Abstract
Aims
Our previous study found that A83-01, a small molecule type 1 TGFβ receptor inhibitor, could induce proliferation of postnatal Nkx2.5+ cardiomyoblasts in vitro and enhance their cardiomyogenic differentiation. The present study addresses whether A83-01 treatment in vivo could increase cardiomyogenesis and improve cardiac function after myocardial infarction through an Nkx2.5+ cardiomyoblast-dependent process.
Methods and results
To determine the effect of A83-01 on the number of Nkx2.5+ cardiomyoblasts in the heart after myocardial injury, we treated transgenic Nkx2.5 enhancer-GFP reporter mice for 7 days with either A83-01 or DMSO and measured the number of GFP+ cardiomyoblasts in the heart at 1 week after injury by flow cytometry. To determine the degree of new cardiomyocyte formation after myocardial injury and the effect of A83-01 in this process, we employed inducible Nkx2.5 enhancer-Cre transgenic mice to lineage label postnatal Nkx2.5+ cardiomyoblasts and their differentiated progenies after myocardial injury. We also examined the cardiac function of each animal by intracardiac haemodynamic measurements. We found that A83-01 treatment significantly increased the number of Nkx2.5+ cardiomyoblasts at baseline and after myocardial injury, resulting in an increase in newly formed cardiomyocytes. Finally, we showed that A83-01 treatment significantly improved ventricular elastance and stroke work, leading to improved contractility after injury.
Conclusion
Pharmacological inhibition of TGFβ signalling improved cardiac function in injured mice and promoted the expansion and cardiomyogenic differentiation of Nkx2.5+ cardiomyoblasts. Direct modulation of resident cardiomyoblasts in vivo may be a promising strategy to enhance therapeutic cardiac regeneration.
Keywords: Nkx2.5, Cardiomyoblast, TGFβ, Regeneration, Myocardial infarction
1. Introduction
Recent studies have reported the existence of multiple stem cell populations in the mammalian heart.1–7 Despite this, the rate of cardiomyocyte renewal is extremely low and declines progressively throughout life.8 Consequently, heart failure due to myocardial injury is a major cause of morbidity and mortality in the Western world. Conceptually, it would be highly appealing to functionally repopulate the resident cardiac progenitor cells to compensate for the lost myocardium. This may also prevent adverse remodelling of the injured heart. Therefore, the availability of an effective strategy to activate endogenous cardiomyogenic cell populations for cardiac repair is highly appealing.
We have previously identified an embryonic Nkx2.5+ cell population that represents the common precursor for cardiovascular lineages in the developing heart.9,10 Using transgenic Nkx2.5 cardiac enhancer-eGFP mice (Nkx2.5-GFP) to label Nkx2.5+ cardiomyoblasts in the postnatal heart, we showed recently that a population of postnatal cardiac cells that express GFP from the Nkx2.5 cardiac enhancer can proliferate in vitro in response to treatment by A83-01, a pharmacological inhibitor of type I TGFβ receptor (TGFβRI).11 Furthermore, this compound inhibited smooth muscle cell differentiation of Nkx2.5+ cardiomyoblasts while stimulating their differentiation into cardiomyocytes.
Signalling by the TGFβ superfamily occurs via formation of heteromeric complexes consisting of two type II receptors (TGFβRIIs) and two TGFβRIs.12–14 The TGFβRII receptors have been shown to phosphorylate the TGFβRI, resulting in the activation of both Smad-dependent and Smad-independent signalling.15–19 TGFβRIs, referred to as activin receptor-like kinase 5 (ALK5), transduce TGFβ signals to intracellular regulators of transcription known as Smad proteins.15,16,20 Of the known ALK receptors, ALK1–7 have been identified in mammals21,22 and A83-01 has been shown to inhibit ALK4,5,7 receptors.23
Given our previous demonstration of the role of A83-01 on postnatal Nkx2.5 cardiomyoblasts in vitro,11 we speculate that the treatment of hearts with A83-01 following injury may activate resident Nkx2.5+ cardiomyoblasts and promote their ability to repair an injured heart. Indeed, recent studies have demonstrated the ability of neonatal heart to regenerate after experimentally induced injury,24 and Nkx2.5+ cardiomyoblasts may participate in this process.9 In this study, we found that A83-01 treatment leads to an expansion of Nkx2.5+ cardiomyoblasts in both injured and uninjured hearts. By prospective lineage tracing using inducible Nkx2.5 cardiac enhancer-Cre transgenic mice, we show that A83-01 treatment gives rise to a two- to three-fold increase in new cardiomyocyte formation compared with DMSO treatment. Furthermore, we found that the A83-01-mediated increase in new cardiomyocyte formation directly correlates with cardiac functional improvement. Altogether, our data support the role of TGFβ receptor inhibition to enhance cardiac regeneration and improve cardiac function after myocardial infarction (MI).
2. Methods
2.1. Chemical reagents
A83-01 (a small molecule ALK4,5,7 inhibitor) was purchased from Stemgent, Inc. (Cambridge, MA, USA) and recombinant human TGFβ1 was purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
2.2. Animals
Nkx2.5 enhancer-eGFP transgenic mice (abbreviated as Nkx2.5-GFP)10 were used at 8–10 weeks of age for the in vivo quantification and isolation and at 2–3 weeks of age for in vitro culturing of cardiac Nkx2.5+ cardiomyoblasts. For genetic fate mapping of Nkx2.5 enhancer-lineage cells in the postnatal hearts, the doxycycline-regulated Nkx2.5 enhancer-Cre/eGFP mice (abbreviated as Nkx2.5 enh-Cre) were crossed with ROSA26-mTmG reporter mice (Jackson Laboratory, USA). The ROSA26-mTmG mouse contains membrane-targeted tdTomato (mT) cassette in the ROSA26 locus and expresses strong red fluorescence in all tissues and cell types at baseline. When the ROSA26-mTmG mouse is interbred with the Nkx2.5 enhancer-Cre mice, Cre recombinase is expressed in Nkx2.5+ cardiomyoblasts leading to excision of the floxed mT cassette, thereby converting the expression of tdTomato to GFP in Nkx2.5 enhancer-Cre expressing cells and their descendants. The pregnant females and all descendants were treated with doxycycline (1 mg/mL in drinking water) from conception until 1 week before drug treatment to prevent embryonic labelling of Nkx2.5+ cardiomyoblasts during early embryonic development. Nkx2.5 enh-Cre/mTmG mice at 8–10 weeks of age were used for testing drug effect. After mice were treated with DMSO (1 mL/kg, i.p.) or A83-01 (10 mg/kg, i.p.) for 7 days, mice were sacrificed by CO2-induced euthanasia and the hearts were harvested and then embedded in optimal cutting temperature (OCT) for frozen sections (8 µm per section). The frozen sections were first blocked with donkey anti-mouse IgG antibody (1 : 40) overnight at 4°C. The cells expressing GFP were identified by primary antibody against GFP (rabbit polyclone, Thermo Fisher Scientific, Inc., IL, USA) subsequently labelled with the Alexa Fluor 488-conjugated secondary antibody. GFP+ cells were further stained by primary antibodies against sarcomeric actinin-α (SA-α; mouse monoclonal, Sigma-Aldrich Co. LLC, St Louis, MO, USA) or against smooth muscle actin-α (mouse monoclonal, Sigma-Aldrich Co. LLC) to clarify whether they were cardiomyocytes or smooth muscle cells, respectively. Allophycocyanin-conjugated secondary antibody against mouse IgG was subsequently used to label the target cells. Nuclei were counterstained by 4′,6-diamino-2-phenylindole (DAPI). Immunostaining was performed in six frozen sections with the intramyocardial distance of 80 µm among them in each heart and three different hearts in each group (DMSO vs. A83-01). The images of myocytes or smooth muscle cells expressing GFP with DAPI-counterstained nucleus were scanned by the TissueFAXS system (TissueGnostics GmbH, Vienna, Austria) via ×20 lens mounted on Zeiss Axio Imager Z2 microscopy (Zeiss, Oberkochen, Germany). The number of the cells expressing both GFP and SA-α/or smooth muscle actin-α was analysed by TissueQuest (TissueGnostics GmbH). Nuclear DAPI was defined as the master channel to identify all measure events. By comparing negative vs. positive controls, cut-off values can be defined and the percentages of positive/negative populations are computed. Comparison of the mean relative intensity of a particular object to an appropriate negative control allows determining the level of specificity. The percentages of co-expression are computed. The fluorescent images of GFP+ cells expressing SA-α/or smooth muscle actin-α were further confirmed by confocal microscopy (TCS SP5, Leica Microsystem K.K., Tokyo, Japan). All animal experiments were performed according to the US National Institutes of Health or European Commission guidelines, and have been approved by Subcommittee on Research Animal Care at Massachusetts General Hospital and the Institutional Animal Care and Use Committee (IACUC) of the National Taiwan University College of Medicine and College of Public Health.
2.3. Murine model of myocardial infarction
Adult mice at age of 8 weeks were anaesthetized by 1% isoflurane inhalation via intratracheal intubation and were received buprenorphine (0.1 mg/kg) by subcutaneous injection for analgesia. Subsequently, midline thoracotomy followed by left coronary artery (LAD) ligation was performed with a 7-0 polyethylene suture to induce MI. The induction of myocardial ischaemia was confirmed by the appearance of blanching and dyskinesia in the left ventricular (LV) apex. Following the procedure, mice were extubated and recovered under a warming lamp until they have regained normal activity. For postoperative analgesia, buprenorphine (0.1 mg/kg) was applied once daily for 3 days post-surgery. Two days later after LAD ligation surgery, the post-MI mice were randomized into either the DMSO- or A83-01-treated groups in a blinded fashion. After cardiac functional evaluation and immunostaining have been performed, the samples were unblinded for final statistical analysis.
2.4. Cell culture
Nkx2.5+ cardiomyoblasts were isolated from the hearts of 2- to 3-week-old postnatal Nkx2.5-GFP transgenic mice (sacrificed by CO2-induced euthanasia) by enzymatic digestion with collagenase A/B combination (Roche Diagnostics GmbH, Mannheim, Germany) in PBS supplemented with HEPES 10 mM and 20% FBS at 37° C for 30 min, and were sorted by fluorescence-activated cell sorting (FACS). Cardiomyocytes for co-culture assays were isolated from embryonic day 9.5 (E9.5) embryos with the same enzymatic protocol. Cardiomyocytes were co-cultured with Nkx2.5+ cardiomyoblasts in the ratio of 1 : 3 (cardiomyocyte : GFP+ cells), and total of 1.2 × 105 cells were seeded in a six-well plate (Fisher Scientific) coated with 0.1% gelatine. The culture medium included an IMDM-based medium containing 20% FBS, 1% penicillin/streptomycin, 2 mM l-glutamine, 1% ascorbic acid, and 149.5 µM monothioglycerol.
2.5. Flow cytometry and FACS
Single cell suspension from the Nkx2.5-GFP mouse heart was washed and re-suspended in PBS, and passed through a 45 µm filter before they were added with propidium iodide for live cell gating. The percentage of GFP+ cardiomyoblasts in non-myocyte fraction within the heart was quantified by flow cytometry (FACSCalibur or FACSAria II, Beckton Dickinson, San Jose, CA, USA; see Supplementary material online, Figure S1). Nkx2.5+ cardiomyoblasts were sorted by FACSAria II (Beckton Dickinson) for in vitro culturing or RT-qPCR after RNA extraction.
2.6. Immunocytochemistry
For immunostaining of culture cells, cells were fixed with 4% paraformaldehyde for 10 min at room temperature, and then washed with PBS. Cells were then incubated in PBS containing 0.25% Triton X-100 for 10 min, then in 1% BSA containing PBST (Tween 20 0.1% in PBS) for another 1 h. Primary antibodies were diluted as recommended by manufacturer in 1% BSA- and 0.3 M glycine-containing PBST. Cells were incubated overnight at 4°C with primary antibodies against α-smooth muscle actin (mouse monoclonal, Sigma-Aldrich Co. LLC), cardiac troponin T (TnT; rabbit polyclonal, NeoMarkers, Fremont, CA, USA), and smooth muscle myosin heavy chain (rabbit polyclonal, Biomedical Technologies, Inc., Stoughton, MA, USA). After two washes with PBS, fluorochrome-conjugated secondary antibodies (e.g. anti-rabbit Alexa Fluor 594 and anti-mouse Alexa Fluor 488; Molecular Probes, Invitrogen) matching to the species and subclasses of the primary antibodies were used for detection. Nuclei of the cells were counterstained with DAPI (Invitrogen Ltd, Carlsbad, CA, USA). IF images were captured by a Leica DM4000 fluorescence microscope.
2.7. Real-time quantitative PCR analysis
Total RNA was immediately extracted from FACS-purified Nkx2.5-GFP+ cells using TRIzol (Invitrogen Ltd) and subsequently purified by the RNeasy Mini Kit (Qiagen GmbH, Hilden, Germany). Following cDNA synthesis, the relative expression levels of genes of interest were quantitated by real-time PCR.
2.8. RNAi-mediated silencing of TGFβ receptor ALK5
RNAi-mediated silencing of ALK5 expression in Nkx2.5+ cardiomyoblasts was performed by introducing lentiviral shRNA (GE Healthcare Dharmacon, Inc., Buckinghamshire, UK) targeting ALK5 into FACS-purified GFP+ cells from the hearts of neonatal Nkx2.5-GFP mice. The GIPZ-ALK5-shRNA was prepared using the Trans-Lentiviral GIPZ Packaging system and introduced into Nkx2.5+ cardiomyoblasts at an optimal multiplicity of infections of 1.
2.9. Analysis of intracardiac haemodynamic parameters
Mice were anaesthetized by isoflurane inhalation (1–2%) and the right carotid artery was cannulated with a 1.2 F admittance pressure–volume catheter, which was then advanced into the ascending aorta and then left ventricle under pressure monitor. The LV pressure and volume relationship in post-MI mice was then measured using the ADVantage system (Scisense Instruments, ON, Canada). The signals of LV pressure and volume were continuously recorded at a sampling rate of 1000/s using the LabScribe2 data recording system (iWorx Systems, Inc.). Contractility was quantified by Ees [the slope of the end-systolic pressure and volume relation (ESPVR)], stiffness [the slope of the end-diastolic pressure and volume (EDPVR)], and preload-recruitable stroke work (PRSW). Ees gives insights into the dynamic behaviour of the ventricle, and PRSW is a load-independent characteristic of ventricular contractile performance in vivo.
2.10. Histological analysis of cardiac sections
After cardiac haemodynamic analyses, mice were euthanized by CO2, and the hearts were removed. The excised hearts were incubated in sucrose gradient from 10 to 30% in cold PBS buffer until sedimentation. The hearts were then embedded in medium of OCT and stored at −80°C for frozen sections and Masson's trichrome staining to quantify the degree of fibrosis (blue area) by Image J.
2.11. BrdU analysis of post-MI hearts
For BrdU pulse chase labelling of post-MI mice hearts, 12 mice underwent left coronary ligation followed by randomization to either DMSO (n = 6) or A83-01 (n = 6) injections daily during Days 3–10 after MI. BrdU (2 mg) was administered via intraperitoneal injection once per day during Days 8–10. Two weeks after the last BrdU injection, mice were sacrificed by CO2-induced euthanasia and the hearts were excised for frozen section and double staining with anti-SA-α (mouse monoclonal, Sigma-Aldrich Co. LLC) and anti-BrdU (sheep polyclonal, Abcam, Cambridge, MA, USA) antibodies followed by DyLight-488-streptavidine (BioLegend, Inc., San Diego, CA, USA) or tetramethyl rhodamine isothiocyanate (TRITC)-conjugated secondary antibodies against mouse IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). Nuclei of the cells were counterstained with DAPI (Life Technologies, Molecular Probes, Carlsbad, CA, USA). DNA denaturation with HCl was performed to expose BrdU-incorporated sites and was neutralized with borate buffer (pH 9.0) before immunostaining. The whole ventricle was cut into longitudinal sections (8 µm thick), and the percentage of BrdU+ cardiomyocyte within the total DAPI-stained cells was quantified from six different sections containing infarcted region in each heart. IF images were captured with a Leica TCS SP5 confocal microscope system with ×40 and ×63 oil immersion objective lenses using 405 nm, 488 nm Ar, and 543 nm HeNe lasers. The total and BrdU+ cell number was quantified using the Image J software.
3. Results
3.1. In vivo expansion of Nkx2.5+ cardiomyoblasts following pharmacological inhibition of TGFβ receptors
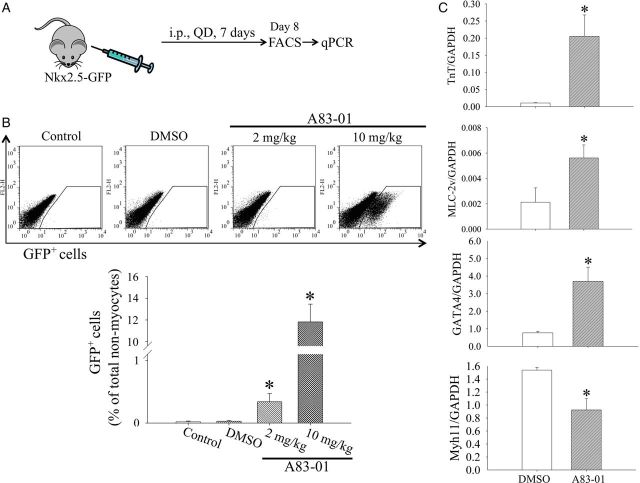
To assess whether the blockade of TGFβ signalling can lead to expansion of postnatal Nkx2.5+ cardiomyoblasts in vivo, we treated 8-week-old Nkx2.5-GFP mice with A83-01 (2 and 10 mg/kg/day) or DMSO (0.1%) by daily intraperitoneal injections for 7 days (Figure 1A). Untreated control mice were also studied in parallel. On Day 8, A83-01 and DMSO-treated hearts along with untreated hearts were harvested and enzymatically digested to deplete cardiomyocytes and quantitate the abundance of Nkx2.5+ cardiomyoblasts (Nkx2.5-GFP+ cells) within the heart. We found that cardiac GFP+ cardiomyoblasts represented 0.019 ± 0.012% of non-myocytes in adult mice in the absence of treatment and 0.029 ± 0.014% in the DMSO-injected group (Figure 1B). Interestingly, treatment with A83-01 at 2 mg/kg increased GFP+ cells in a dose-dependent fashion to 0.34 ± 0.13% of non-myocytes (n = 4, P < 0.05 vs. DMSO group) and 11.82 ± 1.64% of non-myocytes (n = 4, P < 0.05 vs. DMSO group) at 10 mg/kg, respectively (Figure 1B). To characterize further Nkx2.5+ cardiomyoblasts, we pulse-labelled FACS-purified GFP+ cells with BrdU and found an increase in BrdU uptake in A83-01-treated cells compared with DMSO-treated control (see Supplementary material online, Figure S2). In addition, we obtained total RNA from freshly isolated Nkx2.5+ cardiomyoblasts from in vivo A83-01- or DMSO-treated Nkx2.5-GFP mice for RT-qPCR analysis of cardiomyogenic gene expression. As shown in Figure 1C, the expression of cardiac TnT, cardiac myosin light chain 2 (MLC-2v), and Gata4 were markedly increased, whereas the expression of smooth muscle myosin heavy chain (Myh11) was decreased in the A83-01-treated group. Furthermore, we performed a genome-wide transcriptional analysis on Nkx2.5+ cardiomyoblasts with and without A83-01 treatment to examine the genes that are differentially-expressed in the absence of TGFβ signalling (see Supplementary material online, Figure S3 and S4). Interestingly, we found that the expression of cell cycle activators such as FGF receptor type 2 (FIGFR2), CDC42, and CCNE2, as well as trophic factors such as FIGF, and Birc5 were up-regulated significantly. We also found down-regulation among signalling molecules such as Wnt (e.g. Fzd6 and WISP1) and TGFβ (e.g. TGFβ1 and SMURF1) that support smooth muscle differentiation.11 These results confirm our finding that A83-01 treatment enhances Nkx2.5+ cardiomyoblast proliferation and cardiomyogenic differentiation.
Figure 1.
A83-01 treatment promotes expansion and cardiomyogenic differentiation of postnatal cardiac Nkx2.5-GFP+ cells in vivo. (A) Schematic diagram of drug administration in Nkx2.5-GFP mice (Tg+). Mice were injected daily with DMSO (1 mL/kg) or A83-01 at 2 or 10 mg/kg for 7 days. The hearts were harvested on Day 9 for flow cytometric analysis and FACS purification of GFP+ cells. (B) Representative flow cytometric plots (top) and quantification (bottom) of cardiac Nkx2.5-GFP+ cells (Nkx2.5+ cardiomyoblasts) from mice injected with either DMSO or A83-01 (10 mg/kg). Data represent mean ± SEM from four mice in each group. *P < 0.05 vs. DMSO group (by one-way ANOVA with post hoc test by Dunnett's test). (C) Quantitative PCR analysis of FACS-purified GFP+ cells for cardiac TnT, MLC2v, GATA-4, and Myh11 expression. *P < 0.05 vs. control (n = 3 in each group, by unpaired t-test).
3.2. shRNA knock-down of ALK5 recapitulates the effect of A83-01 in Nkx2.5+ cardiomyoblasts
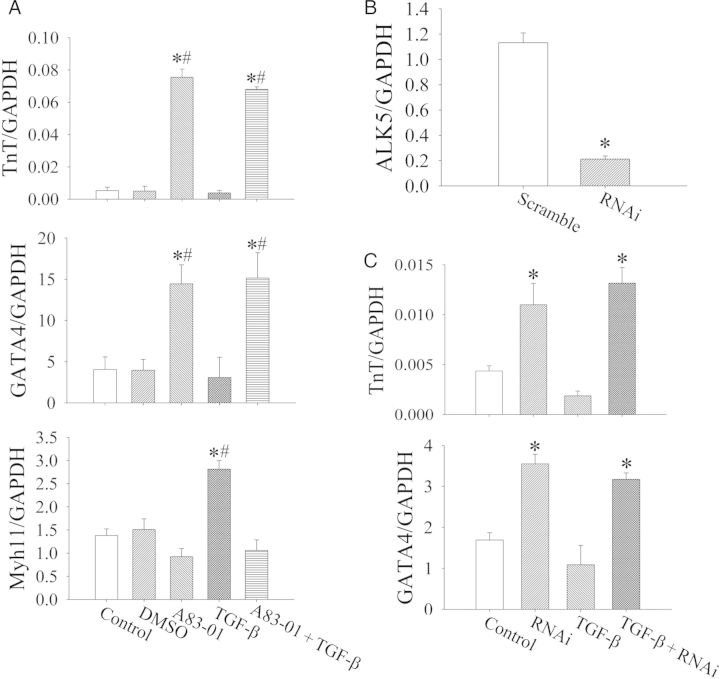
Since A83-01 is known to inhibit ALK4,5,7 receptors but ALK5 is the key mediator of TGFβ signalling, we investigated whether the shRNA-mediated knock-down of ALK5 alone would phenocopy the enhanced cardiomyogenic differentiation by A83-01 treatment. We isolated Nkx2.5+ cardiomyoblasts from neonatal hearts by FACS and treated them in vitro with lentiviruses expressing shRNA that target the ALK5 transcript. Following in vitro culture, we then assessed the expression profile of Nkx2.5+ cardiomyoblasts by quantitative PCR. We found that TGFβ1 treatment increases the expression of Myh11, consistent with our previous finding that TGFβ signalling activates smooth muscle cell differentiation.11 Furthermore, the blockade of TGFβ signalling with either A83-01 (Figure 2A) or ALK5-shRNA (Figure 2B) resulted in a similar degree of increase in cardiomyogenic differentiation as measured by the expression of cardiac TnT and Gata4. This suggests that the blockade of ALK5 signalling is the primary mechanism for the effect of A83-01 on Nkx2.5+ cardiomyoblast differentiation. Of note, there was no significant difference between DMSO-treated and medium-treated (Control) groups, and that the inhibitory effect of TGFβ1 treatment on cardiomyogenic differentiation (as measured by Gata4 and TnT expression) in Nkx2.5 cardiomyoblasts appears to be blunted. This reduced TGFβ1 inhibitory effect may be due to the existence of basal TGFβ1 signalling effects in these cells from TGFβ1 in the serum/media. Hence, the treatment of Nkx2.5 cardiomyoblasts with A83-01 or ALK5-targeted RNAi resulted in a greater change in Gata4 and TnT expression than additional treatment with TGFβ1 (Figure 2A and C).
Figure 2.
Analysis of cardiomyocyte and smooth muscle gene expression by Nkx2.5-GFP+ cells after exposure to TGFβ, A83-01, and ALK5-shRNA in vitro. (A) Treatment of FACS-purified Nkx2.5-GFP+ cells with A83-01 for 7 days increases the expression of TnT and Gata4, whereas treatment with TGFβ increases the expression of Myh11 without affecting TnT or Gata4 expression. *P < 0.05 vs. control and #P < 0.05 vs. DMSO by one-way ANOVA with the post hoc test by Dunnett's test (n = 3 in each group). (B) The effect of A83-01 treatment on Nkx2.5-GFP+ cells is recapitulated by shRNA-mediated knock-down of ALK5. (C) Note the increase in cardiac TnT and Gata4 expression when ALK5 expression is reduced by >75%. Results represent mean from three independent experiments. *P < 0.05 vs. scramble by the unpaired t-test (n = 3 in each group).
3.3. Cardiomyogenic differentiation of postnatal Nkx2.5+ cardiomyoblasts is enhanced by A83-01 treatment in an embryonic cardiomyocyte co-culture
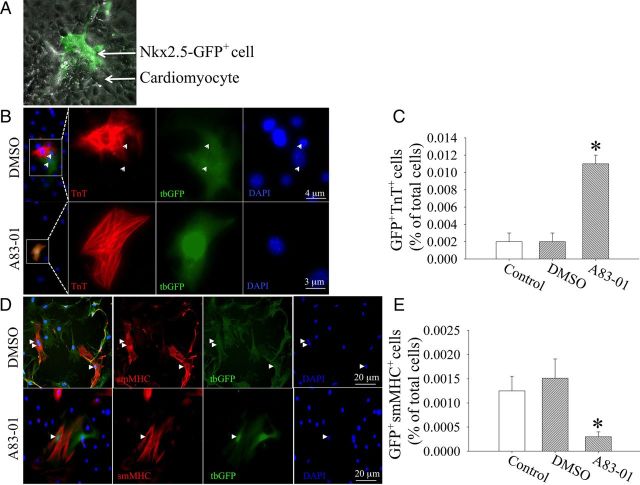
We have previously shown that neonatal Nkx2.5+ cardiomyoblast differentiation in vitro into beating cardiomyocytes requires the presence of embryonic cardiomyocytes in a co-culture.11 We asked whether A83-01 treatment can enhance cardiomyogenic differentiation of postnatal Nkx2.5+ cardiomyoblasts into striated cardiomyocytes in this co-culture assay. We first purified neonatal Nkx2.5-GFP+ cardiomyoblasts by FACS and then labelled these cells permanently with a lentiviral-turbo-GFP marker to distinguish these cells from the unlabelled embryonic cardiomyocytes because the expression of GFP driven by the Nkx2.5-enhancer diminishes during cardiomyocyte maturation.10 These turbo-GFP+ cells were then incubated with embryonic day 9.5 (e9.5) cardiomyocytes in the presence of either A83-01 or DMSO (Figure 3A). After 8 days of co-culture, the cell mixture was enzymatically dispersed into single cells and re-plated onto gelatin-coated glass slides. IF staining shows that the treatment with A83-01 resulted in an increase in GFP+TnT+ cells that exhibit striation (Figure 3B), whereas treatment with DMSO led to fewer GFP+TnT+ cells (P < 0.05, n = 3, from three independent experiment) as shown in Figure 3C. There was no significant difference in the percentage of GFP+TnT+ cells between DMSO (0.002 ± 0.001%, n = 3, from three independent experiment) and media-only treated (Control) groups (0.002 ± 0.001%, n = 3, from three independent experiment, P > 0.05 vs. DMSO; Figure 3C). With DMSO treatment, a notable number of GFP+ cardiomyoblasts could become smooth muscle myosin heavey chain (smMHC) (Figure 3D, upper panel), whereas treatment with A83-01 reduced the number of GFP+smMHC+ cells (P < 0.05, n = 3, from three independent experiment, as shown in Figure 3E).
Figure 3.
A83-01 treatment promotes cardiomyocyte differentiation of Nkx2.5+ cardiomyoblasts in a co-culture assay with embryonic cardiomyocytes. (A) FACS-purified Nkx2.5-GFP+ cells (arrowhead) were labelled with lentiviral-turbo-GFP and co-cultured with embryonic cardiomyocytes (arrows). (B) After 8 days in the presence of DMSO (0.1%) or A83 (1 µM), all cells were disbursed with collagenase into single cells and replated at low cell density on 0.1% gelatin. Cells were then fixed and immunostained for cardiac TnT (red). Note the single nuclei in GFP+TnT+ cell supporting the absence of cell fusion. (C) Quantification of GFP+TnT+ cells derived from A83-01 or DMSO treatment. *P < 0.05 vs. DMSO by one-way ANOVA with Dunnett's post hoc test (n = 3). Data obtained from three independent experiments. (D) Smooth muscle cell differentiation of Nkx2.5+ cardiomyoblasts (arrowheads). (E) Quantification of GFP+smMHC+ cells after A83-01 or DMSO treatment. *P < 0.05 vs. DMSO by one-way ANOVA with Dunnett's post hoc test (n = 3). Data obtained from three independent experiments.
3.4. Genetic fate mapping of Nkx2.5+ cardiomyoblasts after A83-01 treatment
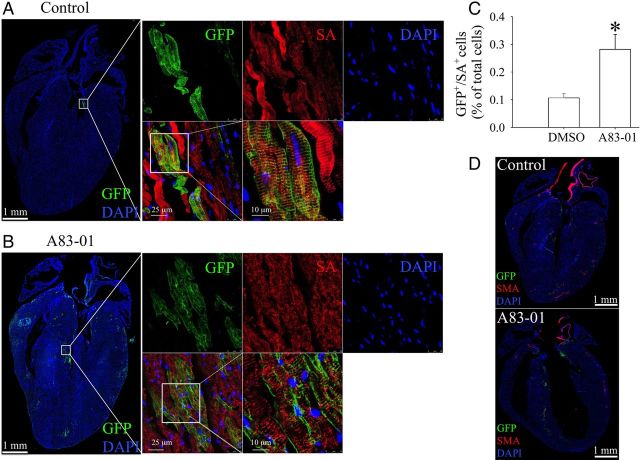
To determine whether A83-01 treatment can increase in vivo cardiomyogenesis by Nkx2.5+ cardiomyoblasts, we interbred previously described doxycycline-suppressible Nkx2.5 cardiac enhancer-Cre mice (Nkx2.5 enh-Cre) and ROSA26-mTmG reporter mice to generate double-transgenic Nkx2.5 enh-Cre/ROSA26-mTmG mice that can lineage trace Nkx2.5+ cardiomyoblasts and their progeny in a doxycycline-regulated fashion. We first treated the pregnant females and all descendants with doxycycline (1 mg/mL in drinking water) from conception until 1 week before A83-01 treatment to prevent embryonic labelling of Nkx2.5+ cardiomyoblasts during early development. We then stopped doxycycline treatment at 1 week before drug treatment to allow Nkx2.5+ cardiomyoblasts labelling in the postnatal heart. This was followed by treatment with either DMSO (1 mL/kg, daily, ip, n = 3) or A83-01 (10 mg/kg, daily, ip, n = 3) for 7 days. At 7 days after the final injection, hearts were then harvested for frozen sectioning and IF analysis. We found that there is no significant difference in the ratio of heart weight over body weight between DMSO-treated (5.75 ± 0.06 mg/g, n = 3) and A83-01-treated (5.87 ± 0.17 mg/g, n = 3) groups (P > 0.1). Interestingly, we found that A83-01 treatment compared with DMSO treatment resulted in an approximately two-fold increase in the number of double GFP+/SA-α+ cells (Figure 4A–C). This was assessed quantitatively by measuring the percentage of double GFP+/SA-α+ cells within the total nuclei counted from six sections in each heart. Interestingly, little to no double GFP+/SMA-α+ smooth muscle cell was observed in 36 cardiac sections from 6 hearts, suggesting a low baseline capacity for smooth muscle differentiation in vivo (Figure 4D).
Figure 4.
A83-01 treatment enhances the expansion and cardiomyogenic differentiation of Nkx2.5+ cardiomyoblasts in vivo. Eight-week-old double-transgenic Nkx2.5 enh-Cre and ROSA26-flox-stop-flox-mTmG reporter mice (Nkx2.5 enh-Cre/ROSA26-mTmG) were treated with doxycycline from conception to 1 week before A83-01 treatment to suppress Cre labelling of embryonic Nkx2.5+ cardiomyoblasts. A83-01 (10 mg/kg, i.p.) or DMSO was then administrated daily for 7 days. At 7 days after the last drug injection, each mouse heart was harvested for frozen section and fluorescence imaging. Nuclei were counterstained with DAPI. Cardiomyocytes were stained by primary antibody against SA-α and Allophycocyanin (APC)-conjugated secondary antibody. Cells expressing GFP were resolved by antibody against GFP with Alexa 488-conjugated secondary antibody. The whole heart images of the fluorescence signals including DAPI, GFP, and APC were respectively scanned via ×20 objective lens under the control of TissueFAXS system, and then re-built the whole heart pictures for further analysis by TissueQuest. The representative superimposed fluorescent images of DAPI and GFP signals from (A) DMSO-treated and (B) A83-01-treated mouse heart are shown. The magnified pictures in the white box were acquired by confocal microscopy with the magnification lens of ×40 or ×100× to confirm that these cells are derived from GFP+ Nkx2.5 cardiomyoblast. (C) Quantification of the percentage of GFP+/SA-α+ cell number from total nuclei in each section by TissueQuest. *P < 0.05 vs. control by the unpaired t-test (n = 3 hearts in each treated group). (D) Smooth muscle cells were stained by primary antibody against smooth muscle actin-α (SMA-α) with an APC-conjugated secondary antibody. Data shown are representative from six sections in each heart and three different hearts per group.
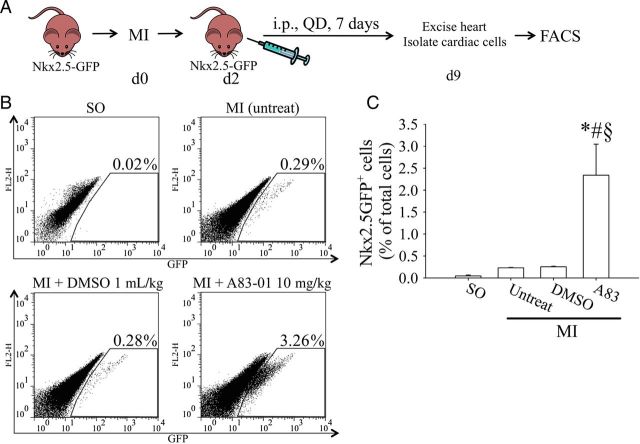
3.5. A83-01 treatment following MI results in the expansion of Nkx2.5+ cardiomyoblasts
Given the ability of A83-01 to induce proliferation in Nkx2.5+ cardiomyoblasts in an uninjured postnatal heart (Figure 1), we asked whether A83-01 treatment could also expand Nkx2.5+ cardiomyoblasts following experimental MI. We performed LAD ligation in 8-week-old Nkx2.5-GFP transgenic mice to induce MI and then administered A83-01 or DMSO once daily for 7 days (Figure 5A). The hearts were then harvested and enzymatically dispersed into single cell suspension for quantification of Nkx2.5+ cardiomyoblast population via flow cytometry. In normal age-matched mice, the percentage of cardiac Nkx2.5+ cardiomyoblast was not different between DMSO-treated (0.018 ± 0.01%) and untreated (0.016 ± 0.01%) groups (Figure 1) compared with sham-operated (SO) mice (0.018 ± 0.002% of non-myocyte fraction, n = 4, P > 0.05; Figure 5B). MI treatment caused a ∼15-fold increase in GFP+ cells with or without DMSO treatment (Figure 5B). Furthermore, treatment with A83-01 (10 mg/kg) in post-MI mice resulted in an additional nine-fold increase in Nkx2.5+ cardiomyoblasts compared with DMSO treatment (n = 4, P < 0.05).
Figure 5.
A83-01 treatment promotes cardiac Nkx2.5-GFP+ cell expansion post-MI. (A) Diagram of experimental set-up and drug treatment. Nkx2.5-GFP+ mice were given daily injections of DMSO (1 mL/kg) or A83-01 (10 mg/kg) starting from the second day following MI for 7 days. The heart was then isolated and the GFP+ cells quantified by flow cytometry. (B) Representative flow cytometry plots of Nkx2.5-GFP+ heart cells after DMSO- or A83-01 treatment following MI (left panels). (C) Quantification of the percentage of GFP+ cells (right panel). *P < 0.05 vs. SO group, #P < 0.05 vs. untreated-MI-group, §P < 0.05 vs. DMSO-MI-group by one-way ANOVA with the Bonferroni post hoc test (n = 4 hearts in each group).
3.6. Treatment with A83-01 improves cardiac function after MI
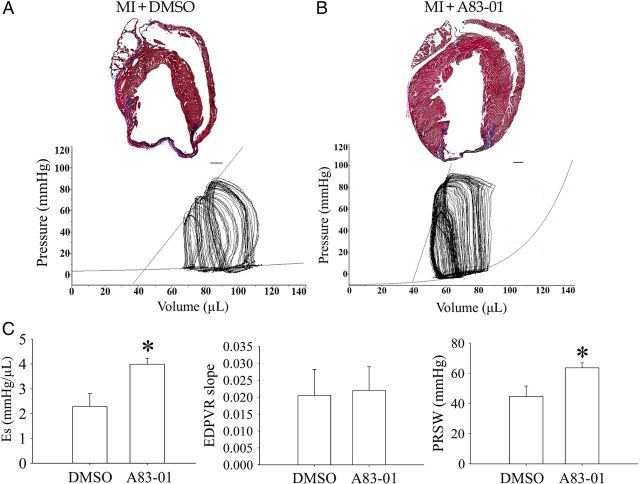
Since A83-01 treatment led to an expansion of Nkx2.5+ cardiomyoblasts in postnatal hearts (Figure 5B) and enhanced their cardiomyogenic differentiation (Figures 2B and 4C), we postulate that the treatment of hearts with A83-01 after MI may lead to improvement in cardiac function. To examine this, wild-type mice underwent left coronary ligation and were assigned randomly to either DMSO (1 mL/kg, n = 4) or A83-01 treatment (10 mg/kg, n = 4) for 7 days via daily intraperitoneal injection. Their LV pressure–volume relationship was examined by catheter-based physiological examination. We confirmed that both DMSO- and A83-01-treated hearts show evidence of massive infarction and fibrosis using Masson's trichrome staining (Figure 6A). Interestingly, the overall myocardial area in A83-01-treated hearts was ∼26.7% larger than that in the DMSO-treated group (390 517 ± 10 166 vs. 307 726 ± 13 099 pixels, P < 0.05, n = 4; Figure 6A and B).
Figure 6.
Quantification of fibrosis and haemodynamic parameters in DMSO- and A83-01-treated hearts following MI. (A) Typical image of Masson's trichrome staining of heart sections from wild-type mice treated with DMSO following MI (upper panel). Representative pressure–volume loops of DMSO-treated mice hearts (lower panel). (B) Same as (A) except that mice were treated with A83-01 injections instead of DMSO. A total of six sections were stained from each heart and three hearts were examined in each group. (C) Comparison of cardiac characteristics, including ventricular contractility (Es), stiffness (EDPVR slope), and preload-recruitable stroke work (PRSW), between DMSO- and A83-01-treated groups. The regression curves of ESPVR and EDPVR were fitted. *P < 0.05 for A83-01 vs. DMSO group by the unpaired t-test (n = 4 hearts in each group).
To determine whether the increase in myocyte area correlated with an improvement in cardiac haemodynamic parameters, we measured the pressure–volume loops during a brief duration of abdominal inferior vena cava occlusion and plotted the regression curves for ESPVR and EDPVR. The cardiac haemodynamic properties of DMSO-treated mice exhibited a significant decrease in the maximum and minimum rate of change in LV pressure (dP/dt) (i.e. contractility) after MI. On the other hand, the contractile function was better preserved in the A83-01-treated mice (Figure 6B). Figure 6C shows that ventricular elastance (Es, the slope of ESPVR) and PRSW were significantly increased in the A83-01-treated group without any change in ventricular stiffness (the slope of EDPVR). While there was a trend towards increased stroke volume, cardiac output, and stroke work in A83-01-treated animals, these did not reach statistical significance (Table 1).
Table 1.
Cardiac haemodynamic parameters of DMSO- or A83-01-treated mice following MI
| Sham OP | DMSO | A83-01 | |
|---|---|---|---|
| HR (bpm) | 333.1 ± 14.7 | 352.7 ± 22.7 | 410.6 ± 22.0 |
| ESP (mmHg) | 115.6 ± 3.7 | 84.5 ± 3.8# | 85.7 ± 8.3# |
| EDP (mmHg) | 6.6 ± 0.4 | 4.6 ± 0.3# | 3.3 ± 0.3# |
| dP/dt, max | 8195.5 ± 530.7 | 5954.5 ± 342.7# | 7859.6 ± 830.5 |
| dP/dt, min | −6382.5 ± 316.7 | −4046.7 ± 506.9# | −6411.0 ± 339.7* |
| SV (µL) | 29.2 ± 3.1 | 21.3 ± 1.9# | 27.9 ± 4.2 |
| CO (µL) | 9595.1 ± 1467.9 | 7446.8 ± 226.8# | 11 649.2 ± 2200.4 |
| SW (mJ) | 0.415 ± 0.041 | 0.151 ± 0.003# | 0.236 ± 0.044# |
| Es (mmHg/µL) | 4.4 ± 0.2 | 2.3 ± 0.3# | 3.9 ± 0.2* |
| EDPVR slope (mmHg/µL) | 0.026 ± 0.014 | 0.021 ± 0.008 | 0.022 ± 0.007 |
| PRSW (mmHg) | 76.2 ± 2.7 | 44.6 ± 4.9# | 63.6 ± 3.0*,# |
HR, heart rate; ESP, end-systolic pressure; EDP, end-diastolic pressure; dP/dt, max, maximum rate of change in LV pressure; dP/dt, min, minimum rate of change in LV pressure; SV, stroke volume; CO, cardiac output; SW, stroke work.
*P < 0.05 vs. DMSO and #P < 0.05 vs. sham operation (sham op) (n = 4 in each group) by one-way ANOVA with Bonferroni post hoc tests.
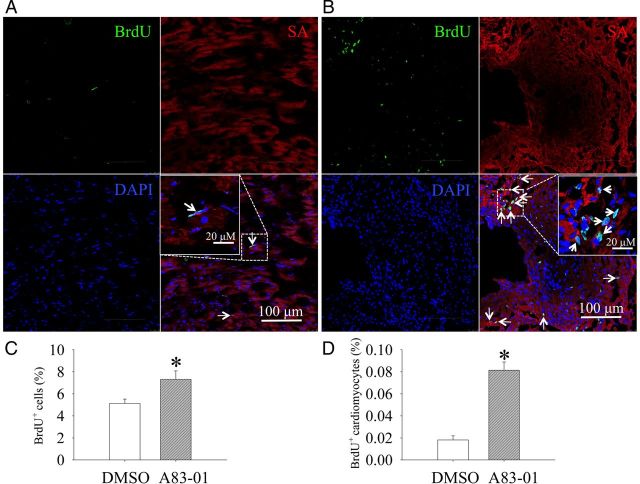
3.7. A83-01 treatment increases BrdU+ labelling of Nkx2.5+ cardiomyoblasts and cardiomyocytes in post-MI mice
To determine whether A83-01 treatment in vivo increases the proliferation rate of cardiomyoblasts and their cardiomyocyte progenies, we performed a pulse labelling experiment on post-MI mice hearts during the last 3 days of A83-01 treatment. Figures 7A and B shows the typical appearance of BrdU+ cells in the border zone of the post-MI mice treated with DMSO or A83-01. We found that A83-01-treatment significantly increased the total number of BrdU+ cells (Figure 7C), including BrdU+ cardiomyocytes (Figure 7D) in the left ventricle of post-MI hearts.
Figure 7.
A83-01 treatment increased the number of BrdU+ cardiomyocytes in post-MI mice. The treatment of adult mice with DMSO (A) and A83-01 (B) followed by BrdU resulted in the labelling of newly formed cardiomyocytes (white arrows) that also express SA-α (red) and incorporated BrdU+ (green) in the border zone of infarct myocardium. Nuclei of the cells were counterstained with DAPI. The percentage of (C) BrdU+ cells and (D) BrdU+ and SA+ myocytes in the MI hearts was quantified as the percentage of the total cells from six different sections per heart. *P < 0.05 for A83-01 vs. DMSO group by the unpaired t-test (n = 6 mouse hearts in each group).
4. Discussion
The present study explored the effect of A83-01 treatment on Nkx2.5+ cardiomyoblast proliferation and expansion in vivo and their ability to improve cardiac contractility. We found that A83-01 treatment resulted in a significant expansion of Nkx2.5+ cardiomyoblasts in the postnatal heart and formed new cardiomyocytes that ultimately improved cardiac function. We also confirmed the ability of TGFβ1 to induce smooth muscle cell differentiation by Nkx2.5+ cardiomyoblasts in vitro and participate in the post-infarct remodelling by promoting cardiac fibrosis.25,26 We anticipate that pharmacological modulation of Nkx2.5+ cardiomyoblasts may help improve cardiac function after MI and may be clinically useful in the setting of ischaemic cardiomyopathy.
We showed previously that TGFβ1 treatment promoted Nkx2.5+ cardiomyoblasts to undergo smooth muscle cell differentiation in vitro as evident by the increased expression of smooth muscle myosin heavy chain. On the other hand, the addition of A83-01 to TGFβ1-treated cells inhibited smooth muscle cell differentiation and increased cell proliferation.11 Here, we demonstrate that A83-01 treatment to FACS-purified Nkx2.5+ cardiomyoblasts enhanced their differentiation into cardiomyocytes in the absence of cell fusion with co-cultured cardiomyocytes. The results from the genetic fate mapping studies using Nkx2.5 enh-Cre/mTmG mice and post-MI hearts with Masson's trichrome staining showed that A83-01 treatment led to an increase in overall cardiomyocyte area. This finding was further supported by studies in the injured heart where A83-01 was found to significantly increase the population of Nkx2.5+ cardiomyoblasts. However, it should be recognized that the cell cycle rate found in the infarcted heart is too low to account for the difference in the overall architecture and function in the A83-01-treated animals. Despite finding an increase in new cardiomyocytes derived from our lineage-labelled Nkx2.5+ cardiomyoblasts in the Nkx2.5 enh-Cre/ROSA25-mTmG mice, this amount remains quite low and the data from our lineage tracing studies alone cannot distinguish between the direct effects of A83-01 on cardiomyoblast proliferation and subsequent cardiomyogenic differentiation in vivo from its indirect effects on other non-myocytes (e.g. smooth muscle cell and endothelial cells) that supports cardiomyocyte survival or expansion. It is likely that effects from A83-01 on cells other than Nkx2.5 cardiomyoblasts are responsible for a significant portion of the overall improvement in cardiac function, given the recent finding that very low contribution of new cardiomyocyte in the adult heart comes from non-cardiomyocytes.27 Nevertheless, we believe that the increase in the percentage of GFP+ cardiomyoblasts should play at least a contributory role in myocardial repair process.
The finding that modulation of TGFβ signalling pathway with an ALK4,5,7 inhibitor can increase cardiomyogenesis raises interesting questions regarding the mechanism of this effect. TGFβ expression is rapidly up-regulated during early stages of MI and pressure-overload25,26,28–34 and in patients with dilated or hypertrophic cardiomyopathy.25,26 In the infarcted heart, TGFβ deactivates inflammatory macrophages while promoting fibroblast expansion and matrix synthesis as a part of its fibrogenic actions via a Smad3-dependent pathway.25,26 A83-01 was administrated on Day 3 after passing the acute inflammatory stage of MI. We show that A83-01 acts directly on postnatal Nkx2.5+ cardiomyoblasts to increase proliferation (Figure 2), and this effect is associated with the inhibition of Smad2/3 phosphorylation (see Supplementary material online, Figures S5 and S7). The expansion and subsequent cardiomyogenic differentiation of Nkx2.5+ cardiomyoblasts likely contributed to the maintenance of ventricular wall thickness and overall cardiac function in post-MI mice (Table 1). These results raise the prospect that modulation of TGFβ signalling may represent a novel therapeutic strategy for endogenous repair and regeneration of the infarcted myocardium.
In Nkx2.5+ cardiomyoblasts, A83-01 treatment appears to up-regulate FIGFR2, cell cycle genes (CDC42 and CCNE2), and trophic factors (FIGF and Birc5), and to down-regulate signalling molecules along the Wnt (Fzd6 and WISP1) and TGFβ1 (TGFβ1, SMURF1) pathways (see Supplementary material online, Figures S3 and S4). These changes may be responsible for the proliferative effects of A83-01 on Nkx2.5+ cardiomyoblasts. To further understand the mechanism of cardiac functional improvement with A83-01 treatment, we examined the effect of ALK receptor silencing on cardiomyogenic differentiation as measured by cardiac TnT and Gata4 expression in vitro (Figure 2) and Smad phosphorylation in vitro and in vivo (see Supplementary material online, Figures S5–S7). Our results showed that ALK5 receptor inhibition promoted cardiomyocyte differentiation of Nkx2.5 cardiomyoblasts which, interestingly, correlated with the result from in vivo treatment of mouse hearts with A83-01, where it lead to expansion of Nkx2.5+ cardiomyoblasts and their cardiomyocyte differentiation based on our in vivo genetic fate mapping studies (Figure 4). It is worth noting that TGFβ1 treatment has been described to increase Gata4 expression in colon cancer cells and promote cardiomyocyte differentiation (as measured by increased Gata4 expression) by increasing mesodermal induction.35,36 This apparent paradox with our data showing that inhibition instead of stimulation of ALK5 receptor leads to greater Gata4 expression is most likely due to the context-dependent effect of TGFβ1 signalling on cells.35,37 These findings support the notion that ALK receptor inhibition with A83-01 could promote the cardiomyogenic differentiation of cardiac Nkx2.5-GFP+ cells.
In conclusion, we found a remarkable ability for A83-01, an inhibitor of ALK receptors, to expand postnatal Nkx2.5+ cardiomyoblasts and promote their cardiomyogenic differentiation in adult mice. In post-infarct hearts, A83-01 may also reduce adverse cardiac remodelling by increasing the number of functional cardiomyocytes in situ. These data support a role for pharmacological inhibition of TGFβ signalling as a treatment strategy for post-MI heart failure in the future.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work is supported by NIH/NHLBI (K08 HL081086 and U01 HL009976-05) NIH Director's New Innovator's Award DP2 OD004411, and endowed Faculty Scholar Award from Stanford Child Health Research Institute (to S.M.W.) and Taiwan Ministry of Science and Technology (NSC-100-B2-003 and NSC-101-2325-B002-025 to W.-P.C.).
Acknowledgements
We thank Marcus Vallaster, Rebecca Feistritzer, and Karolina Plonowska for assistance with preparing cardiac samples and immunostaining.
Conflict of interest: none declared.
References
- 1.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 2.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P, Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci USA. 2006;103:9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang GN, Thatcher JE, McAnally J, Kong Y, Qi X, Tan W, DiMaio JM, Amatruda JF, Gerard RD, Hill JA, Bassel-Duby R, Olson EN. C/EBP transcription factors mediate epicardial activation during heart development and injury. Science. 2012;338:1599–1603. doi: 10.1126/science.1229765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfister O, Oikonomopoulos A, Sereti KI, Sohn RL, Cullen D, Fine GC, Mouquet F, Westerman K, Liao R. Role of the ATP-binding cassette transporter Abcg2 in the phenotype and function of cardiac side population cells. Circ Res. 2008;103:825–835. doi: 10.1161/CIRCRESAHA.108.174615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudat C, Kispert A. Wt1 and epicardial fate mapping. Circ Res. 2012;111:165–169. doi: 10.1161/CIRCRESAHA.112.273946. [DOI] [PubMed] [Google Scholar]
- 6.Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, Pu WT, Riley PR. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unno K, Jain M, Liao R. Cardiac side population cells: moving toward the center stage in cardiac regeneration. Circ Res. 2012;110:1355–1363. doi: 10.1161/CIRCRESAHA.111.243014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu SM, Chien KR, Mummery C. Origins and fates of cardiovascular progenitor cells. Cell. 2008;132:537–543. doi: 10.1016/j.cell.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Chen WP, Wu SM. Small molecule regulators of postnatal nkx2.5 cardiomyoblast proliferation and differentiation. J Cell Mol Med. 2012;16:961–965. doi: 10.1111/j.1582-4934.2011.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 14.Derynck R. TGF-beta-receptor-mediated signaling. Trends Biochem Sci. 1994;19:548–553. doi: 10.1016/0968-0004(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 15.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang JS, Liu C, Derynck R. New regulatory mechanisms of TGF-beta receptor function. Trends Cell Biol. 2009;19:385–394. doi: 10.1016/j.tcb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Derynck R, Zhang YE. Smad-dependent and smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 19.Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 20.Goumans MJ, Liu Z, ten Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 21.Graham H, Peng C. Activin receptor-like kinases: structure, function and clinical implications. Endocr Metab Immune Disord Drug Targets. 2006;6:45–58. doi: 10.2174/187153006776056585. [DOI] [PubMed] [Google Scholar]
- 22.Lebrin F, Deckers M, Bertolino P, Ten Dijke P. TGF-beta receptor function in the endothelium. Cardiovasc Res. 2005;65:599–608. doi: 10.1016/j.cardiores.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 23.Tojo M, Hamashima Y, Hanyu A, Kajimoto T, Saitoh M, Miyazono K, Node M, Imamura T. The ALK-5 inhibitor A-83-01 inhibits Smad signaling and epithelial-to-mesenchymal transition by transforming growth factor-beta. Cancer Sci. 2005;96:791–800. doi: 10.1111/j.1349-7006.2005.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan SM, Zhang Y, Connelly KA, Gilbert RE, Kelly DJ. Targeted inhibition of activin receptor-like kinase 5 signaling attenuates cardiac dysfunction following myocardial infarction. Am J Physiol Heart Circ Physiol. 2010;298:H1415–H1425. doi: 10.1152/ajpheart.01048.2009. [DOI] [PubMed] [Google Scholar]
- 29.Okada H, Takemura G, Kosai K, Li Y, Takahashi T, Esaki M, Yuge K, Miyata S, Maruyama R, Mikami A, Minatoguchi S, Fujiwara T, Fujiwara H. Postinfarction gene therapy against transforming growth factor-beta signal modulates infarct tissue dynamics and attenuates left ventricular remodeling and heart failure. Circulation. 2005;111:2430–2437. doi: 10.1161/01.CIR.0000165066.71481.8E. [DOI] [PubMed] [Google Scholar]
- 30.Deten A, Holzl A, Leicht M, Barth W, Zimmer HG. Changes in extracellular matrix and in transforming growth factor beta isoforms after coronary artery ligation in rats. J Mol Cell Cardiol. 2001;33:1191–1207. doi: 10.1006/jmcc.2001.1383. [DOI] [PubMed] [Google Scholar]
- 31.Li JM, Brooks G. Differential protein expression and subcellular distribution of TGFbeta1, beta2 and beta3 in cardiomyocytes during pressure overload-induced hypertrophy. J Mol Cell Cardiol. 1997;29:2213–2224. doi: 10.1006/jmcc.1997.0457. [DOI] [PubMed] [Google Scholar]
- 32.Wei H, Bedja D, Koitabashi N, Xing D, Chen J, Fox-Talbot K, Rouf R, Chen S, Steenbergen C, Harmon JW, Dietz HC, Gabrielson KL, Kass DA, Semenza GL. Endothelial expression of hypoxia-inducible factor 1 protects the murine heart and aorta from pressure overload by suppression of TGF-beta signaling. Proc Natl Acad Sci USA. 2012;109:E841–E850. doi: 10.1073/pnas.1202081109. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Meyer A, Wang W, Qu J, Croft L, Degen JL, Coller BS, Ahamed J. Platelet TGF-beta1 contributions to plasma TGF-beta1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood. 2012;119:1064–1074. doi: 10.1182/blood-2011-09-377648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, Mankowski J, Zhang D, Nakamura T, Takimoto E, Kass DA. Pivotal role of cardiomyocyte TGF-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121:2301–2312. doi: 10.1172/JCI44824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai W, Guzzo RM, Wei K, Willems E, Davidovics H, Mercola M. A nodal-to-TGFbeta cascade exerts biphasic control over cardiopoiesis. Circ Res. 2012;111:876–881. doi: 10.1161/CIRCRESAHA.112.270272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haveri H, Ashorn M, Iltanen S, Wilson DB, Andersson LC, Heikinheimo M. Enhanced expression of transcription factor GATA-4 in inflammatory bowel disease and its possible regulation by TGF-beta1. J Clin Immunol. 2009;29:444–453. doi: 10.1007/s10875-009-9292-x. [DOI] [PubMed] [Google Scholar]
- 37.Munoz NM, Baek JY, Grady WM. TGF-beta has paradoxical and context dependent effects on proliferation and anoikis in human colorectal cancer cell lines. Growth Factors. 2008;26:254–262. doi: 10.1080/08977190802291667. [DOI] [PMC free article] [PubMed] [Google Scholar]