Abstract
Ageing is a natural phenomenon and decline of physiological and structural changes are incurable in advancing years of human life. When such degenerative changes occur in the brain they may lead to dementia and other memory related conditions. The Ayurvedic classics identified the importance of higher faculties dealing with memory and introduced a separate group of drugs namely Medhya Rasayanas. Regular intake of such drugs will help to prevent the onset of degenerative changes in the brain prematurely. Ayurveda can play a useful role in the management of such geriatric conditions. The current review has been done with a view to update documented Ayurvedic therapeutic modalities for certain geriatric conditions suggested by Ayurvedic classics in the management of diseases called Vātavyādhi (nervous system disorders), which also include conditions related to memory functions. Recent studies have started validating the claims recorded in Ayurvedic texts. The pathogenesis and remedies for Vātavyādhi documented in Ayurvedic classics have been reviewed with special emphasis on disorders related to dementia. A review of recent researches on the herbs mentioned in management of vāta disorders including dementia have been done to understand their role in management of Alzheimer's disease (AD). There are many herbs of ethno-medicinal source studied experimentally for their potential in treatment of AD. A judicious combination of modern research methodology and Ayurvedic principles could go a long way in the management and care of AD which is going to be a heavy burden on the society in the future.
KEY WORDS: Alzheimer's disease, cognitive disorders, memory enhancing drugs
INTRODUCTION
Ageing is multi-factorial which reflects at cellular, organ and organism levels. Through advancements in modern medicine and technology more humans are surviving into later life with the potential to reach their technical life span of approximately 120 years of age.[1] However, part of the disability currently seen[2] in the elderly is due to lifestyle-induced diseases (for example, smoking related to heart disease and stroke). The elderly have a distinct spectrum of diseases which includes stroke, hypertension, coronary heart diseases, parkinsonism, osteoporosis, osteoarthritis, constipation, urinary incontinence, insomnia, vertigo, deafness and dementia including Alzheimer's disease (AD).
Until recently, cognitive decline was considered characteristic of normal ageing. In the past decade, the surveillance studies have noted that 15% of those over the age of 65 have moderate to severe dementia. AD once considered to be rare, is now recognized as the most common disorder of dementia.[3,4]
Dementia is a chronic disorder in which there is progressive decline in intellect, behavior and personality.[5] Certain conditions such as AD, multi infarct dementia, uremia, chronic hepatic encephalopathy, thyroid/adrenal dysfunction, pernicious anemia, pellagra, brain tumors, subdural hematoma and head injury may cause dementia. Among these conditions, AD is being projected as one of the important memory disorder which calls for proper management.
The classic triad of senile plaques, neurofibrillary tangles and granulovascular degeneration are suggested to be pathogenic of AD. Clinical features include memory loss, difficulty in learning and retaining new information, depressive symptoms, aggression, agitation and disorders of perception.
With Parkinson's disease serving as the model for neurotransmitter replacement therapy, great expectations arose for analogous treatment success for victims of AD. Unfortunately, the results of replacement therapy have been notably disappointing.[6]
Keeping the above in view, a careful review has been done about the herbal recipes suggested by Ayurvedic classics in the management of disease called Vātavyādhi (nervous system disorders).
In addition to the above diseased condition an analysis has also been carried out about the memory enhancing drugs categorized under Medhya Rasāyana (memory rejuvenators) group of drugs.
AD-AN AYURVEDIC VIEW
There is no condition as described in Ayurveda which can be clinically equated to AD. But in Ayurvedic classics Smṛtināsha (loss of memory) is mentioned among the prodromal symptom of jarā (ageing).[7] In Jarāvasthā (old age), which starts from 60 years of age as per Caraka, Smṛti and other mental faculties gradually deteriorate naturally. As per Vāgbhaṭa (As.Sam.Sha 8/25) and Sharṅgadhara (Prathama Khaṇḍa 6/20), the functions of mind and Buddhi decline start declining from the 9th decade of life. Mental function declines at 11th decade of life as per Sharṅgadhara. Further, Smṛtibhramsha (disturbed memory) is described as a symptom where Smṛti (memory) is vitiated by rajas (passion) and tamas (obscurity).[8] Thus, senile dementia can be interpreted as Jarājanya Smṛtibhramsha according to Ayurvedic principles.[9] Ayurveda suggests Nasya karma (nasal administration) for all the Jatrugataroga (diseases of head and neck) which may have beneficial therapeutic effect in the patients of AD. Some of the important memory enhancing herbs mentioned in the ancient Ayurvedic literature is enumerated in Table 1.[10]
Table 1.
Medhya Rasāyana (memory enhancers)
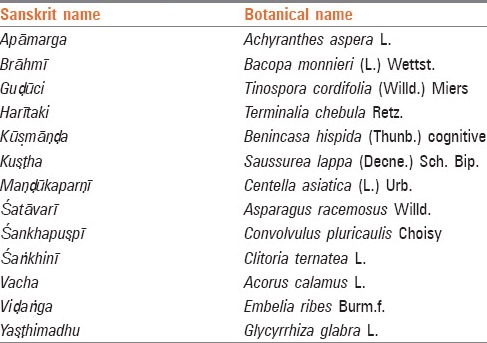
Herbs indicated in the treatment of Vātavyādhi (nervous system disorders)[11] which may help in restoration of declining memory are listed in Table 2.
Table 2.
Memory enhancer herbs

MODERN SCIENTIFIC VALIDATIONS OF HERBAL MEMORY ENHANCERS
Certain medicinal plants and their combinations mentioned in Ayurvedic material medica have been evaluated in various experimental studies for their memory enhancing property are enumerated in Table 3.
Table 3.
Herbs found useful for Alzheimer's disease in experimental studies

From the above list, the herbs which were subjected to extensive evaluation for their activity in the management of dementia are summarized below.
Brāhmī (Bacopa monieri)
Considerable number of studies (animal experiments and clinical trials)[37,38] have been done to evaluate its efficacy in learning and memory. It was concluded that Brāhmī has a positive effect on learning and both short-term and long-term memory. In a recent double-blind, placebo-controlled clinical trial,[39] the above findings were further proved and it was found that maximal effect was evident after 12 weeks of treatment. In another double blind randomized placebo control clinical study covering 76 adults aged 40-60 years and carried out over a 3 month period, it was concluded that the drug has significant beneficial action on retention of new information.[40] Saponins are considered as the active compounds.[41] Ethanolic extract of aerial parts and rhizome from the plant possessed nootropic activity.[42] It has been suggested that bacosides are able to induce membrane dephosphorylation with concomitant increase in protein and ribonucleic acid turnover in specific brain areas.[43] Other probable actions include: (1) Cognitive enhancement through modulatory effect on the cholinergic system,[44] (2) enhancement of protein kinase activity in hippocampus.[45]
Maṇḍūkaparṇī (Centella asiatica)
Maṇḍūkaparṇī is highly prized in Ayurvedic treatises as a Medhya (Memory and intellect promoting) and vayassthāpana (anti-ageing) drug. In Bhāvaprakāsha it has been stated that Maṇḍūkaparṇī and Brāhmī have very similar pharmacological activities.[46] A double blind clinical trial involving 30 mentally retarded children (age, 7-8 years) who received the drug (whole plant, dried in shade) for 3 months, showed improved cognitive function.[47] In recent investigations,[42] it was demonstrated that the aqueous extract of the plant (200 mg/kg, 14 days) improved learning and memory, in both the shuttle box and step through paradigms in male Wister rats. The extract was also found to be effective in preventing cognitive deficits in an intracerebroventricular Streptozotocin model of AD in rats. It is believed that an antioxidant mechanism is involved. Asiatic acid, the principal triterpinoid constituent of C. asiatica has been demonstrated to reduce hydrogen peroxide induced cell death and lower intracellular free radical concentration.[48] Asiatic acid has been patented (Hoechst Aktiengesellschaft) as a therapeutic agent for the treatment of dementia and this compound as well as some related synthetic analogues have been shown to protect cultured cortical neurons from glutamate-induced excite-toxicity.[49] Aqueous extract of the plant at a dose level of 300 mg/kg decreased the pentylenetetazole-kindled seizures in rats thus, indicating antiepileptic action of the herb.[50] The memory enhancing activity of C. asiatica may be due to Acetylcholinesterase inhibiting activity of asiatic acid and betulinic acid that can be potential lead for the symptomatic treatment of AD.[51]
Haridrā (Curcuma longa)
In Caraka Samhitā, Haridrā has been classified as curative of skin diseases (Kuṣṭhaghna), anti-obesity and scarifying (Lekhanīya), antidote to poisoning (Viśaghna) and has been recommended for the treatment of jaundice, cough, coryza, senility and impaired vision.[52] β-Amyloid induced oxidative stress appears to be an important pathway of neuronal cell death in AD. A bioassay guided fractionation of methanol (90%) extract of turmeric led to the isolation of calebin-A and the curcumins which effectively protected (in vitro) neuronal cells against β-Amyloid deposition.[53] In another study curcumin, on oral administration to alcohol-fed rats, caused a significant reversal of brain lipid peroxidation, thus indicating a neuro-protective role.[54]
DISCUSSION
Ācaryas of Ayurveda opine that Jarā (senility) is a natural disease (Svābhāvika vyādhi). Premature senility can be well-prevented by regular intake of Rasāyana or Vayassthāpana (longevity promoter) drugs. It is suggested to introduce Rasāyana drugs during young and middle phases of life. Ayurvedic classics document the decade wise decline in individuals and emphasized further that the decline of intelligence and memory starts at the end of 40th year of the life.
In different phases of life, different dośas predominate: In the early part of life, kaphaja dośa (those arising out of biological humor which maintains structural integrity) predominates in the body; in the middle age, pitta (biological humor which resembles enzymatic and endocrinal functions) related diseases predominate and vāta (biological humor representing nervous system functions) dominance leads to degenerative changes during old age. Rasāyana drugs by their specific activity subdue the vitiated vāta and help in preserving the bodily physiological functions by restoring the feeling of well-being. Everybody is gifted with the capacity to regenerate destroyed cells. At the end of last century the scientists proved that damaged nerve cells can be regenerated contrary to the findings of earlier research studies. Caraka has identified Rāsnā (Pluchea lanceolata) as the prime drug for subduing vāta vitiation. Vāgbhaṭa has identified Lasuna (Allium sativum) as one of the prime drugs to curtail the pathological changes due to vāta dośa. It is also suggested that intake of Harītakī (Terminalia chebula) with different anupānas (vehicles) in different seasons not only helps to prevent seasonal diseases but also helps in regeneration of diseased cells. The combination of three fruits, i.e. Triphala (Three myrobalans) is suggested as one of the important Rasāyana group. Among Triphalā, Caraka identifies Āmalaki (Emblica officinalis) to be the best fruit bestowed with Vayassthāpana activity (youth retaining activity). Both harītakī and āmalakī are attributed with Medhya property. Brāhmī and Maṇḍūkaparṇī share similar pharmacological properties. Caraka enumerates Maṇḍūkaparṇī among Medhya Rasāyanas while Vāgbhaṭa considers Brāhmī as a prime drug from apasmāra which is characterized by loss of Smṛti (memory) and saṃjñā (consciousness). Vāgbhaṭa also considers lasuna as the prime drug to treat vātavyādhi. During old age, body enters the phase of vāta dominance and vata decreases the asthi dhātu and the related majjā dhātu too. Brain tissue is referred as mastuluṅga majjā which goes into the degenerative phase due to interplay of excessive vata. In such conditions Lasūna in kalka dosage form as suggested by Sage Kaśyapa and Śodhala help to prevent degenerative changes in the brain. In a controlled clinical trial with Vayassthāpana group (8 out of 10 herbs suggested by Caraka) it was observed the volunteers who received vayassthāpana compound have shown improvement in the symptoms like anxiety and loss of concentration.[55]
CONCLUSION
Dementia pathologies such as AD are reaching epidemic proportions. They are not being successfully managed effectively by symptomatic treatments.[56] Currently, two centrally acting, reversible anti-cholinesterase drugs, tacrine and donepezil, are used to increase acetylcholine levels in the brain. Beneficial effects of the drugs are most notable in the early stages of the disease and lessen as the disease progresses.[57] There are many herbs from ethno-medicinal source which have been studied for their experimental potential in treating AD. A judicious combination of Ayurvedic methodology along with proved herbs from varied origin can be used in a in the management of AD.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Schernitski P, Bootman JL, Byers J, Likes K, Hughes JH. Demographic characteristics of elderly drug overdose patients admitted to a hospital emergency department. J Am Geriatr Soc. 1980;28:544–6. doi: 10.1111/j.1532-5415.1980.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 2.Nelson RC, Franzi LR. The Medical Clinics of North America. Vol. 73. Philadelphia: W.B. Saunders company; 1989. Nutrition and ageing, Geriatric medicine; p. 1531. [DOI] [PubMed] [Google Scholar]
- 3.Katzman R. Editorial: The prevalence and malignancy of Alzheimer disease. A major killer. Arch Neurol. 1976;33:217–8. doi: 10.1001/archneur.1976.00500040001001. [DOI] [PubMed] [Google Scholar]
- 4.Terry RD, Davies P. Dementia of the Alzheimer type. Annu Rev Neurosci. 1980;3:77–95. doi: 10.1146/annurev.ne.03.030180.000453. [DOI] [PubMed] [Google Scholar]
- 5.Golwalla AF. Mumbai: The National Book Depot; 2003. Medicine for Students. [Google Scholar]
- 6.The Medical Clinics of North America. Vol. 73. W. B. Saunders company; 1989. Germaine Louise Odenheimer Acquired cognitivedisorders of the elderly, Geriatricmedicine; p. 1405. [Google Scholar]
- 7.Upadhyaya Y. In: Jararoga nidan 37/116. Nidan M, editor. Varanasi: Chaukhambha Sanskrit Sansthan; 1993. pp. 500–1. [Google Scholar]
- 8.Agnivesa . ‘Charak Samhita’, revised by Charak and Dradhabala with ‘Ayurved Dipika’ commented by Chakrapanidatta. Cha.Sha. 1/101. In: Acharya VJ, editor. Goapal Mandir Lane. Varanasi, India: Chaukhambha Surbharti Prakashan; 2005. p. 297. [Google Scholar]
- 9.Chaudhuri K, Samarakoon SM, Chandola HM, Kumar R, Ravishankar B. Evaluation of diet and life style in etiopathogenesis of senile dementia: A survey study. Ayu. 2011;32:171–6. doi: 10.4103/0974-8520.92554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varanasi: Chaukhambha Sanskrit Bhawan; 2010. Bhavmishra. Bhavprakasha Samhita edited with ‘Vidyotini’ Hindi comm. by Brahmasankara Mishra and Rupalalji Vaisya, Part II, Rasayana; pp. 822–23. [Google Scholar]
- 11.Bhavmishra . Varanasi: Chaukhambha Sanskrit Bhawan; 2010. Bhavprakasha Samhita edited with ‘Vidyotini’ Hindi comm. by Brahmasankara Mishra and Rupalalji Vaisya, Part II, Vatavyadhi; pp. 227–72. [Google Scholar]
- 12.Datta C. Varanasi: Chaukhambha Surbharti Prakashan; 2006. Chakradatta of Chakrapani Datta, with ‘Padarhtabodhini’ Hindi Comm. By Vd. Ravidatta Sastri, Apasmaradhikar 21/11. Reprint; p. 90. [Google Scholar]
- 13.Vasudevan M, Parle M. Memory enhancing activity of Anwala churna (Emblica officinalis Gaertn.): An Ayurvedic preparation. Physiol Behav. 2007;91:46–54. doi: 10.1016/j.physbeh.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 14.RaiKiranmai S. Neurogenic potential of Clitoriaternatea aqueous root extract - A basis for enhancing learning and memory. World academy of science, Engineering and technology. 2010;4:10–22. [Google Scholar]
- 15.Dhuley JN. Nootropic-like effect of ashwagandha (Withania somnifera L.) in mice. Phytother Res. 2001;15:524–8. doi: 10.1002/ptr.874. [DOI] [PubMed] [Google Scholar]
- 16.Schliebs R, Liebmann A, Bhattacharya SK, Kumar A, Ghosal S, Bigl V. Systemic administration of defined extracts from Withania somnifera (Indian Ginseng) and Shilajit differentially affects cholinergic but not glutamatergic and GABAergic markers in rat brain. Neurochem Int. 1997;30:181–90. doi: 10.1016/s0197-0186(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 17.Sehgal N, Gupta A, Valli RK, Joshi SD, Mills JT, Hamel E, et al. Withania somnifera reverses Alzheimer's disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc Natl Acad Sci U S A. 2012;109:3510–5. doi: 10.1073/pnas.1112209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dev S. New Delhi: Anamaya Publishers; 2006. A Selection of Prime Ayurvedic Plant Drugs-Ancient-Modern Concordance; p. 137. [Google Scholar]
- 19.Drever BD, Anderson WG, Riedel G, Kim DH, Ryu JH, Choi DY, et al. The seed extract of Cassia obtusifolia offers neuroprotection to mouse hippocampal cultures. J Pharmacol Sci. 2008;107:380–92. doi: 10.1254/jphs.08034fp. [DOI] [PubMed] [Google Scholar]
- 20.Kim DH, Yoon BH, Kim YW, Lee S, Shin BY, Jung JW, et al. The seed extract of Cassia obtusifolia ameliorates learning and memory impairments induced by scopolamine or transient cerebral hypoperfusion in mice. J Pharmacol Sci. 2007;105:82–93. doi: 10.1254/jphs.fp0061565. [DOI] [PubMed] [Google Scholar]
- 21.Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr Drug Targets. 2011;12:332–47. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin MS, Hung KS, Chiu WT, Sun YY, Tsai SH, Lin JW, et al. Curcumin enhances neuronal survival in N-methyl-d-aspartic acid toxicity by inducing RANTES expression in astrocytes via PI-3K and MAPK signaling pathways. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:931–8. doi: 10.1016/j.pnpbp.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 23.von Bohlen und Halbach O, Dermietzel R. Weinheim, Germany: Wiley-VCH; 2002. Neurotransmitters and Neuromodulators; p. 41. [Google Scholar]
- 24.Zhang Y, Shoyama Y, Sugiura M, Saito H. Effects of Crocus sativus L. on the ethanol-induced impairment of passive avoidance performances in mice. Biol Pharm Bull. 1994;17:217–21. doi: 10.1248/bpb.17.217. [DOI] [PubMed] [Google Scholar]
- 25.Dev S. Anamaya Publication; 2006. A Selective of Prime Ayurvedic Plant Drugs (Ancient- Modern Concordance) pp. 100–102. [Google Scholar]
- 26.Dhanasekaran M, Holcomb LA, Hitt AR, Tharakan B, Porter JW, Young KA, et al. Centella asiatica extract selectively decreases amyloid beta levels in hippocampus of Alzheimer's disease animal model. Phytother Res. 2009;23:14–9. doi: 10.1002/ptr.2405. [DOI] [PubMed] [Google Scholar]
- 27.Kim SR, Hwang SY, Jang YP, Park MJ, Markelonis GJ, Oh TH, et al. Protopine from Corydalis ternata has anticholinesterase and antiamnesic activities. Planta Med. 1999;65:218–21. doi: 10.1055/s-1999-13983. [DOI] [PubMed] [Google Scholar]
- 28.Preeti K, Rawat MSM. Comparative nootropic effect of Evolvulusalsinoides and Convolvulus pluricaulis. International Journal of Pharma and Bio Sciences. 2011;2:1–16. [Google Scholar]
- 29.Ghosal S, Bhattacharya SK. Desmodium alkaloids. II. Chemical and pharmacological evaluation of D. gangeticum. Planta Med. 1972;22:434–40. doi: 10.1055/s-0028-1099630. [DOI] [PubMed] [Google Scholar]
- 30.Joshi H, Parle M. Antiamnesic effects of Desmodium gangeticum in mice. Yakugaku Zasshi. 2006;126:795–804. doi: 10.1248/yakushi.126.795. [DOI] [PubMed] [Google Scholar]
- 31.Malik J, Karan M, Vasisht K. Nootropic, anxiolytic and CNS-depressant studies on different plant sources of shankhpushpi. Pharm Biol. 2011;49(12):1234–1242. doi: 10.3109/13880209.2011.584539. [DOI] [PubMed] [Google Scholar]
- 32.Ganguly R, Guha D. Alteration of brain monoamines & EEG wave pattern in rat model of Alzheimer's disease & protection by Moringa oleifera. Indian J Med Res. 2008;128:744–51. [PubMed] [Google Scholar]
- 33.Ganguly R, Hazra R, Ray K, Guha D. Effect of Moringa oleiferain experimental model of Alzheimer's disease: Roleofantioxidants. Ann Neurosci. 2005;12:36–9. [Google Scholar]
- 34.Mohan M, Kaul N, Punekar A, Girnar R, Junnare P, Patil L. Nootropicactivity of Moringa oleifera leaves. J Nat Remedies. 2005;5:59–62. [Google Scholar]
- 35.Ghayur MN, Gilani AH, Ahmed T, Khalid A, Nawaz SA, Agbedahunsi JM, et al. Muscarinic, Ca(++) antagonist and specific butyrylcholinesterase inhibitory activity of dried ginger extract might explain its use in dementia. J Pharm Pharmacol. 2008;60:1375–83. doi: 10.1211/jpp/60.10.0014. [DOI] [PubMed] [Google Scholar]
- 36.Obulesu M, Rao DM. Effect of plant extracts on Alzheimer's disease: An insight into therapeutic avenues. J Neurosci Rural Pract. 2011;2:56–61. doi: 10.4103/0976-3147.80102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Handa SS, Kaul MK, editors. Jammu-Tawi, India: Regional Research Laboratory, Council of Scientific & Industrial Research; 1996. Supplementto Cultivation and Utilization of Medicinal Plants; p. 515. [Google Scholar]
- 38.38 Lodha R, Bagga A. Traditional Indian systems of medicine. Ann Acad Med Singapore. 2000;29:37–41. [PubMed] [Google Scholar]
- 39.Stough C, Lloyd J, Clarke J, Downey LA, Hutchison CW, Rodgers T, et al. The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacology (Berl) 2001;156:481–4. doi: 10.1007/s002130100815. [DOI] [PubMed] [Google Scholar]
- 40.Roodenrys S, Booth D, Bulzomi S, Phipps A, Micallef C, Smoker J. Chronic effects of Brahmi (Bacopa monnieri) on human memory. Neuropsychopharmacology. 2002;27:279–81. doi: 10.1016/S0893-133X(01)00419-5. [DOI] [PubMed] [Google Scholar]
- 41.Billore KV, Yelne MB, Dennis TJ, Chaudhari BG. I. New Delhi: Central Council for Research in Ayurveda and Siddha; 2004. Database on Medicinal Plants Used in Ayurveda. [Google Scholar]
- 42.Veerendra Kumar MH, Gupta YK. Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. J Ethnopharmacol. 2002;79:253–60. doi: 10.1016/s0378-8741(01)00394-4. [DOI] [PubMed] [Google Scholar]
- 43.Singh HK, Rastogi RP, Sriman RC, Dhavan BN. Effect of Bacoside A and B on avoidanace response in rats. Phytother Res. 1988;2:70–5. [Google Scholar]
- 44.Stough C, Downey L, Lloyd J, Silber B, Redman S, Hutchison C, et al. Examining the nootropic effects of a special extract of Bacopamonniera on human cognitive functioning: 90 days double blind placebo controlled randomized trial. Phytother Res. 2008:1–6. doi: 10.1002/ptr.2537. [DOI] [PubMed] [Google Scholar]
- 45.Singh HK, Dhawan BN. Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa monniera Linn.(Brahmi) Indian J Pharmacol. 1997;29:359–65. [Google Scholar]
- 46.Bhava Mishra, Chunekar KC. Varanasi: Chaukhambha Bharati Academy; 2010. Commentator, Bhava Prakasha Nighantu, Guduchyadivarga 237, Revised and Enlarged edition. [Google Scholar]
- 47.Appa Rao MV, Srinivasan K, Rao TK. The effect of Mandookaparni (Centellaasiatica) on the general mental ability (Medhya) of mentally retarded children. J Res Indian Med. 1973;8:9–16. [Google Scholar]
- 48.Mook-Jung I, Shin JE, Yun SH, Huh K, Koh JY, Park HK, et al. Protective effects of asiaticoside derivatives against beta-amyloid neurotoxicity. J Neurosci Res. 1999;58:417–25. [PubMed] [Google Scholar]
- 49.Lee MK, Kim SR, Sung SH, Lim D, Kim H, Choi H, et al. Asiatic acid derivatives protect cultured cortical neurons from glutamate-induced excitotoxicity. Res Commun Mol Pathol Pharmacol. 2000;108:75–86. [PubMed] [Google Scholar]
- 50.Gupta YK, Veerendra Kumar MH, Srivastava AK. Effect of Centella asiatica on pentylenetetrazole-induced kindling, cognition and oxidative stress in rats. Pharmacol Biochem Behav. 2003;74:579–85. doi: 10.1016/s0091-3057(02)01044-4. [DOI] [PubMed] [Google Scholar]
- 51.Orhan IE. Centellaasiatica (L) Urban, From traditional medicine to modern medicine with neuroprotective potential. Evid Based Complement Alternat Med. 2012;2012:1–8. doi: 10.1155/2012/946259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garg DS. Vol. 6. Vijaygard, Aligarh, India: Dhanvantari Karyalaya; 1971. Dhanwantari–Vanaushadhivishesh Anka; p. 452. [Google Scholar]
- 53.Park SY, Kim DS. Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: A drug discovery effort against Alzheimer's disease. J Nat Prod. 2002;65:1227–31. doi: 10.1021/np010039x. [DOI] [PubMed] [Google Scholar]
- 54.Rajakrishnan V, Viswanathan P, Rajasekharan KN, Menon VP. Neuroprotective role of curcumin from curcuma longa on ethanol-induced brain damage. Phytother Res. 1999;13:571–4. doi: 10.1002/(sici)1099-1573(199911)13:7<571::aid-ptr494>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 55.Reddy DV, Nishteshwar K. Clinical evaluation of Rasayana effects of Charak's Vayasthapana Dashemani. Int J Res Ayurveda Pharm. 2011;2:20–2. [Google Scholar]
- 56.Howes MJ, Perry E. The role of phytochemicals in the treatment and prevention of dementia. Drugs Aging. 2011;1(28):439–68. doi: 10.2165/11591310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 57.Nagle BT, Hitner H. 5th ed. McGraw Hill; 2005. Pharmacologyan Introduction; p. 76. [Google Scholar]


