Abstract
Common variable immunodeficiency (CVID) can show variant histological patterns in the gastrointestinal system. We present a case of an 11-year-old boy who has been followed up with a diagnosis of CVID since he was 6 months old. He presented with abdominal pain and diarrhoea. Colonoscopic biopsy showed crypt destructive colitis, severe decrease and focal absence of plasma cells. Three months later he suffered from abdominal pain, vomiting and bloody diarrhoea. Macroscopic examination of small intestinal resection material revealed multiple perforation areas, ulcers. Histopathology showed mild–moderate active enteritis with aphthous ulcers, purulent peritonitis, decrease in plasma cells and loss of primary follicles in lymph nodes. Histopathological findings were consistent with inflammatory bowel disease (IBD)-like CVID. Although in 6–10% of patients with CVID an IBD-like presentation is observed, this highly aggressive form is rarely seen. We present this case because of its extraordinary presentation displaying perforating active enteropathy.
Background
Common variable immunodeficiency (CVID) is the most common primary immune deficiency syndrome following IgA deficiency. CVID may present in some non-infectious patients or may develop secondary to autoimmune, granulomatous gastrointestinal diseases, or gastrointestinal neoplasms. Mild watery diarrhoea is seen in 20% and severe enteropathy is seen in 10% of patients with CVID. Villous architecture anomalies, sprue-like atrophy, chronic giardia, colitis and inflammatory bowel diseases are reported in CVID. Our patient has been followed up with the diagnosis of CVID since he was 6 months old. After a long asymptomatic period, at the age of 11 he presented with abdominal pain and diarrhoea. We present this case because of its extraordinary clinical presentation and histopathology.
Case presentation
An 11-year-old boy who had been followed up since he was 6 months old with a diagnosis of CVID presented with abdominal pain, diarrhoea and fever. Colonoscopy revealed ulcers with yellow-white membranous exudates at proximal mucosa. Pathological examination showed extensive epithelial damage, crypt distortion with active colitis, cryptitis, apoptotic bodies in the crypt lumina, severe decrease of plasma cells in the lamina propria and focal absence of plasma cells (figure 1). In addition, on small intestinal mucosa, villous atrophy and mild enteritis were observed (figure 2). Granulomas were not seen. Findings were consistent with crypt destructive inflammatory bowel disease-like colitis. Mild immunopositivity for IgG was observed. The patient's blood analysis revealed white cell count: 10.200 mm3, haemoglobin : 8.8 mm3, haematocrit: 29.0 mm3, platelet: 965 000 mm3, C reactive protein: 2.89 mg/dL (fourfold higher), erythrocyte sedimentation rate: 15 mm higher, IgA: 6.83 IU, total IgE: 16.4 IU, IgG: 292 IU and IgM: 16.9 IU. Laboratory analysis showed CD45: 97%, CD3: 72%, CD4: 62%, CD8: 30%, CD19: 24%, CD20: 23%, HLA-DR: 11%, CD20: 23%. Adenovirus and rotavirus antigens were negative in stool. On microbiological analysis, rotavirus, salmonella, parvovirus, anticytomegalovirus (anti-CMV) IgG 2ISR, anti-CMV IgM, antihepatitis A virus IgG, IgM, anti-Hepatitis B Surface (HBS) 380 and HBS antigens were negative. Three months later the patient re-presented with abdominal pain, vomiting and bloody diarrhoea. Direct upright abdominal X-ray showed free air. Segmental small intestinal resection was performed. Macroscopic evaluation showed superficial and deep ulcers and multiple perforations (figure 3). Microscopic examination revealed mild–moderate active enteritis with extensive aphthous ulcers, purulent peritonitis, decrease in plasma cells and loss of primary follicles in lymph nodes (figure 4).
Figure 1.
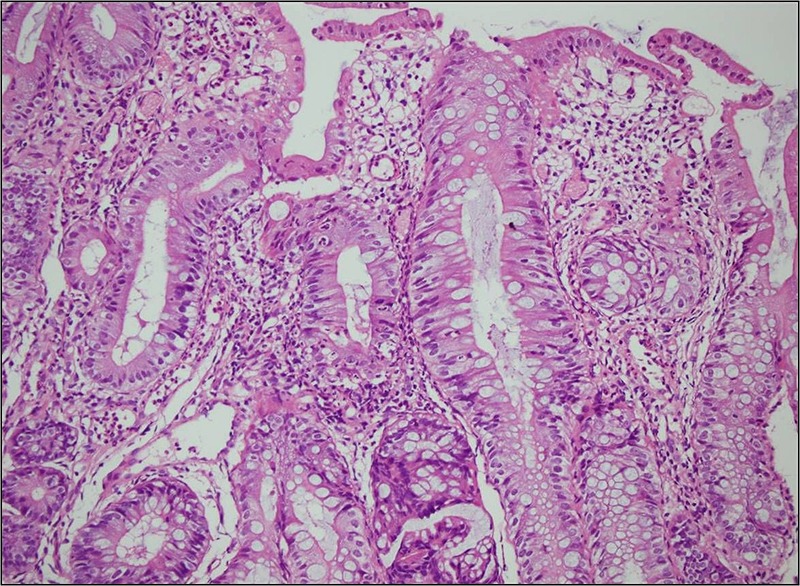
Colonic mucosa biopsies showing extensive epithelial damage, apoptotic bodies in the crypt lumens, crypt distortion, cyriptitis and severe decrease of plasma cells in the lamina propria (H&E, ×200).
Figure 2.
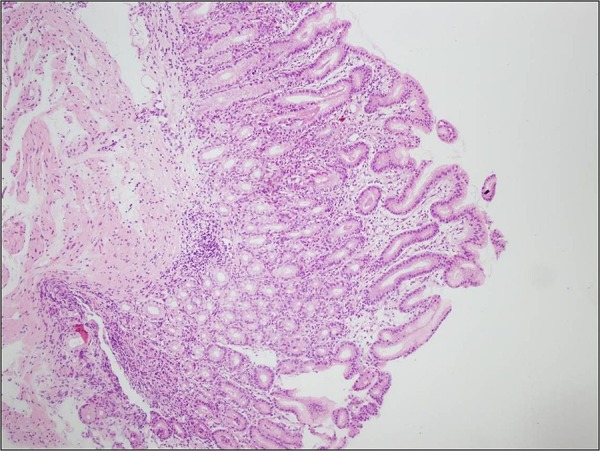
Villous atrophy on small intestine mucosa and mild enteritis (H&E, ×40).
Figure 3.
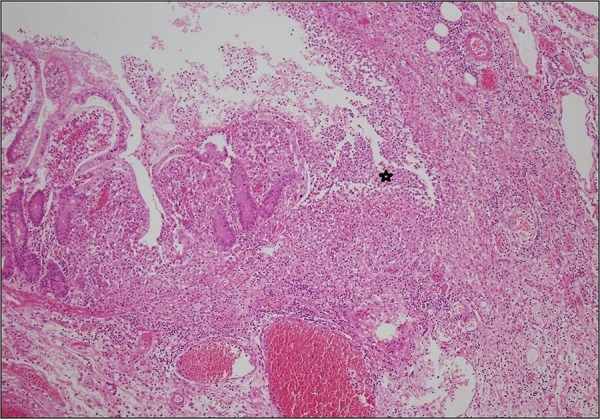
Aphthous ulcers (asterisk) in small intestinal mucosa (H&E, ×40).
Figure 4.
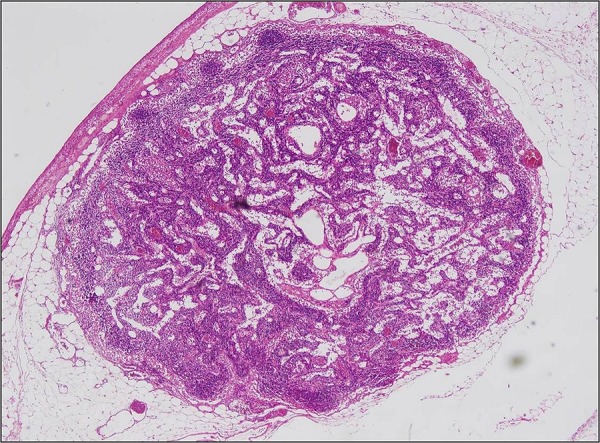
Loss of primary follicles in lymph nodes (H&E, ×40).
Discussion
CVID is the insufficiency of secretion in B cells due to dysfunction of B cell differentiation. Normal population range differs between 1/50 000 and 1/200 000. Most of the cases are sporadic. Nineteen to 22% of cases may be familial.1 2 In our patient, whose parents were blood-relatives, family history was suspicious. Our patient had two siblings who died in the third and seventh months of their lives, respectively. These early deaths may be associated with a familial disease, however, no special examinations were performed. In patients with CVID, gastrointestinal complications are the highest cause of morbidity following respiratory complications. These may be infectious or non-infectious. Gastrointestinal system findings in patients with CVID are chronic watery diarrhoea, oesophagitis, chronic active gastritis, duodenitis, villous architecture anomalies, sprue-like atrophy, chronic giardiasis, colitis, lymphoid hyperplasia, Crohn's disease, ulcerative colitis, inflammatory bowel disease, autoimmune gastritis and collagenous enterocolitis. In the report by Khodadad et al, 19 of 39 cases had gastrointestinal problems. The most common symptom of these patients was diarrhoea. Oesophagitis, gastritis and other gastrointestinal system problems were also reported.3 In a review of 248 CVID cases by Lai Ping So et al, 51 cases were reported as having gastrointestinal problems.4 CVID-related colitis is subdivided into crypt destructive colitis (ulcerative colitis, Crohn's colitis) and non-destructive colitis (lymphocytic colitis, collagenous colitis, graft-versus-host disease (GVHD)-like pattern). An increased range of inflammatory bowel disease is reported in patients with CVID. However, it is hard to differentiate destructive colitis from inflammatory bowel disease.5 Non-destructive colitis was seen in three cases and crypt-destructive colitis in one case of the Khodadad et al3 series. Crypt destructive colitis (Crohn's colitis, ulcerative colitis, inflammatory bowel disease) was reported in seven cases and non-destructive colitis was seen in one case of the Daniels et al6 series. In this series, chronic secretory diarrhoea in a 19-year-old patient who suffered from this problem since birth is the most severe gastrointestinal symptom presentation.6 This patient had partial colectomy and the histopathological evaluation showed severe colitis without crypt distortion. In addition, goblet cell loss, increased apoptosis and decrease in villous configuration were reported.6 In our case, at the age of 11, the patient had an endoscopic biopsy following abdominal pain and bloody diarrhoea, and the biopsy showed crypt distortion with active colitis. In addition, cryptitis, goblet cell loss, increased apoptosis, lymphoid follicle presence and severe loss or absence of plasma cells in lamina propria were observed. These findings were consistent with crypt destructive colitis.
Intravenous immunoglobulin treatment does not provide total healing in crypt destructive colitis cases, as Khodadad et al3 have pointed out. It was shown that T-cell defect and autoimmune phenomena play a big role in the mechanism of gastrointestinal pathology of patients with CVID, in addition to hypogammaglobulinaemia.3 7 8 These findings support the facts that infections trigger the immune deficiency in inflammatory bowel disease aetiology and plasma cell loss or absence is not seen in patients with CVID.
Granulomas are reported in CVID cases. Three cases with granulomas were seen in the Daniels et al6 series. Differential diagnosis may be necessary for Crohn's disease.6 Origin of granulomas in patients with CVID may be related to infection or sarcoidosis.9 We did not observe granulomas in our patient.
Several different findings in intestinal biopsies of patients with CVID were seen, such as: duodenitis, villous atrophy and nodular lymphoid hyperplasia (NLH). In the literature, 24–50% increase in intraepithelial lymphoid cells and villous atrophy has been reported in duodenal biopsies of patients with CVID.7 8 Eight cases with villous atrophy were reported in the series by Khodadad et al.3 Three cases with coeliac disease were reported in the series from Daniels et al.6 The percentage of CVID with coeliac disease histology was 31%, with 2% in the work of Luzi and colleagues.10 The percentage of coeliac disease in the normal population is reported as 0.5–1%.9 A relation to anaemia, malnutrition and decreased CD4 lymphocytes in blood is seen in patients with CVID with villous atrophy,8 however, Khodadad et al3 did not observe this relationship. In our patient's biopsies, villous atrophy was seen but intraepithelial lymphocytes were not increased.
CVID may cause increase in bacteria and tropical sprue-like histology, or there may be a relation between CVID and coeliac disease.3 9 Decrease and even absence of plasma cells in some areas were seen in our case. Decreased plasma cells were observed in 68% of the Daniels et al6 series. Increased opportunistic pathogens (CMV, cryptosporidium, giardia lamblia) were reported.9 These infections were not seen in our patient.
Fifty per cent of patients with CVID who have this enteropathy were reported to have responded well to a gluten-free diet. Villous atrophy may be related to an autoimmune process, giardia lamblia infection or bacterial overgrowth.7 8 These findings may require differential diagnosis for distinction from coeliac sprue.
Lymphoid follicular hyperplasia was reported in colitis cases that were related to CVID. Some researchers reported the relationship between hypogammaglobulinemia and NLH.8 10 In the Khodadad et al3 series, three cases were reported to have NLH. NLH is not specific for CVID. It can be found in the normal population. NLH is usually seen as a generalised form of CVID, differing from the normal population.4 7 Lymphoma is reported in a small number of NLH cases. However, the relationship between NLH and intestinal lymphoma is not clear.11 12 In the resection material of our patient, decreased plasmacytes and primary follicle loss in lymph nodes were seen. Lymphoid follicles are most commonly related with infections and giardia.3 Giardia infection was not observed in our case.
Coeliac sprue is related to microscopic colitis (lymphocytic and collagenous). Collagenous colitis results in chronic watery diarrhoea and does not appear pathological on endoscopy. Collagenous colitis is characterised by increased intraepithelial lymphocytes and increased subepithelial collagen. Its aetiology is not clear. The reason for this is thought to be the agents triggering the immune system. Byrne et al13 first showed CVID-related atypical collagenous colitis pattern. In a case from the Daniels et al series, thickening of subepithelial collagen in the small intestine and colon is reported. This finding is described as being similar to collagenous colitis.6 Some other researchers observed collagenous colitis in cases with IgG and IgA deficiency.14 Collagenous colitis was not observed in our case.
Histologically, increased acute GVHD-like intraepithelial apoptosis was reported in CVID. Increased apoptosis in the oesophagus, stomach, and small and large intestine was reported in most cases in the Daniels et al series.6 Increased apoptosis was observed in our patient's small and large intestine. The patient's transplantation history must warn the pathologist about a GVHD-like pattern of CVID.3
There is a strong relationship between CVID and IgA deficiency. The relationship between CVID and IgA deficiency was described to be in the gene locus on major histocompatibility complex, especially on the sixth chromosome, which carries the locus for D821/D823 and HLA-B8 markers. TACI mutations, which are expressed on B cells, coded by TNFRSF13B on 17th chromosome and belonging to the TNF-like receptor family, were observed in some patients with CVID. Plasma cell maturation and immunoglobulin production cannot take place with a deficiency of BAFF and APRIL, which are known as TACI ligands. Five and 10% of patients with CVID show at least one of the defined TNFRSF13B mutations. B cells that lack TACI are resistant to IgG production.15 16 IgA levels were below normal in our case. In addition, maternal antibodies, which are produced against IgA, are effective on IgA deficiency in children and it leads us to think that this mechanism is valid on patients with CVID who have genetic susceptibility.17
In summary, CVID is a heterogeneous disease that shows TACI, BAFF and APRIL deficiencies. Often, in these patients, appearance of Crohn's-like inflammatory bowel disease findings in the intestine supports immunodeficiency findings that are triggered by infections of inflammatory bowel disease aetiology. CVID may show different histological patterns in the gastrointestinal system. These are lymphocytic colitis, collagenous colitis, coeliac disease, lymphocytic gastritis, granulomatous bowel disease, acute GVHD and inflammatory bowel disease. Our patient is an individual with CVID who presented with inflammatory bowel disease-like findings. He had no symptoms after partial intestinal resection. A high frequency of inflammatory bowel disease-like features in patients with CVID shows us that immunological mechanisms play a role in the aetiopathogenesis of inflammatory bowel diseases. New research on inflammatory bowel disease should be planned for this group of patients.
Learning points.
In this report we presented a male patient with CVID having gastrointestinal symptoms 11 years after his diagnosis, remaining asymptomatic during this period.
In 6–10% of patients with CVID, an inflammatory bowel disease (IBD)-like presentation is observed.
Highly aggressive form, displaying deep aphthous ulcers and perforation in the small intestine, is rarely seen.
Clinicians should be aware that an IBD-like presentation might express itself, after a long asymptomatic period, in a very aggressive form.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sneller MC. Common variable immunodeficiency. Am J Med Sci 2001;321:42–8. 10.1097/00000441-200101000-00007 [DOI] [PubMed] [Google Scholar]
- 2.Vorechovský I, Zetterquist H, Paganelli R et al. Family and linkage study of selective IgA deficiency and common variable immunodeficiency. Clin Immunol Immunopathol 1995;77:185–92. 10.1006/clin.1995.1142 [DOI] [PubMed] [Google Scholar]
- 3.Khodadad A, Aghamohammadi A, Parvaneh N et al. Gastrointestinal manifestations in patients with common variable immunodeficiency. Dig Dis Sci 2007;52:2977–83. 10.1007/s10620-006-9736-6 [DOI] [PubMed] [Google Scholar]
- 4.Lai Ping So A, Mayer L. Gastrointestinal manifestations of primary immunodeficiency disorders. Semin Gastrointest Dis 1997;8:22–32. [PubMed] [Google Scholar]
- 5.Carpenter HA, Talley NJ. The importance of clinicopathological correlation in the diagnosis of inflammatory conditions of the colon: histological patterns with clinical implications. Am J Gastroenterol 2000;95:878–96. 10.1111/j.1572-0241.2000.01924.x [DOI] [PubMed] [Google Scholar]
- 6.Daniels JA, Lederman HM, Maitra A et al. Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID): a clinicopathologic study and review. Am J Surg Pathol 2007;31:1800–12. 10.1097/PAS.0b013e3180cab60c [DOI] [PubMed] [Google Scholar]
- 7.Washington K, Stenzel TT, Buckley RH et al. Gastrointestinal pathology in patients with common variable immunodeficiency and X-linked agammaglobulinemia. Am J Surg Pathol 1996;20:1240–52. 10.1097/00000478-199610000-00010 [DOI] [PubMed] [Google Scholar]
- 8.Luzi G, Zullo A, Iebba F et al. Duodenal pathology and clinical-immunological implications in common variable immunodeficiency patients. Am J Gastroenterol 2003;98:118–21. 10.1111/j.1572-0241.2003.07159.x [DOI] [PubMed] [Google Scholar]
- 9.Morimoto Y, Routes JM. Granulomatous disease in common variable immunodeficiency. Curr Allergy Asthma Rep 2005;5:370–5. 10.1007/s11882-005-0008-x [DOI] [PubMed] [Google Scholar]
- 10.Bästlein C, Burlefinger R, Holzberg E et al. Common variable immunodeficiency syndrome and nodular lymphoid hyperplasia in the small intestine. Endoscopy 1988;20:272–5. 10.1055/s-2007-1018192 [DOI] [PubMed] [Google Scholar]
- 11.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol 1999;92:34–48. 10.1006/clim.1999.4725 [DOI] [PubMed] [Google Scholar]
- 12.Castellano G, Moreno D, Galvao O et al. Malignant lymphoma of jejunum with common variable hypogammaglobulinemia and diffuse nodular hyperplasia of the small intestine. A case study and literature review. J Clin Gastroenterol 1992;15:128–35. 10.1097/00004836-199209000-00010 [DOI] [PubMed] [Google Scholar]
- 13.Byrne MF, Royston D, Patchett SE. Association of common variable immunodeficiency with atypical collagenous colitis. Eur J Gastroenterol Hepatol 2003;15:1051–3. 10.1097/00042737-200309000-00019 [DOI] [PubMed] [Google Scholar]
- 14.Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med 2002;346:180–8. 10.1056/NEJMra010852 [DOI] [PubMed] [Google Scholar]
- 15.Salzer U, Chapel HM, Webster AD et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet 2005;37:820–8. 10.1038/ng1600 [DOI] [PubMed] [Google Scholar]
- 16.Salzer U, Grimbacher B. Common variable immunodeficiency: the power of co-stimulation. Semin Immunol 2006;18:337–46. 10.1016/j.smim.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 17.Schäffer AA, Pfannstiel J, Webster AD et al. Analysis of families with common variable immunodeficiency (CVID) and IgA deficiency suggests linkage of CVID to chromosome 16q. Hum Genet 2006;118:725–9. 10.1007/s00439-005-0101-1 [DOI] [PMC free article] [PubMed] [Google Scholar]


