Abstract
Quality Ayurvedic herbal medicines are potential, low-cost solutions for addressing contemporary healthcare needs of both Indian and global community. Correlating Ayurvedic herbal preparations with modern processing principles (MPPs) can help develop new and use appropriate technology for scaling up production of the medicines, which is necessary to meet the growing demand. Understanding the fundamental Ayurvedic principles behind formulation and processing is also important for improving the dosage forms. Even though Ayurvedic industry has adopted technologies from food, chemical and pharmaceutical industries, there is no systematic study to correlate the traditional and modern processing methods. This study is an attempt to provide a possible correlation between the Ayurvedic processing methods and MPPs. A systematic literature review was performed to identify the Ayurvedic processing methods by collecting information from English editions of classical Ayurveda texts on medicine preparation methods. Correlation between traditional and MPPs was done based on the techniques used in Ayurvedic drug processing. It was observed that in Ayurvedic medicine preparations there were two major types of processes, namely extraction, and separation. Extraction uses membrane rupturing and solute diffusion principles, while separation uses volatility, adsorption, and size-exclusion principles. The study provides systematic documentation of methods used in Ayurveda for herbal drug preparation along with its interpretation in terms of MPPs. This is the first step which can enable improving or replacing traditional techniques. New technologies or use of existing technologies can be used to improve the dosage forms and scaling up while maintaining the Ayurvedic principles similar to traditional techniques.
KEY WORDS: Ayurvedic extraction principle, Ayurvedic herbal drug preparation, Ayurvedic separation principle, Modern processing principle
INTRODUCTION
Ayurveda uses 1587 plants[1] for many well-documented herbal medicines formulated based on Ayurvedic principles to cure several diseases.[2,3] This can become a good source for developing more scientifically advanced herbal products. There are several elaborate dosage forms of herbal medicines in Ayurveda such as cūrṇa, svarasa, kaṣāya, asava, ariṣṭa, ghṛtam, lehyam and tailam[2,3,4] used by the people owing to its affordability and easy access.[5,6,7]
Probably until the 19th century, Ayurvedic medicines were prepared by practitioners at their homes. This practice still continues for very specialized preparations passed down family traditions. Household level drug preparation using traditional tools and principles[2,3] cannot meet the growing demand for herbal products both in terms of numbers as well as convenience in dosage forms. The 20th century however witnessed Arya Vaidya Sala, Kottakkal pioneering the attempt to industrialize Ayurveda. Today, there are more than 9000 traditional drug manufacturing units in the country.[1] The processes used have mostly been a direct mechanization of traditional processing equipment or have been adapted from the food, chemical or pharmaceutical sector. This sometimes makes Ayurvedic manufacturing laborious and time-consuming.
The trend in traditional medicine research is mainly focused on understanding the use of medicinal plants for developing new allopathic and herbal drugs. Immense potential exists in terms of innovations in pharmaceutical dosage forms if one can understand the basic processing methods involved in Ayurvedic herbal drug preparations (AHDPs). At the same time, new technological principles exist in modern science. Marrying the two can give rise to new technologies and processes that could help upscale production, decrease raw materials requirements, etc. Currently, there is very little research in this area. This review paper attempts to highlight traditionally known AHDP methods and provide possible correlation with modern processing principles (MPPs). This can enable future research to compare the products developed by using AHDP and modern chemical technology as well as provide logic for selection of appropriate technologies for Ayurvedic drugs production scale up.
METHODS
Traditional AHDP methods were selected from English translated versions of two classical Ayurvedic books, namely Bhaiṣajya Kalpanā Vijñānam and Śārṅgadhara Samhitā of Śārṅgadharācarya, which are the main books describing AHDP methods. Extraction and separation steps were identified for each AHDP method by correlating the activities used in every step of the process with modern extraction and separation techniques. Possible MPPs for each method was identified based on the understanding of the traditional techniques.
Division of Ayurvedic herbal drug preparation process into steps
The process for each AHDP method was divided on the lines of modern biopharmaceutical drug preparation processes into three steps namely preprocessing, extraction and separation.[8] Preprocessing step consists of those activities such as washing, drying, cleaning and powdering of herbs in which the medicinal plant used is prepared for processing. Extraction step is composed of those activities in which the medicinal plant with/without preprocessing is processed to release bioactive (or, undesirable) compounds from the medicinal plant into the medium. Separation step is defined for those activities in which desirable compounds (in any form) are separated from the unwanted plant parts/components.
Identification of extraction step and techniques
The extraction step was identified by identifying the activities in each AHDP method, which uses techniques, which are either identical or similar to the modern extraction techniques. Some of the commonly used modern extraction techniques used in modern non Ayurvedic herbal industry which were used in this study for correlation were mechanical pounding/mashing, hot solvent extraction, maceration, pressurized liquid extraction, microwave-assisted extraction, ultrasound-assisted extraction, supercritical fluid extraction, solid phase extraction, reflux and fermentation.[9,10] These techniques attunes with various enhancers such as heating,[9,11,12] pressurization,[9,10] change of pH,[13] agitation,[14,15] ultrasound,[9] surfactant,[16] enzyme,[9,17] microbe[18] and microwave[9] to accelerate the process of extraction.
Identification of separation step and techniques
The separation step was identified by identifying the activities in AHDP method, which uses a technique(s), which is (are) either identical or similar to the modern separation techniques. Some of the commonly used modern separation techniques in modern non Ayurvedic herbal industry which were used in this study for correlation were filtration,[9] chromatography,[19] liquid-liquid extraction,[20] electrophoresis[21,22] and solid phase micro-extraction.[23] The separation techniques can use various enhancers like temperature,[24] pressure[19] and potential gradient[25] to accelerate the process of separation.
Identification of modern processing principle for extraction step of each dosage form
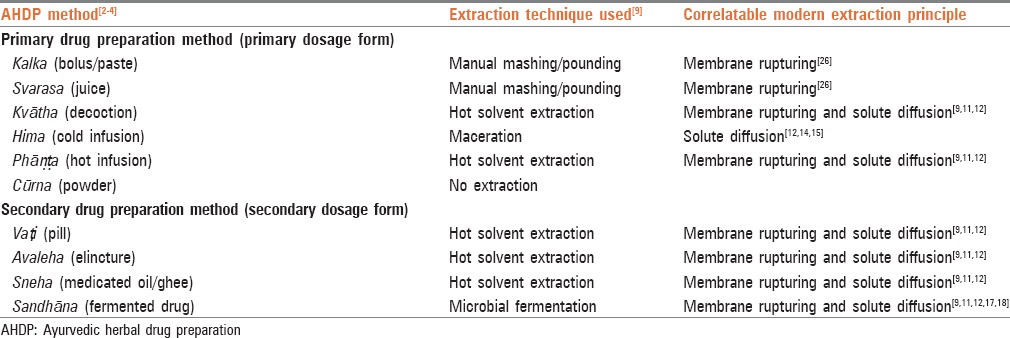
Modern processing principle followed by the identified extraction techniques of each AHDP method extraction step was determined using the MPP, followed by those techniques’ modern counterpart. Modern extraction techniques considered for this study follow membrane rupturing and solute diffusion principles. Mechanical pounding/mashing technique uses “membrane rupturing” principle as pounding/mashing cause mechanical shear and stress on the cells,[26] which can cause cell membrane disruption and release of cellular components in the surrounding medium.
Maceration technique uses solute diffusion principle as the solute (compounds soluble in the solvent) move from high concentration medium (cell cytoplasm) to low concentration medium (solvent/water).[12] This process is accelerated by the use of agitation, which aids in increasing the interaction between the solvent and the raw material by increasing the contact surface area.[14,15] In addition, the solvent used may aid in membrane rupturing either by creating osmotic shock or by cell membrane disruption/dissolution. Hot solvent extraction technique uses both membrane rupturing and solute diffusion principles owing to the use of both heating and solvent as they would cause increased cell permeability and higher mobility of compounds.[9,11,12]
Fermentation technique uses both “membrane rupturing” and “solute diffusion” principles as use of exogenous enzymes either synthesized by exogenous enzyme producing micro-organisms[18] or by the use of purified enzymes.[17] This allows rupturing of the plant cells. In addition, use of solvent, which is either produced in-situ (like ethanol and vinegar)[27,28] or added separately allows compounds to diffuse from cells into the medium. The use of heating with solvent extraction increases cell membrane permeability/breakdown and also an increase in molecular kinetic energy, which allow higher mobility of the compounds across concentration gradient.[9,11,12]
Identification of modern processing principle for separation step of each dosage form
Modern processing principle followed by the identified separation technique of each AHDP method separation step was determined using the MPP, followed by those techniques’ modern counterpart. Modern separation techniques considered for this study follow size-exclusion, affinity, charge, partition-coefficient/solubility, adsorption, and volatility principles. Based on the matrix used, chromatography can use most of the above-mentioned principles.[19] Filtration utilizes the size-exclusion principle for separating compounds by allowing only particles of a particular size to pass through the filter.[9] This process can be enhanced by the application of vacuum by creating negative pressure difference between unfiltered liquid and filtrate.[29]
Liquid-liquid extraction uses the principle of the solubility in which the compounds dissolve based on their relative solubility between two immiscible liquids.[20] Electrophoresis separates the compounds of different size and charge by using the principle of size-exclusion and compound charge.[22,30] Solid phase micro-extraction relies on the principle of adsorption and volatility for the separation of volatile compounds from the raw material.[23]
RESULTS AND DISCUSSION
Ayurvedic herbal drug preparation methods
The results from the analysis of the English translated versions of two classical Aurvedic books, namely, Bhaiṣajya Kalpanā Vijñānam and Śārṅgadhara Saṃhitā of Śārṅgadharācārya showed that AHDP are based on drug dosage forms and have been classified by us into Primary Herbal Drug preparation method (or, Primary dosage forms) and Secondary Herbal Drug preparation Method (or, Secondary dosage forms) [Table 1]. These methods use plant based raw materials which are processed to prepare drugs.
Table 1.
Primary and secondary drug preparation methods

The primary dosage forms are svarasa (juice), kalka (bolus/paste), kvātha (Decoction), hima (Cold Infusion), phāṇṭa (Hot infusion) and cūrna (Powder). They are the dosage forms in which the herbs are used directly. These drugs except cūrṇa have short shelf life of up to the day. Secondary dosage forms are vaṭi (pill), avaleha (linctures), sneha (Medicated oils/ghee) and sandhāna (Fermented drugs). Secondary dosage forms process both herbs and primary drugs to prepare drugs. These drugs have long shelf life of up to a year.[2,3,4]
Preprocessing step in different Ayurvedic herbal drug preparation methods
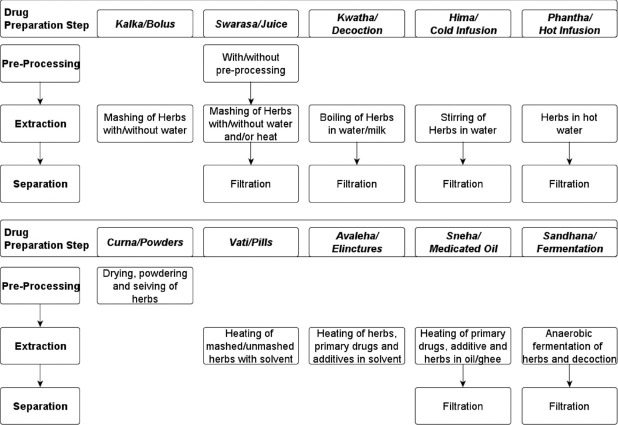
The preprocessing step considered for this study involves various preliminary activities such as washing, drying, powdering and cleaning of herbs. The study of literature revealed that the activities which could be considered for preprocessing step have been explicitly mentioned only for the svarasa and cūrna preparation [Figure 1]. In the case of svarasa preparation, preprocessing activity was performed for fresh herbs which were cleaned by washing. In the case of cūrna preparation, all the herbs undergo preprocessing activity of drying and powdering.[2,3,4]
Figure 1.
Different Ayurvedic herbal drug preparation mentioned in Ayurvedic literature
Extraction step and its modern processing principle in different Ayurvedic herbal drug preparation methods
The extraction step considered for this study involves those activities which are performed in any of the modern techniques namely mechanical pounding/mashing, hot solvent extraction, maceration, pressurized liquid extraction, microwave-assisted extraction, ultrasound-assisted extraction, supercritical fluid extraction, solid phase extraction, reflux and fermentation. The study of literature found that activities which could be considered for extraction were present in all AHDP methods except cūrna[2,3,4] as shown in Figure 1.
The AHDP methods correlate with different techniques and MPP for extraction as shown in Table 2. In kalka and svarasa method, the activity of mashing or pounding of the medicinal plant for the release of the juice from the plant cells is similar to the modern extraction technique of mechanical pounding/mashing. This indicates that membrane rupturing is predominant in kalka and svarasa method.
Table 2.
Extraction principles used by various AHDP methods
In the hima method, the activity of immersing herbs in water (solvent) for release of plant juice into the water indicates similarity with maceration which is a modern extraction technique. This indicates that the hima technique uses both solute diffusion and membrane rupturing principles.
In the sandhāna method, the activity of immersed herbs in water along with other adjuvants in a closed container for around 60 days indicates similarity with the modern extraction technique of fermentation. This indicates that the sandhāna uses both solute diffusion and membrane rupturing principles.
In the remaining drug preparation methods, the activity of keeping herbs immersed in solvent (such as water, milk and ghee) along with heating for short duration unlike sandhāna (where duration is for months) indicates similarity with the modern extraction technique, viz., hot solvent extraction. This indicates that the other methods use both solute diffusion and membrane rupturing principles. The methods are different based on the number of steps of extraction, type of solvent and intensity of heating [Figure 1]. The use of solvent extraction based extraction techniques by most of the AHDP methods indicates that chemical extraction is the most preferred route of drug preparation.
Separation step and its modern processing principle in different Ayurvedic herbal drug preparation methods
The separation step considered for this study involves those activities which are performed in any of the modern techniques such as: Filtration, chromatography, liquid-liquid extraction, electrophoresis and solid phase micro-extraction. The study of literature revealed that activities which could be considered for separation step were present in those methods which require only the liquid filtrate namely svarasa, kvātha, hima, phāṇṭa, sneha and sandhāna[2,3,4] as shown in Figure 1.
The AHDP methods correlate with different techniques and MPP for separation as shown in Table 3. In svarasa and hima method, the activity of filtering the solvent to separate it from the unwanted plant components indicates that it is similar to the filtration, the modern extraction technique. Size-exclusion is the principle used in the svarasa and hima methods.
Table 3.
Separation principles used by various AHDP methods
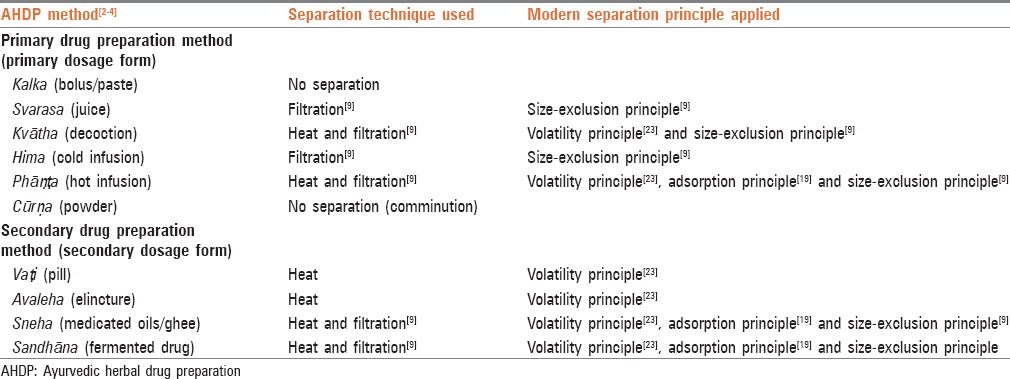
In kvātha, the action of filtering the solvent to separate it from the unwanted plant components also indicates that it is similar to the modern extraction technique, filtration, and it uses the size-exclusion principle as MPP. In addition, use of heating during extraction step could cause removal of volatile compounds from the solvent as well as prevent the adsorption of the extracted compounds on nonsoluble part of the herb (like cell wall). This shows us that volatility of the compounds is used without the use of any special separation step.
In phāṇṭa, sneha and sandhāna method, the activity of filtering the solvent to separate it from the unwanted plant components also indicates similarity with the modern separation technique namely filtration and use of size-exclusion principle as MPP. In addition, the use of heat with solvent and gradual cooling of solvent in the extraction step could cause additional compound separation in two phases. In the initial phase when solvent is hot, volatile compounds could be removed from the solvent by evaporation and in the second phase when the solvent is cooled down, adsorption of various extracted compounds on the surface of the nonsoluble part of the herb could happen. The possibility of adsorption phenomenon can be understood by use of various plant product based matrices such as banana peel,[21] empty fruit bunches of oil-palm,[31] activated neem leaves,[32] cactus pear leaves,[33] sawdust[34,35] and used tea leaves[36] in literature for performing adsorption chromatography. The adsorption phenomenon may become more dominant with use of multiple herbs as different herbs can provide different solid phase matrix. This indicates the use of volatility and adsorption principles.
In the preparation of vaṭi and avaleha dosage forms, no special activity was found to indicate use of any separation technique, except for use of heat with solvent during extraction and this indicates the use of volatility principle to remove volatile compounds from the desired compounds.
The results indicate the use of filtration based separation techniques by most of the AHDP methods. Further, the results indicate the use of volatility and adsorption principles by all techniques without the use of any special separation step.
Correlation between Ayurvedic herbal drug preparation and modern techniques
The selection of AHDP method/Ayurvedic dosage form depends on several factors like the desired drug characteristics (like nature of raw drug, nature of medicinal fraction that needs to get extracted, shelf life, potency and response time), environment (season etc.) and patient's prevalent condition (like digestive capability and disease stage). Irrespective of the bio-interaction of the drug with the body, the fact remains that a certain process is being performed on the drug material before use. An attempt has been made to correlate this to a pharmaceutical principle/MPP in this article. MPP interpreted in the extraction and separation steps for each AHDP method/Ayurvedic dosage form resulted in predicting potential modern techniques that could be used for each AHDP method/Ayurvedic dosage form as shown in Table 4.
Table 4.
Correlation between AHDP and modern techniques
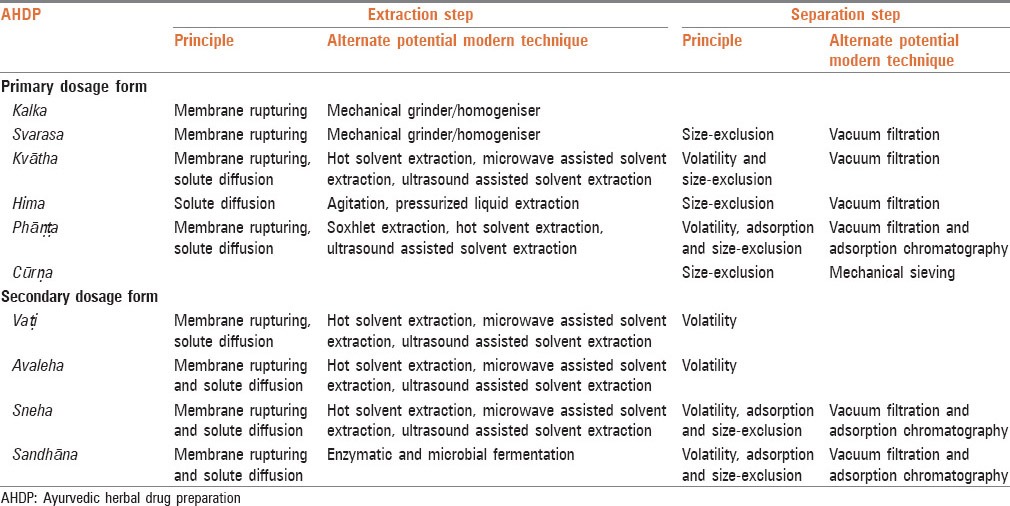
Among the primary dosage forms, kalka and svarasa extraction steps may be performed using homogenizer/mechanical grinder as these two dosage forms use physical methods for breakdown of cell wall. Kvātha extraction step involves extraction using solvent with heat as an enhancer to increase the solubility of compounds in a solvent and to breakdown the cell wall. This phenomenon can also be seen in a hot solvent extraction using normal heating or heating using microwave. Further, heat indirectly aids in the separation by removing the volatile compounds. Ultrasound-assisted extraction can also be used as the extraction technique along with heat to speed up the cell breakdown.
Hima uses solvent extraction but without using heat as an enhancer which results in no loss of the volatile compounds. This Ayurvedic dosage form may use modern extraction techniques namely agitation or pressurized liquid extraction to enhance the extraction rate using agitation or high pressure.
Phāṇṭa Ayurvedic dosage form uses solvent extraction along with heat followed by cooling over a period of time, this dosage form can use modern extraction techniques which can be performed at less than boiling temperature like hot solvent extraction, soxhlet extraction and ultrasound-assisted extraction. The potential loss of volatile compounds is possible owing to the use of hot solvent, which indirectly aids in separation step.
Among the secondary dosage forms, vaṭi, avaleha and sneha uses the similar extraction method by solvent extraction with the aid of heat, which indicates that they may use modern extraction techniques similar to them such as hot solvent extraction, microwave-assisted extraction and ultrasound-assisted extraction. In the case of sandhāna, the solvent extraction is aided with heat and microbes, this dosage form can use enzymatic or microbial fermentation methods for extraction.
The separation steps exist for primary dosage forms (namely svarasa, kvātha, hima and phāṇṭa) and secondary dosage forms (namely sneha and sandhāna), which use filtering mechanism for separating filtrate from unwanted plant components. The separation process may use vacuum filtration technique to enhance separation rate. In the case of phāṇṭa, multi-herb hima, svarasa, sneha or sandhāna dosage forms, the possibility of adsorption based separation exists and that is why, adsorption chromatography may also be used as a separation technique.
CONCLUSION
This review provides various techniques used in AHDP for extraction and separation steps based on which MPPs have been proposed for respective steps. In extraction, both membrane rupturing and solute diffusion principles are commonly employed in AHDP. In separation, the principles employed in AHDP are volatility, adsorption and size-exclusion. Further, the use of separation principles without using any special separation technique indicates that only separation techniques based identification of separation principles may not be sufficient. This study is the first step to enable new technology development for processing traditional medicines to improve the dosage forms and scaling up while maintaining the principles similar to that of traditional techniques. For example, conversion of kaṣāyams to freeze-dried/vacuum dried powder/tablets are in vogue in today's Ayurveda pharmacies.
The different principles used for the separation step of AHDP are less than the total number of principles which can be employed for the modern herbal drugs preparation, indicating the potential scope of research in the field of AHDP. Further, the modern techniques for extraction and separation steps that work on similar principles as identified for AHDP may be explored for innovating/upscaling the traditional techniques and methods. The potential correlation provided in the study is not completely exhaustive, and many more correlations can be explored with more research and data availability. Future work may involve the experimental/laboratory validation of identified MPP in extraction and separation steps of different AHDP methods. Further, the various factors controlled in modern techniques for product quality maintenance can be compared with the various factors mentioned in AHDP, which can help in better selection of modern techniques for Ayurvedic herbal drug manufacturing.
ACKNOWLEDGMENT
We would like to express our gratitude to IAIM librarian Mrs. Nandini KK for allowing Rahi Jain to study in library and use its facilities.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Ved DK, Goraya GS. Dehradun: Bishen Singh Mahendra Pal Singh Publishers, Dehradun and FRLHT, Bengaluru; 2008. Demand and Supply of Medicinal Plants in India. [Google Scholar]
- 2.Rao G. New Delhi: Chaukhambha Sanskrit Sansthan; 2008. A Textbook of Bhaisajya Kalpana Vignanam. [Google Scholar]
- 3.Murthy PH. Varanasi: Chowkhamba Sanskrit Series Office; 2007. Sarngadhara Samhita of Sarngadharacarya. [Google Scholar]
- 4.Reddy KR. Varanasi: Chaukhambha Sanskrit Sansthan; 2005. Bhaisajya Kalpana Vijnanam. [Google Scholar]
- 5.Patwardhan B. Ayurveda for all: 11 action points for 2011. J Ayurveda Integr Med. 2010;1:237–9. doi: 10.4103/0975-9476.74416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.New Delhi: 2011. Planning Commission of India. Report of Steering Committee on AYUSH for 12th Five Year Plan (2012-17) [Google Scholar]
- 7.Priya R, Shweta AS. New Delhi: National Rural Health Mission (NRHM); 2010. Status and Role of AYUSH and Local Health Traditions. [Google Scholar]
- 8.Rates SM. Plants as source of drugs. Toxicon. 2001;39:603–13. doi: 10.1016/s0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 9.Handa SS, Khanuja SP, Longo G, Rakesh DD. Trieste, Italy: International Centre for Science and High Technology; 2008. Extraction Technologies for Medicinal and Aromatic Plants. [Google Scholar]
- 10.Katakdond AB, Vinjamur M, Zambre S, Shah N. Mumbai: Centre for Technology Alternatives for Rural Areas (CTARA), Indian Institute of Technology Bombay (IITB; 2006. Process Improvement Study of Preparation of Medicinal Oil from Nirgundi. [Google Scholar]
- 11.Yang L, Jiang JG, Li WF, Chen J, Wang DY, Zhu L. Optimum extraction process of polyphenols from the bark of Phyllanthus emblica L. based on the response surface methodology. J Sep Sci. 2009;32:1437–44. doi: 10.1002/jssc.200800744. [DOI] [PubMed] [Google Scholar]
- 12.Escribano-bailon MT, Santos-buelga C. Polyphenol extraction from foods. In: Williamson G, editor. Methods in Polyphenol Analysis. Cambridge: The Royal Society of Chemistry; 2003. pp. 1–16. [Google Scholar]
- 13.Ruenroengklin N, Zhong J, Duan X, Yang B, Li J, Jiang Y. Effects of various temperatures and pH values on the extraction yield of phenolics from litchi fruit pericarp tissue and the antioxidant activity of the extracted anthocyanins. Int J Mol Sci. 2008;9:1333–41. doi: 10.3390/ijms9071333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gjelstad A, Andersen TM, Rasmussen KE, Pedersen-Bjergaard S. Microextraction across supported liquid membranes forced by pH gradients and electrical fields. J Chromatogr A. 2007;1157:38–45. doi: 10.1016/j.chroma.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Su Y, Chen G, Zhao Y, Yuan Q. Intensification of liquid-liquid two-phase mass transfer by gas agitation in a microchannel. AIChE J. 2009;55:1948–58. [Google Scholar]
- 16.Eng AT, Heng MY, Ong ES. Evaluation of surfactant assisted pressurized liquid extraction for the determination of glycyrrhizin and ephedrine in medicinal plants. Anal Chim Acta. 2007;583:289–95. doi: 10.1016/j.aca.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Mudgett RE, Rufner R, Bajracharya R, Kim K, Rajagopalan K. Enzymatic effects on cell rupture in plant protien recovery. J Food Biochem. 1978;2:185–207. [Google Scholar]
- 18.Demeyer DI. Rumen microbes and digestion of plant cell walls. Agric Environ. 1981;6:295–337. [Google Scholar]
- 19.Upadhyay A, Upadhyay K, Nath N. Biophysical Chemistry: Principles and Techniques. Mumbai: Himalaya Publishing House; 2005. Chromatography; pp. 344–421. [Google Scholar]
- 20.Rydberg J, Cox M, Musikas C, Choppin GR. 2nd ed. New York: Marcel Dekker Inc; 2004. Solvent Extraction Principles and Practice. [Google Scholar]
- 21.Upadhyay A, Upadhyay K, Nath N. Biophysical Chemistry: Principles and Techniques. Mumbai: Himalaya Publishing House; 2005. Electrophoresis; pp. 422–78. [Google Scholar]
- 22.Dennison C. A Guide to Protein Isolation. Netherlands: Springer; 2002. Principles of electrophoresis; pp. 115–49. [Google Scholar]
- 23.Marsili R. New York, USA: CRC Press; 2001. Flavor, Fragrance, and Odor Analysis. [Google Scholar]
- 24.Gupta R, Mauri R, Shinnar R. Liquid-liquid extraction using the composition-induced phase separation process. Ind Eng Chem Res. 1996;5885:2360–8. [Google Scholar]
- 25.Eeltink S, Rozing GP, Kok WT. Recent applications in capillary electrochromatography. Electrophoresis. 2003;24:3935–61. doi: 10.1002/elps.200305638. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg S. Mechanical/physical methods of cell disruption and tissue homogenization. In: Posch A, editor. 2D PAGE: Sample Preparation and Fractionation. New York, USA: Humana Press; 2008. pp. 3–22. [Google Scholar]
- 27.Woods DR. The genetic engineering of microbial solvent production. Trends Biotechnol. 1995;13:259–64. doi: 10.1016/S0167-7799(00)88960-X. [DOI] [PubMed] [Google Scholar]
- 28.Liew ST, Arbakariya A, Rosfarizan M, Raha AR. production of solvent (acetone-butanol-ethanol) in continuous fermentation by clostridium saccharobutylicum DSM 13864 using gelatinised sago starch as a carbon source. Malays J Microbiol. 2006;2:42–50. [Google Scholar]
- 29.Matteson M, Orr C. 2nd ed. New York: CRC Press; 1986. Filtration: Principles and Practices. [Google Scholar]
- 30.Achak M, Hafidi A, Ouazzani N, Sayadi S, Mandi L. Low cost biosorbent “banana peel” for the removal of phenolic compounds from olive mill wastewater: Kinetic and equilibrium studies. J Hazard Mater. 2009;166:117–25. doi: 10.1016/j.jhazmat.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 31.Alam MZ, Muyibi SA, Mansor MF, Wahid R. Activated carbons derived from oil palm empty-fruit bunches: Application to environmental problems. J Environ Sci (China) 2007;19:103–8. doi: 10.1016/s1001-0742(07)60017-5. [DOI] [PubMed] [Google Scholar]
- 32.Babu BV, Gupta S. Adsorption of Cr(VI) using activated neem leaves: Kinetic studies. Adsorption. 2008;14:85–92. [Google Scholar]
- 33.Gebrekidan A, Nicolai H, Vincken L, Teferi M, Asmelash T, Dejenie T, et al. Pesticides removal by filtration over cactus pear leaves: A cheap and natural method for small-scale water purification in semi-arid regions. CLEAN-Soil Air Water. 2013;41:235–43. [Google Scholar]
- 34.Jadhav DN, Vanjara AK. Removal of phenol from wastewater using sawdust, polymerized sawdust and sawdust carbon. Indian J Chem Technol. 2004;11:35–41. [Google Scholar]
- 35.Mebirouk BM, Sbai L, Lopez M, Gonzalez J, Mohammadia E, Minéral DG, et al. The absorption of polyphenols from olive oil mill wastewaters by sawdust and biodegradation by the fungus Phanerochaetae chrysosporium. Grasaa Y Aceites. 2007;58:366–71. [Google Scholar]
- 36.Tee TW, Khan RM. Removal of lead, cadmium and zinc by waste tea leaves. Enivron Technol Lett. 2008;9:1223–32. [Google Scholar]