAL4-VP16 through the region from residue 22 to residue 91, a domain necessary and sufficient for the coactivator activity of PC4 (21, 27). Furthermore, the PC4 mutants PC4(1-62) and PC4(63-127), which do not overlap with each other, interacted with both TFIIH and GAL4-VP16, suggesting that these interactions occur redundantly at multiple sites within the coactivator domain of PC4. The interactions of the different PC4 mutants with GAL4-VP16 were equally strong, whereas those with TFIIH showed variations; for example, PC4(43-127) interacted with TFIIH more strongly than wild-type PC4 did, while PC4(22-127), PC4(1-62), and PC4(1-42) interacted with TFIIH more weakly than wild-type PC4 did (Fig. 4B).
FIG. 4.
The interaction of TFIIH and GAL4-VP16 with the coactivator domain of PC4. (A) Schematic representation of the domain structure of PC4. Two serine-rich domains, termed SEAC, are present between amino acid residues 7 and 22 and between residues 43 and 63. The domain for binding single-stranded DNA (dotted line) is localized between residues 63 and 127, and the 89th tryptophan residue is critical for its activity. The coactivator domain (dotted line) is localized to the region between residues 63 and 91, partially overlapping the ssDNA-binding domain. The lower panel shows the tested deletion mutants and the results of the GST pull-down assays for their interactions with PC4 or GAL4-VP16. The 127-amino-acid full-length PC4 is indicated by “wt.” Binding and nonbinding are indicated by “+” and “−,” respectively, on the right side of the lower panel. (B) GST pull-down assays for PC4 deletion mutants, as detected with Western blots. Note the variation in the amounts of bound TFIIH, which was reproducible, in marked contrast to the constant level of GAL4-VP16 binding.
Thus, colocalization of the interaction region to the functionally defined coactivator domain (21, 27) argues that these interactions are functionally relevant for the coactivator activity of PC4. Moreover, the redundancy of these interactions is consistent with the role of PC4 as a coactivator, which is expected to interact with activators and the basal transcriptional machinery at the same time.
Distinct regulation of the interactions of PC4 with GAL4-VP16 and TFIIH.
Since the interaction of TFIIH with PC4 mutants appeared to differ slightly from that of GAL4-VP16, we further explored the difference between these two interactions. The interaction between PC4 and GAL4-VP16 was previously shown to be negatively regulated by phosphorylation of the N-terminal region of PC4 (14, 27); therefore, we sought to determine whether the same was true for the interaction between PC4 and TFIIH. To make this determination, we used PC4-GST, in which the C terminus of PC4 is fused to the N terminus of GST, since GST-PC4 could not be phosphorylated efficiently by casein kinase II, presumably because the N-terminal phosphorylation sites of PC4 within GST-PC4 were sterically inaccessible to the casein kinase II (Fig. 5C). PC4-GST, expressed in E. coli and retained on glutathione-Sepharose, showed essentially the same binding to TFIIH and GAL4-VP16 as GST-PC4 did (data not shown). As shown in Fig. 5B, PC4-GST could be readily phosphorylated, and the phosphorylation slowed the migration of PC4-GST on the SDS gel, a shift of migration similar to that observed for nonfused PC4 (Fig. 5A), indicating that PC4-GST was phosphorylated in essentially the same manner as PC4 was. Pull-down assays with PC4-GST indicated not only that TFIIH interacted with both phosphorylated and nonphosphorylated PC4, but also that its interaction with PC4 was slightly enhanced by the phosphorylation of PC4 (Fig. 5D). In marked contrast, the interaction between GAL4-VP16 and PC4 was completely abolished upon phosphorylation of PC4, as reported previously (13, 27). Thus, although TFIIH and GAL4-VP16 interact with PC4 through the same coactivator domain, these interactions show markedly distinct regulation through the phosphorylation of PC4.
FIG. 5.
PC4 interacts with TFIIH in a manner distinct from that of GAL4-VP16. (A) Phosphorylation of PC4 with casein kinase II (CKII). Purified PC4 was phosphorylated by casein kinase II (New England Biolabs), and the shifted mobility of PC4 was observed upon phosphorylation. The positions of phosphorylated PC4 (p-PC4) and nonphosphorylated PC4 (PC4) are shown on the right. (B) Phosphorylation of PC4-GST. The positions of phosphorylated PC4-GST (p-PC4-GST) and nonphosphorylated PC4-GST (PC4-GST) are indicated on the right. (C) As shown schematically, PC4 fused to the N terminus of GST was efficiently phosphorylated by casein kinase II. Casein kinase II phosphorylated PC4 fused to the N terminus, but not to the C terminus, of GST (data not shown), presumably because the phosphorylation sites within the N-terminal region of PC4 were sterically masked by GST. A GST molecule and PC4 are schematically represented, and a phosphate molecule and the amino and carboxyl termini of PC4 are indicated by P, N, and C, respectively. (D) Interaction of phosphorylated PC4 with TFIIH. GST pull-down assays with PC4-GST revealed that TFIIH interacted with both nonphosphorylated and phosphorylated forms of PC4 but that GAL4-VP16 interacted only with the nonphosphorylated form of PC4. Note that approximately twofold more TFIIH bound to p-PC4-GST than to PC4-GST.
The number of GAL4 binding sites determines the degree to which each step of the transcriptional process is stimulated upon activation.
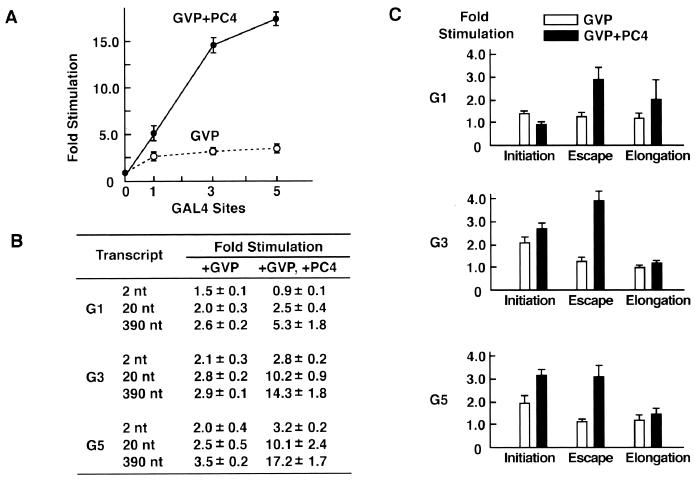
The multiple interactions of GAL4-VP16 and PC4 with the basal transcription machinery, as demonstrated here and elsewhere, and the observed stimulatory effects before and after initiation suggest that each GAL4-VP16 dimer bound to the five GAL4 sites may have a distinct role in activated transcription. To gain further insight into a potential relationship between each GAL4-VP16 dimer and the effects on distinct steps, as well as the role of PC4 in this process, we determined the degree to which each step of transcription is stimulated in the presence and absence of PC4 when the number of bound GAL4-VP16 dimers was reduced (Fig. 6A). To this end, we created the templates with one, three, and five GAL4-binding sites (G1, G3, and G5 templates, respectively, binding 2, 6, and 10 GAL4-VP16 dimers) (Fig. 6A) and performed in vitro transcription analyses. As shown in Fig. 6 and quantified in Fig. 7, GAL4-VP16 alone stimulated the level of the 390-nt transcripts from the G1, G3, and G5 templates 2.6-, 2.9-, and 3.5-fold, respectively, showing that increasing the number of bound GAL4-VP16 dimers does not necessarily lead to robust transcriptional activation when PC4 is absent from the reactions. In the presence of PC4, however, stimulation of the 390-nt transcript increased dramatically to 5.3-, 14.3-, and 17.2-fold for the G1, G3, and G5 templates, respectively (Fig. 6B and 7A), revealing that the effect of PC4 becomes more apparent as the number of GAL4-VP16 dimers is increased. Moreover, DNase I footprint analyses showed that all of the GAL4 sites on the G1, G3, and G5 templates were occupied almost completely by 25 ng of GAL4-VP16 (Fig. 6E, lane 6), the amount that was used for in vitro transcription reactions. Thus, it is unlikely that transcriptional activation for the G3 and G5 templates derives from the PC4-induced cooperative binding of GAL4-VP16 to its cognate sites. More likely, however, is the possibility that PC4 increases the number, or the effectiveness, of the interactions between GAL4-VP16 and the basal transcription machinery to allow synergistic effects of multiply bound GAL4-VP16 dimers (Fig. 7A).
FIG. 6.
Effect of the number of GAL4 sites on the degree of stimulation of the 390-, 20-, and 2-nt transcripts. (A) Templates used for in vitro transcription. Three templates, pG1HMC2AT, pG3HMC2AT, and pG5HMC2AT, contained one, three, and five GAL4 binding sites, respectively. For in vitro transcription for 20-nt transcripts, the same set of the templates with a G residue at the +20 position (not shown) was used. The amount of GAL4-VP16 added to the transcription reaction was the same for all reactions (25 ng). (B to D) The effects of one, three, and five GAL4 sites on the stimulation of the 390-nt (B), 20-nt (C), and 2-nt (D) transcripts. G1, G3, and G5 indicate pG1HMC2AT, pG3HMC2AT, and pG5HMC2AT, respectively. (E) Binding of GAL4-VP16 to the G1, G3, and G5 templates. Increasing amounts of GAL4-VP16 were tested for binding to the DNA fragments containing one, three, and five GAL4 sites. The added amounts of GAL4-VP16 were 0 ng (lane 1), 1.6 ng (lane 2), 3.1 ng (lane 3), 6.3 ng (lane 4), 12.5 ng (lane 5), 25 ng (lane 6), and 50 ng (lane 7). The positions of GAL4 binding sites (G1, G3, and G5), the TATA box (TATA), and the initiation site (+1) are indicated on the right.
FIG. 7.
The stimulation of initiation, promoter escape, and elongation on the templates with different numbers of GAL4-sites. (A) The effect of coactivation by PC4 became more pronounced as the number of GAL4 sites was increased. The levels of the 390-nt transcripts shown in Fig. 6 were quantified by using Fujix BAS 2000, and the values of stimulation (n-fold) were calculated. The standard deviations for three independent experiments are indicated. (B) The values of activation (n-fold) for the 2-, 20-, and 390-nt transcripts were determined from three independent experiments and are shown as means ± standard deviations. (C) The number of GAL4 sites influences the activation of initiation, promoter escape, and elongation. The values for stimulation (n-fold) of initiation, promoter escape, and elongation were calculated as those presented in Fig. 1 were. On the templates with a single GAL4 site, PC4 stimulated promoter escape rather than initiation of GAL4-VP16-dependent transcription, while on the templates with three or five GAL4 sites, PC4 stimulated both initiation and promoter escape to similar extents. There were small but reproducible effects on elongation in all experiments.
Next, to determine the relative stimulation of initiation, promoter escape, and elongation in activated transcription from the G1, G3, and G5 templates, we assayed and quantified the amounts of the 2- and 20-nt transcripts from these templates (Fig. 6C and D) and then ascribed the effects of GAL4-VP16 or of GAL4-VP16 and PC4 to three distinct steps (Fig. 7B and C). The analyses of the transcripts from the G1 template in the presence of GAL4-VP16 alone revealed minor stimulation of initiation, with little stimulation of promoter escape and elongation. However, markedly increased levels of stimulation of promoter escape and, to a lesser extent, elongation were observed when PC4 was included in these reactions (Fig. 7C, top panel). Interestingly, no stimulation whatsoever of initiation from the G1 template was observed in the presence of both GAL4-VP16 and PC4 (Fig. 7C, top panel). In contrast, robust activation of transcription from the G3 and G5 templates by GAL4-VP16 and PC4 was attributed largely to the marked stimulation of both initiation and promoter escape (Fig. 7C, middle and bottom panels). Low levels of transcriptional activation for these templates in the presence of GAL4-VP16 alone, however, resulted mainly from the stimulation of initiation.
These data demonstrate the following points. First, GAL4-VP16 alone can effect a low level of stimulation of the initiation step but little, if any, promoter escape, regardless of the number of its binding sites. Second, PC4 increases the degrees to which GAL4-VP16 stimulates initiation and promoter escape, having a more pronounced effect on promoter escape than on initiation. Third, promoter escape appears to be preferentially stimulated by GAL4-VP16 in the presence of PC4 when GAL4-VP16 is bound on a single GAL4 site. Together, these observations suggest that each GAL4-VP16 dimer bound on the promoter may stimulate a distinct step of transcription.
DISCUSSION
Although a large body of evidence indicates the functional significance of coactivators in regulating transcription in vitro and in vivo (2, 18, 20, 36, 41), far less is known about the precise mechanism(s) by which these coactivators stimulate transcription in conjunction with activators, especially in the context of naked DNA templates. In the present study, we took advantage of a well-defined reconstituted in vitro transcription system (10, 12) and demonstrated a crucial role for a coactivator, PC4, in stimulating promoter escape in activated transcription, in part through direct interaction with TFIIH.
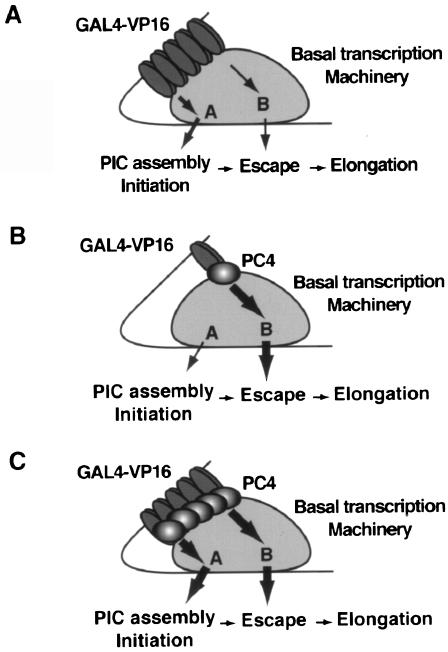
Figure 8 depicts how PC4 enables GAL4-VP16 to achieve a high level of transcriptional activation. This model postulates at least two targets, termed targets A and B, in the basal transcription machinery, to which signals from activators are transmitted. These signals, in turn, permit target A and target B to regulate the steps leading to initiation (PIC assembly, promoter opening, and initiation) and promoter escape, respectively. Each target postulated in the model is meant to represent multiple factors rather than a single factor, and, conversely, a single factor may constitute a part of more than one target. For instance, since TFIIA and TFIID are important for facilitating both PIC assembly (7, 8, 24, 25) and promoter escape (Fig. 2), each factor must constitute parts of both target A and target B. In addition, PC4 and TFIIH (Fig. 3, 4, and 5), whose ERCC3 helicase activity is also essential for stimulating promoter escape (10), are likely to constitute the target B that regulates promoter escape. This complex network of multiple interactions may induce conformational changes, including isomerization of the DA complex (7), that lead to stimulation of individual steps of the transcriptional process.
FIG. 8.
A model of PC4 coactivator activity. In this model, we postulate that the basal transcription machinery contains at least two targets, termed targets A and B, through which the signals from activators are transmitted either directly or via PC4 to the individual steps of the transcription process. Target A regulates the steps leading to initiation (PIC assembly, promoter opening, and initiation) and is likely to consist of more discrete targets, whereas target B regulates promoter escape. (A) In the absence of PC4, GAL4-VP16 elicits transcriptional activation through the predominant effect on target A, regardless of the number of bound GAL4-VP16 molecules. (B) When PC4 is present, GAL4-VP16 bound at a single GAL4 site provides substantial transcriptional activation through target B. (C) When PC4 is present, multiply bound GAL4-VP16 achieves robust transcription through the synergized effects on both target A and target B, enhanced by PC4.
In the absence of PC4, GAL4-VP16 appears to function mainly through target A, and even increasing the number of GAL4-VP16 dimers bound on the template does not lead to robust transcriptional activation (Fig. 8A). In the presence of PC4, however, GAL4-VP16 can function through target B and also augment the effect through target A. Of the two postulated targets, target B seems to be preferred by the combination of GAL4-VP16 and PC4, since PC4 directs GAL4-VP16 to function predominantly through target B when the amount of GAL4-VP16 is limited, as on the G1 template (Fig. 8B). In contrast, when multiply bound GAL4-VP16 dimers are present, as on the G3 and G5 templates, PC4 permits distinct GAL4-VP16 molecules to function through both target A and target B (Fig. 8C), providing a mechanism for transcriptional synergy (6, 17, 34, 35, 46).
In this model, it is implicitly assumed that GAL4-VP16 and PC4 are capable of multiple interactions with the basal transcription machinery, interactions that obligate GAL4-VP16 and PC4 to adopt different conformations depending upon the target to which they bind. This assumption is supported not only by numerous interaction studies but also by recent structural studies showing that transcriptional activation domains, including that of VP16, are poorly structured in their free form but undergo an induced structural transition when complexed with their targets (5, 9, 30, 45, 51-53, 57, 61). In addition, the VP16 activation domain can adopt different structures whether it is bound to TBP or TFIIB (53). Moreover, this structural flexibility is also displayed by a mediator-like coactivator complex, CSRP (42, 56). Thus, given the lack of a stable three-dimensional structure within its coactivator domain (3), PC4 may form a stable structure only upon binding to activators and the basal transcription machinery. Through these interactions, PC4 could bestow activators with extra surfaces and an added conformational flexibility that permit more functionally effective links between activators and the basal transcription machinery.
Our model of PC4 action appears to contradict the widely accepted notion that PIC assembly is the primary target for activated transcription, as demonstrated by various in vivo and in vitro studies (47). In particular, using a similar in vitro transcription system, Chi et al. (7, 8) demonstrated that PIC assembly, especially DA complex assembly, is necessary and sufficient for activation, an observation supported by others (24, 25, 54, 55). Furthermore, Jacob and Luse (19) failed to detect any stimulatory effect on promoter escape by GAL4-VP16 by using HeLa nuclear extract. We believe, however, that this apparent contradiction can be reconciled for the following reasons. First, the effect on PIC assembly as inferred by the order-of-addition experiments does not necessarily dictate the actual time point at which the assembled PIC acts on steps of transcription. Thus, the effects of the assembled PIC, such as the isomerized DA complex (7), may remain far beyond the time point of their assembly. Second, we also observed the predominant effects on initiation (which may reflect PIC assembly in our assays) to overall stimulation of transcription when the amounts of factors were reduced. We suspect that, under these conditions, the stimulatory effect on promoter escape may be easily overlooked. Third, since PC4 acts as a coactivator only in its nonphosphorylated form (14, 27) and also in a highly concentration-dependent manner (13), PC4 may not have been functional as a coactivator in the transcription systems involving crude fractions (7, 8, 19, 24, 25), in which the majority of PC4 is phosphorylated (14, 27). Given these considerations, our results are not inconsistent with earlier observations that emphasized the predominant role of PIC assembly in transcriptional activation.
The exact mechanism by which PC4 assists the ERCC3 helicase of TFIIH during promoter escape remains an enigma. One attractive possibility is that PC4 stabilizes the ssDNA region exposed during promoter escape through its ssDNA-binding ability (62), thereby indirectly assisting the ERCC3 helicase. It is generally known that ssDNA-binding proteins stimulate the activities of DNA polymerases and helicases (7), and indeed, PC4 facilitates DNA replication mediated by SV40 T antigen (44). However, the possibility of this mechanism seems remote because a PC4 mutant, W89A, which has little ssDNA-binding ability (63), shows essentially the same effect on promoter escape as wild-type PC4 does (10). Therefore, we favor alternative mechanisms by which PC4 facilitates the recruitment of TFIIH (29) or directly stabilizes the ATP-induced conformational change of TFIIH per se through protein-protein interactions, a mechanism consistent with the fact that TFIIH does not function as a classical helicase (22). Related to this idea, HBx, a coactivator-like transcriptional regulator of the hepatitis B virus (15), stimulates TFIIH helicase activities independently of its ssDNA-binding ability (48).
In conclusion, we have shown that PC4 assists GAL4-VP16 in stimulating the multiple steps of transcription and facilitates synergy by multiply bound GAL4-VP16 dimers. Future studies should address more detailed mechanistic aspects of the coactivator activity of PC4 and identify the precise factors within the basal transcription machinery that are targeted by individual GAL4-VP16 and PC4 molecules bound multiply on a single promoter. These studies may offer a paradigm for further functional analyses of diverse coactivators.
Acknowledgments
We thank K. Nakagawa and Y. Miyagi for technical assistance and other members of the laboratory for materials, advice, discussion, and critical reading of the manuscript.
This study was supported by grants-in-aid from the Ministry of Education, Science, Sports and Culture, by the Maruki Memorial Prize of Saitama Medical School, and by a grant from the Sumitomo Foundation. A.F. was a Research Fellow of the Japan Society for the Promotion of Science.
REFERENCES
- 1.Akhtar, A., G. Faye, and D. L. Bentley. 1996. Distinct activated and non-activated RNA polymerase II complexes in yeast. EMBO J. 15:4654-4664. [PMC free article] [PubMed] [Google Scholar]
- 2.Berk, A. J. 1999. Activation of RNA polymerase II transcription. Curr. Opin. Cell Biol. 11:330-335. [DOI] [PubMed] [Google Scholar]
- 3.Brandsen, J., S. Werten, P. C. van der Vliet, M. Meisterernst, J. Kroon, and P. Gros. 1997. C-terminal domain of transcription cofactor PC4 reveals dimeric ssDNA binding site. Nat. Struct. Biol. 4:900-903. [DOI] [PubMed] [Google Scholar]
- 4.Brown, C. E., T. Lechner, L. Howe, and J. L. Workman. 2000. The many HATs of transcription coactivators. Trends Biochem. Sci. 25:15-19. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, K. M., A. R. Terrell, P. J. Laybourn, and K. J. Lumb. 2000. Intrinsic structural disorder of the C-terminal activation domain from the bZIP transcription factor Fos. Biochemistry 39:2708-2713. [DOI] [PubMed] [Google Scholar]
- 6.Carey, M., Y. S. Lin, M. R. Green, and M. Ptashne. 1990. A mechanism for synergistic activation of a mammalian gene by GAL4 derivatives. Nature 345:361-364. [DOI] [PubMed] [Google Scholar]
- 7.Chi, T., and M. Carey. 1996. Assembly of the isomerized TFIIA-TFIID-TATA ternary complex is necessary and sufficient for gene activation. Genes Dev. 10:2540-2550. [DOI] [PubMed] [Google Scholar]
- 8.Chi, T., P. Lieberman, K. Ellwood, and M. Carey. 1995. A general mechanism for transcriptional synergy by eukaryotic activators. Nature 377:254-257. [DOI] [PubMed] [Google Scholar]
- 9.Dahlman-Wright, K., H. Baumann, I. J. McEwan, T. Almlof, A. P. Wright, J. A. Gustafsson, and T. Hard. 1995. Structural characterization of a minimal functional transactivation domain from the human glucocorticoid receptor. Proc. Natl. Acad. Sci. USA 92:1699-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukuda, A., Y. Nogi, and K. Hisatake. 2002. The regulatory role for the ERCC3 helicase of general transcription factor TFIIH during promoter escape in transcriptional activation. Proc. Natl. Acad. Sci. USA 99:1206-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuda, A., S. Tokonabe, M. Hamada, M. Matsumoto, T. Tsukui, Y. Nogi, and K. Hisatake. 2003. Alleviation of PC4-mediated transcriptional repression by the ERCC3 helicase activity of general transcription factor TFIIH. J. Biol. Chem. 278:14827-14831. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda, A., J. Yamauchi, S. Y. Wu, C. M. Chiang, M. Muramatsu, and K. Hisatake. 2001. Reconstitution of recombinant TFIIH that can mediate activator-dependent transcription. Genes Cells 6:707-719. [DOI] [PubMed] [Google Scholar]
- 13.Ge, H., and R. G. Roeder. 1994. Purification, cloning, and characterization of a human coactivator, PC4, that mediates transcriptional activation of class II genes. Cell 78:513-523. [DOI] [PubMed] [Google Scholar]
- 14.Ge, H., Y. Zhao, B. T. Chait, and R. G. Roeder. 1994. Phosphorylation negatively regulates the function of coactivator PC4. Proc. Natl. Acad. Sci. USA 91:12691-12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haviv, I., D. Vaizel, and Y. Shaul. 1996. pX, the HBV-encoded coactivator, interacts with components of the transcription machinery and stimulates transcription in a TAF-independent manner. EMBO J. 15:3413-3420. [PMC free article] [PubMed] [Google Scholar]
- 16.Henry, N. L., D. A. Bushnell, and R. D. Kornberg. 1996. A yeast transcriptional stimulatory protein similar to human PC4. J. Biol. Chem. 271:21842-21847. [DOI] [PubMed] [Google Scholar]
- 17.Hori, R., and M. Carey. 1994. The role of activators in assembly of RNA polymerase II transcription complexes. Curr. Opin. Genet. Dev. 4:236-244. [DOI] [PubMed] [Google Scholar]
- 18.Ito, M., and R. G. Roeder. 2001. The TRAP/SMCC/Mediator complex and thyroid hormone receptor function. Trends Endocrinol. Metab. 12:127-134. [DOI] [PubMed] [Google Scholar]
- 19.Jacob, G. A., and D. S. Luse. 1996. GAL4-VP16 stimulates two RNA polymerase II promoters primarily at the preinitiation complex assembly step. Gene Expr. 5:193-203. [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser, K., and M. Meisterernst. 1996. The human general co-factors. Trends Biochem. Sci. 21:342-345. [PubMed] [Google Scholar]
- 21.Kaiser, K., G. Stelzer, and M. Meisterernst. 1995. The coactivator p15 (PC4) initiates transcriptional activation during TFIIA-TFIID-promoter complex formation. EMBO J. 14:3520-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, T. K., R. H. Ebright, and D. Reinberg. 2000. Mechanism of ATP-dependent promoter melting by transcription factor IIH. Science 288:1418-1422. [DOI] [PubMed] [Google Scholar]
- 23.Knaus, R., R. Pollock, and L. Guarente. 1996. Yeast SUB1 is a suppressor of TFIIB mutations and has homology to the human co-activator PC4. EMBO J. 15:1933-1940. [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi, N., T. G. Boyer, and A. J. Berk. 1995. A class of activation domains interacts directly with TFIIA and stimulates TFIIA-TFIID-promoter complex assembly. Mol. Cell. Biol. 15:6465-6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi, N., P. J. Horn, S. M. Sullivan, S. J. Triezenberg, T. G. Boyer, and A. J. Berk. 1998. DA-complex assembly activity required for VP16C transcriptional activation. Mol. Cell. Biol. 18:4023-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraus, W. L., and J. T. Kadonaga. 1998. p300 and estrogen receptor cooperatively activate transcription via differential enhancement of initiation and reinitiation. Genes Dev. 12:331-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kretzschmar, M., K. Kaiser, F. Lottspeich, and M. Meisterernst. 1994. A novel mediator of class II gene transcription with homology to viral immediate-early transcriptional regulators. Cell 78:525-534. [DOI] [PubMed] [Google Scholar]
- 28.Krumm, A., L. B. Hickey, and M. Groudine. 1995. Promoter-proximal pausing of RNA polymerase II defines a general rate-limiting step after transcription initiation. Genes Dev. 9:559-572. [DOI] [PubMed] [Google Scholar]
- 29.Kumar, K. P., S. Akoulitchev, and D. Reinberg. 1998. Promoter-proximal stalling results from the inability to recruit transcription factor IIH to the transcription complex and is a regulated event. Proc. Natl. Acad. Sci. USA 95:9767-9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, H., K. H. Mok, R. Muhandiram, K. H. Park, J. E. Suk, D. H. Kim, J. Chang, Y. C. Sung, K. Y. Choi, and K. H. Han. 2000. Local structural elements in the mostly unstructured transcriptional activation domain of human p53. J. Biol. Chem. 275:29426-29432. [DOI] [PubMed] [Google Scholar]
- 31.Lee, Y. C., J. M. Park, S. Min, S. J. Han, and Y. J. Kim. 1999. An activator binding module of yeast RNA polymerase II holoenzyme. Mol. Cell. Biol. 19:2967-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemon, B., and R. Tjian. 2000. Orchestrated response: a symphony of transcription factors for gene control. Genes Dev. 14:2551-2569. [DOI] [PubMed] [Google Scholar]
- 33.Lieberman, P. M., J. Ozer, and D. B. Gursel. 1997. Requirement for transcription factor IIA (TFIIA)-TFIID recruitment by an activator depends on promoter structure and template competition. Mol. Cell. Biol. 17:6624-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin, Y. S., M. Carey, M. Ptashne, and M. R. Green. 1990. How different eukaryotic transcriptional activators can cooperate promiscuously. Nature 345:359-361. [DOI] [PubMed] [Google Scholar]
- 35.Lin, Y. S., and M. R. Green. 1991. Mechanism of action of an acidic transcriptional activator in vitro. Cell 64:971-981. [DOI] [PubMed] [Google Scholar]
- 36.Malik, S., and R. G. Roeder. 2000. Transcriptional regulation through Mediator-like coactivators in yeast and metazoan cells. Trends Biochem. Sci. 25:277-283. [DOI] [PubMed] [Google Scholar]
- 37.Malik, S., A. E. Wallberg, Y. K. Kang, and R. G. Roeder. 2002. TRAP/SMCC/mediator-dependent transcriptional activation from DNA and chromatin templates by orphan nuclear receptor hepatocyte nuclear factor 4. Mol. Cell. Biol. 22:5626-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meisterernst, M., A. L. Roy, H. M. Lieu, and R. G. Roeder. 1991. Activation of class II gene transcription by regulatory factors is potentiated by a novel activity. Cell 66:981-993. [DOI] [PubMed] [Google Scholar]
- 39.Moqtaderi, Z., Y. Bai, D. Poon, P. A. Weil, and K. Struhl. 1996. TBP-associated factors are not generally required for transcriptional activation in yeast. Nature 383:188-191. [DOI] [PubMed] [Google Scholar]
- 40.Myers, L. C., and R. D. Kornberg. 2000. Mediator of transcriptional regulation. Annu. Rev. Biochem. 69:729-749. [DOI] [PubMed] [Google Scholar]
- 41.Naar, A. M., B. D. Lemon, and R. Tjian. 2001. Transcriptional coactivator complexes. Annu. Rev. Biochem. 70:475-501. [DOI] [PubMed] [Google Scholar]
- 42.Naar, A. M., D. J. Taatjes, W. Zhai, E. Nogales, and R. Tjian. 2002. Human CRSP interacts with RNA polymerase II CTD and adopts a specific CTD-bound conformation. Genes Dev. 16:1339-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oelgeschlager, T., Y. Tao, Y. K. Kang, and R. G. Roeder. 1998. Transcription activation via enhanced preinitiation complex assembly in a human cell-free system lacking TAFIIs. Mol. Cell 1:925-931. [DOI] [PubMed] [Google Scholar]
- 44.Pan, Z. Q., H. Ge, A. A. Amin, and J. Hurwitz. 1996. Transcription-positive cofactor 4 forms complexes with HSSB (RPA) on single-stranded DNA and influences HSSB-dependent enzymatic synthesis of simian virus 40 DNA. J. Biol. Chem. 271:22111-22116. [DOI] [PubMed] [Google Scholar]
- 45.Parker, D., U. S. Jhala, I. Radhakrishnan, M. B. Yaffe, C. Reyes, A. I. Shulman, L. C. Cantley, P. E. Wright, and M. Montminy. 1998. Analysis of an activator:coactivator complex reveals an essential role for secondary structure in transcriptional activation. Mol. Cell 2:353-359. [DOI] [PubMed] [Google Scholar]
- 46.Ptashne, M. 1988. How eukaryotic transcriptional activators work. Nature 335:683-689. [DOI] [PubMed] [Google Scholar]
- 47.Ptashne, M., and A. Gann. 1997. Transcriptional activation by recruitment. Nature 386:569-577. [DOI] [PubMed] [Google Scholar]
- 48.Qadri, I., J. W. Conaway, R. C. Conaway, J. Schaack, and A. Siddiqui. 1996. Hepatitis B virus transactivator protein, HBx, associates with the components of TFIIH and stimulates the DNA helicase activity of TFIIH. Proc. Natl. Acad. Sci. USA 93:10578-10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roeder, R. G. 1998. Role of general and gene-specific cofactors in the regulation of eukaryotic transcription. Cold Spring Harb. Symp. Quant. Biol. 63:201-218. [DOI] [PubMed] [Google Scholar]
- 50.Roeder, R. G. 1996. The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem. Sci. 21:327-335. [PubMed] [Google Scholar]
- 51.Schmitz, M. L., M. A. dos Santos Silva, H. Altmann, M. Czisch, T. A. Holak, and P. A. Baeuerle. 1994. Structural and functional analysis of the NF-kappa B p65 C terminus. An acidic and modular transactivation domain with the potential to adopt an alpha-helical conformation. J. Biol. Chem. 269:25613-25620. [PubMed] [Google Scholar]
- 52.Shen, F., S. J. Triezenberg, P. Hensley, D. Porter, and J. R. Knutson. 1996. Critical amino acids in the transcriptional activation domain of the herpesvirus protein VP16 are solvent-exposed in highly mobile protein segments. An intrinsic fluorescence study. J. Biol. Chem. 271:4819-4826. [DOI] [PubMed] [Google Scholar]
- 53.Shen, F., S. J. Triezenberg, P. Hensley, D. Porter, and J. R. Knutson. 1996. Transcriptional activation domain of the herpesvirus protein VP16 becomes conformationally constrained upon interaction with basal transcription factors. J. Biol. Chem. 271:4827-4837. [DOI] [PubMed] [Google Scholar]
- 54.Shykind, B. M., J. Kim, and P. A. Sharp. 1995. Activation of the TFIID-TFIIA complex with HMG-2. Genes Dev. 9:1354-1365. [DOI] [PubMed] [Google Scholar]
- 55.Shykind, B. M., J. Kim, L. Stewart, J. J. Champoux, and P. A. Sharp. 1997. Topoisomerase I enhances TFIID-TFIIA complex assembly during activation of transcription. Genes Dev. 11:397-407. [DOI] [PubMed] [Google Scholar]
- 56.Taatjes, D. J., A. M. Naar, F. Andel III, E. Nogales, and R. Tjian. 2002. Structure, function, and activator-induced conformations of the CRSP coactivator. Science 295:1058-1062. [DOI] [PubMed] [Google Scholar]
- 57.Uesugi, M., O. Nyanguile, H. Lu, A. J. Levine, and G. L. Verdine. 1997. Induced alpha helix in the VP16 activation domain upon binding to a human TAF. Science 277:1310-1313. [DOI] [PubMed] [Google Scholar]
- 58.Verrijzer, C. P., and R. Tjian. 1996. TAFs mediate transcriptional activation and promoter selectivity. Trends Biochem. Sci. 21:338-342. [PubMed] [Google Scholar]
- 59.Walker, S. S., J. C. Reese, L. M. Apone, and M. R. Green. 1996. Transcription activation in cells lacking TAFIIS. Nature 383:185-188. [DOI] [PubMed] [Google Scholar]
- 60.Wang, W., M. Carey, and J. D. Gralla. 1992. Polymerase II promoter activation: closed complex formation and ATP-driven start site opening. Science 255:450-453. [DOI] [PubMed] [Google Scholar]
- 61.Warnmark, A., A. Wikstrom, A. P. Wright, J. A. Gustafsson, and T. Hard. 2001. The N-terminal regions of estrogen receptor alpha and beta are unstructured in vitro and show different TBP binding properties. J. Biol. Chem. 276:45939-45944. [DOI] [PubMed] [Google Scholar]
- 62.Werten, S., F. W. Langen, R. van Schaik, H. T. Timmers, M. Meisterernst, and P. C. van der Vliet. 1998. High-affinity DNA binding by the C-terminal domain of the transcriptional coactivator PC4 requires simultaneous interaction with two opposing unpaired strands and results in helix destabilization. J. Mol. Biol. 276:367-377. [DOI] [PubMed] [Google Scholar]
- 63.Werten, S., G. Stelzer, A. Goppelt, F. M. Langen, P. Gros, H. T. Timmers, P. C. Van der Vliet, and M. Meisterernst. 1998. Interaction of PC4 with melted DNA inhibits transcription. EMBO J. 17:5103-5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu, S. Y., E. Kershnar, and C. M. Chiang. 1998. TAFII-independent activation mediated by human TBP in the presence of the positive cofactor PC4. EMBO J. 17:4478-4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu, L., C. K. Glass, and M. G. Rosenfeld. 1999. Coactivator and corepressor complexes in nuclear receptor function. Curr. Opin. Genet. Dev. 9:140-147. [DOI] [PubMed] [Google Scholar]
- 66.Yankulov, K., J. Blau, T. Purton, S. Roberts, and D. L. Bentley. 1994. Transcriptional elongation by RNA polymerase II is stimulated by transactivators. Cell 77:749-759. [DOI] [PubMed] [Google Scholar]
- 67.Yudkovsky, N., J. A. Ranish, and S. Hahn. 2000. A transcription reinitiation intermediate that is stabilized by activator. Nature 408:225-229. [DOI] [PubMed] [Google Scholar]