This is the first assessment of microbiologic changes associated with ivacaftor treatment of cystic fibrosis patients with the G551D mutation, and demonstrates a significant reduction in Pseudomonas aeruginosa, suggesting that cystic fibrosis transmembrane conductance regulator modulation may modify disease course by altering respiratory microbiology.
Keywords: cystic fibrosis, CFTR modulator, ivacaftor, P. aeruginosa
Abstract
Background. Ivacaftor improves outcomes in cystic fibrosis (CF) patients with the G551D mutation; however, effects on respiratory microbiology are largely unknown. This study examines changes in CF respiratory pathogens with ivacaftor and correlates them with baseline characteristics and clinical response.
Methods. The G551D Observational Study enrolled a longitudinal observational cohort of US patients with CF aged 6 years and older with at least 1 copy of the G551D mutation. Results were linked with retrospective and prospective culture data in the US Cystic Fibrosis Foundation's National Patient Registry. Pseudomonas aeruginosa infection category in the year before and year after ivacaftor was compared and correlated with clinical findings.
Results. Among 151 participants prescribed ivacaftor, 29% (26/89) who were culture positive for P. aeruginosa the year prior to ivacaftor use were culture negative the year following treatment; 88% (52/59) of those P. aeruginosa free remained uninfected. The odds of P. aeruginosa positivity in the year after ivacaftor compared with the year prior were reduced by 35% (odds ratio [OR], 0.65; P < .001). Ivacaftor was also associated with reduced odds of mucoid P. aeruginosa (OR, 0.77; P = .013) and Aspergillus (OR, 0.47; P = .039), but not Staphylococcus aureus or other common CF pathogens. Patients with intermittent culture positivity and higher forced expiratory volume in 1 second (FEV1) were most likely to turn culture negative. Reduction in P. aeruginosa was not associated with change in FEV1, body mass index, or hospitalizations.
Conclusions. Pseudomonas aeruginosa culture positivity was significantly reduced following ivacaftor treatment. Efficacious CFTR modulation may contribute to lower frequency of culture positivity for P. aeruginosa and other respiratory pathogens, particularly in patients with less established disease.
Cystic fibrosis (CF) is an ion transport disorder resulting from heritable mutations of the cystic fibrosis transmembrane conductance regulator (CFTR) gene. The disease is characterized by inadequate mucociliary clearance of the lung, chronic infection, inflammation, and structural damage of the airways; most CF patients eventually experience lung function decline and premature death [1]. The CFTR potentiator ivacaftor treats the protein defect in CF patients with a G551D or other gating mutation by improving CFTR function, augmenting anion transport and reducing sweat chloride concentrations—a diagnostic criterion for CF [2, 3]. Clinical trials evaluating ivacaftor in CF patients with the G551D CFTR mutation showed significant improvements in lung function, exacerbation rate, weight gain, and CF-specific quality-of-life measures [2, 4], leading to drug approval and widespread use in the US population.
Pseudomonas aeruginosa is one of the hallmark pathogens in the CF lung and is strongly associated with increased morbidity [5–7] and mortality [8]. Preliminary findings from an observational study of ivacaftor in CF patients with at least 1 copy of the G551D mutation (the G551D ObservationAL [GOAL] study) indicated a marked decrease in P. aeruginosa culture positivity after initiation of ivacaftor [9]. The objectives of this analysis were to (1) comprehensively describe microbiologic changes in common CF pathogens, including P. aeruginosa, in G551D participants after starting ivacaftor; (2) determine baseline clinical characteristics associated with reduced frequency of P. aeruginosa culture positivity after initiation of ivacaftor; and (3) examine whether changes in P. aeruginosa positivity were associated with clinical outcomes following initiation of ivacaftor. We hypothesized that ivacaftor treatment would be associated with a significant reduction in P. aeruginosa culture positivity.
METHODS
Study Design
A longitudinal observational cohort study was initiated in 2012 at 28 US Cystic Fibrosis Therapeutic Development Network–accredited sites to follow CF patients aged ≥6 years with at least 1 copy of the G551D mutation [9]. Each participant/guardian gave written informed consent, and site institutional review boards approved the study. All enrolled participants had no prior exposure to ivacaftor. Upon the decision to prescribe ivacaftor, clinical assessments included spirometry, weight, height, and sweat chloride analysis at baseline and at 1, 3, and 6 months after ivacaftor initiation. Study data were augmented by linked microbiology, hospitalization, and CF medication data collected in the Cystic Fibrosis Foundation's National Patient Registry (CFFNPR) [10]. Investigators were encouraged to follow CF treatment guidelines [11, 12] including surveillance respiratory cultures every 3 months or with change in clinical status.
Outcomes and Analysis Variables
CFFNPR data from the year before and the year after ivacaftor initiation were used to ascertain number of respiratory cultures, specimen type (sputum or oropharyngeal [OP] swab), number of culture positives for CF pathogens (P. aeruginosa, mucoid P. aeruginosa, methicillin-susceptible Staphylococcus aureus [MSSA], methicillin-resistant Staphylococcus aureus [MRSA], Stenotrophomonas maltophilia, Haemophilus influenzae, Burkholderia cepacia complex, and Aspergillus species), number of hospitalizations (for any reason), number of pulmonary exacerbations (treated in hospital or via home intravenous antibiotics), and use of maintenance CF therapies. Respiratory culture data from 2 years before ivacaftor were also obtained to serve as a control comparator.
Participants' P. aeruginosa infection categories were defined within each year interval according to modified Leed criteria as infection free (no positive cultures), intermittent infection (≤50% culture positive), or persistent infection (>50% culture positive) [13]. Because these criteria are dependent on the number of cultures performed, a sensitivity analysis was done on the subset of participants with ≥3 cultures in each year. For associations with clinical outcomes, each participant was categorized as either experiencing a reduction in P. aeruginosa after initiation of ivacaftor (eg, shift of P. aeruginosa infection category from persistent to intermittent or free, or intermittent to free) or as experiencing no change or increased frequency of positive cultures.
The change in forced expiratory volume in 1 second (FEV1) percentage predicted [14, 15] and body mass index (BMI) were calculated from baseline through 12 months after starting ivacaftor, whereas change in sweat chloride was calculated from baseline through 6 months. Hospitalization rate (for any reason) for each participant each year (pre- and postivacaftor) accounted for observed follow-up time, as did pulmonary exacerbation rate (treated in hospital or at home with intravenous antibiotics).
Statistical Analysis
Summary statistics (eg, mean, standard deviation [SD], proportion) were used to describe the cohort. Analysis of variance, t test, and Fisher exact test were used to compare groups, and McNemar test for paired data was used to compare pre-ivacaftor to post-ivacaftor microbiologic findings, including shift in P. aeruginosa infection category, which tests the null hypothesis of no distributional change in category by time period. Repeated measures logistic regression with a compound symmetric error structure for each individual estimated the odds of culture positivity, accounting for number of and type of respiratory culture specimens, and use of concomitant chronic CF therapies.
Sensitivity analyses were performed that assumed those participants with missing respiratory cultures or with <3 cultures in the year post-ivacaftor had positive cultures for P. aeruginosa at the missing time points. Multivariable regression was used to determine the association of change in P. aeruginosa (infection category and continuous reduction) after ivacaftor with changes in FEV1, BMI, and hospitalization rate—adjusting for sex, age, P. aeruginosa in year prior, baseline FEV1, and baseline sweat chloride. All covariates were selected a priori as known potential predictors of clinical response. P values and confidence intervals (CIs) are 2-sided, with .05 significance level; analyses were performed with SAS software, version 9.2 (SAS Institute, Cary, North Carolina).
RESULTS
Cohort Description
There were 153 participants in the GOAL study; 2 did not receive ivacaftor and were not analyzed further; thus, 151 were prescribed and initiated ivacaftor from February through September 2012. The cohort was 46% female and 54% aged ≥18 years. Lung function distribution showed that 30% had baseline FEV1 <70% predicted, 26% were 70%–89% and 90% or higher predicted, and 44% had a baseline FEV1 90% or higher predicted; more detailed characteristics are reported elsewhere [9]. Median follow-up in the CFFNPR at the time of analysis was 12.5 months after initiation of ivacaftor. Seventy-six percent (n = 115) had ≥10 months’ follow-up in the year after ivacaftor initiation. Baseline characteristics presented by P. aeruginosa infection category in the year before ivacaftor are presented in Table 1. Among the 148 of 151 participants (98%) with respiratory cultures in the year prior to ivacaftor, 40% (59/148) had persistent infection (>50% of cultures in the year P. aeruginosa positive), 20% (30/148) intermittent infection (1%–50% of cultures P. aeruginosa positive), and 40% (59/148) were infection free. Participants with persistent infection were older, had lower FEV1, and higher hospitalization rates at baseline (Table 1).
Table 1.
Characteristics of Study Participants Prior to Ivacaftor Initiation, by Pseudomonas aeruginosa Infection Category
| Characteristic |
Pa Infection Category in Year Prior to Ivacaftora |
NAb (n = 3) | P Value | ||
|---|---|---|---|---|---|
| Free (n = 59) | Intermittent (n = 30) | Persistent (n = 59) | |||
| Female | 26 (44.1) | 17 (56.7) | 27 (45.8) | 0 (0.0) | .26 |
| Age, mean (SD) | 18.3 (11.5) | 17.6 (11.3) | 25.6 (10.1) | 23.0 (3.3) | .001 |
| 6–11 y | 22 (37.3) | 10 (33.3) | 6 (10.2) | 0 (0) | <.001 |
| 12–17 y | 16 (27.1) | 8 (26.7) | 8 (13.6) | 0 (0) | |
| ≥18 y | 21 (35.6) | 12 (40.0) | 45 (76.3) | 3 (100) | |
| FEV1 % predicted at baseline, mean (SD) | 88.4 (24.3) | 93.6 (23.2) | 72.6 (24.1) | 57.5 (28.3) | <.001 |
| FEV1 predicted at baseline <90% | 28 (47.5%) | 10 (33.3) | 43 (72.9) | 3 (100) | <.001 |
| Genotype class of other allele | .77 | ||||
| I–II | 45 (76.3) | 25 (83.3) | 51 (86.4) | 3 (100) | |
| III–V | 8 (13.6) | 2 (6.7) | 4 (6.8) | 0 (0) | |
| Unknown | 6 (10.2) | 3 (10.0) | 4 (6.8) | 0 (0) | |
| BMI, kg/m2, mean (SD) | 21.1 (5.9) | 20.1 (3.2) | 22.1 (3.4) | 21.4 (2.7) | .28 |
| BMI z scorec, No., mean (SD) | 41, 0.08 (0.99) | 22, 0.18 (0.99) | 19, 0.20 (0.69) | 0, NA | .87 |
| Hospitalization rate, count/person/year, mean (SD) | 0.42 (0.70) | 0.60 (0.81) | 1.0 (1.5) | 0 (0) | .01 |
| Pulmonary exacerbation rate, count/person/year, mean (SD) | 0.53 (0.97) | 0.60 (0.86) | 1.26 (1.88) | 0 (0) | .01 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 second; NA, not available; Pa, Pseudomonas aeruginosa; SD, standard deviation.
a Free: 0% of respiratory cultures Pa positive; intermittent: 1%–50% Pa positive; persistent: >50% Pa positive.
b Three participants did not have recorded respiratory culture results in year prior to ivacaftor.
c BMI z scores for those aged <20 years at baseline using US Centers for Disease Control and Prevention growth charts.
Changes in the Prevalence of CF Pathogens Before and After Ivacaftor Use
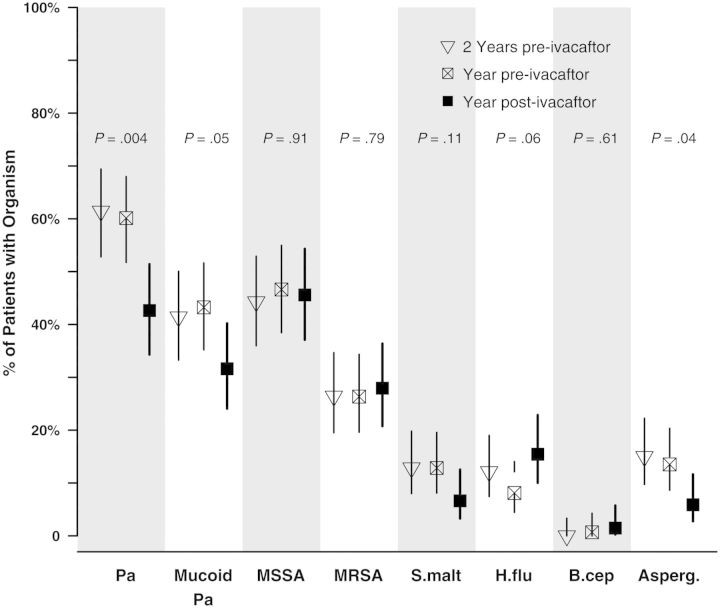
The prevalence of common CF pathogens among respiratory cultures obtained in the 2 years before and the year after ivacaftor use are shown in Figure 1. The prevalence of P. aeruginosa, mucoid P. aeruginosa, and Aspergillus species decreased significantly with ivacaftor use, whereas there was no significant change in MSSA, MRSA, H. influenzae, or S. maltophilia. Paired comparisons among the 134 with respiratory results in both years yield identical findings; notably, 27% (15/55) of those with mucoid P. aeruginosa prior to ivacaftor did not have mucoid P. aeruginosa in the year after (P < .01). There were no differences in prevalence between 2 years and 1 year pre-ivacaftor.
Figure 1.
Prevalence of culture positivity for cystic fibrosis pathogens before (open triangle and square) and after (solid square) initiation of ivacaftor. Prevalence reported among those with at least 1 respiratory culture in each year: 93% 2 years prior, 98% year prior, and 90% year after. P value reported for test comparing prevalence of each organism in year after to year prior. Vertical lines are 95% confidence intervals of the prevalence. Abbreviations: Asperg., Aspergillus species; B.cep, B. cepacia complex; H.flu, H. influenzae; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; Pa, P. aeruginosa; S.malt, S. maltophilia.
Because there were more respiratory cultures overall (mean, 3.7 [SD, 1.8] vs 3.0 [SD, 1.7]; P < .001) and more sputum cultures (mean, 2.4 [SD, 1.9] vs 1.9 [SD, 1.8]; P = .01) in the years prior to ivacaftor use vs after, multivariable logistic regression was performed to estimate the odds of respiratory culture positivity after ivacaftor, adjusting for number and type of respiratory culture (Table 2). Ivacaftor reduced the odds of P. aeruginosa culture positivity by 35% (odds ratio [OR], 0.65; P < .001), adjusting for sputum (vs OP swabs) and number of cultures performed in each year treatment interval. Similarly, ivacaftor significantly reduced the odds of culture positivity for mucoid P. aeruginosa (23% reduction), and Aspergillus (53% reduction) but not MSSA, MRSA, or S. maltophilia (Table 2). In contrast, there was a significant increase in H. influenzae following ivacaftor therapy (OR, 2.13; 95% CI, 1.15–3.96; P = .017). Chronic CF therapies were not significantly altered after ivacaftor (Supplementary Table 1), and adjustment for inhaled tobramycin, macrolide, oral antibiotic, dornase alfa, and hypertonic saline use produced similar results (data not shown).
Table 2.
Odds Ratios for Respiratory Culture Positivity of Prevalent Cystic Fibrosis Pathogens
| Pathogen | Covariate | Value | OR | (95% CI) | P Value |
|---|---|---|---|---|---|
| Pseudomonas aeruginosa | Treatment | Pre-ivacaftor | … | … | … |
| Ivacaftor | 0.65 | (.53–.79) | <.001 | ||
| Culture type | OP swab | … | … | … | |
| Sputum | 1.46 | (1.16–1.85) | .001 | ||
| Culture counta | Each additional | 0.93 | (.85–1.03) | .15 | |
| Mucoid P. aeruginosa | Treatment | Pre-ivacaftor | … | … | … |
| Ivacaftor | 0.77 | (.62–.95) | .013 | ||
| Culture type | OP swab | … | … | … | |
| Sputum | 1.17 | (.91–1.49) | .22 | ||
| Culture counta | Each additional | 0.87 | (.74–1.02) | .083 | |
| MSSA | Treatment | Pre-ivacaftor | … | … | … |
| Ivacaftor | 0.93 | (.71–1.21) | .59 | ||
| Culture type | OP swab | … | … | … | |
| Sputum | 0.84 | (.65–1.10) | .21 | ||
| Culture counta | Each additional | 0.92 | (.82–1.03) | .17 | |
| MRSA | Treatment | Pre-ivacaftor | … | … | … |
| Ivacaftor | 1.13 | (.84–1.52) | .44 | ||
| Culture type | OP swab | … | … | … | |
| Sputum | 1.55 | (1.08–2.23) | .016 | ||
| Culture counta | Each additional | 1.08 | (.93–1.25) | .30 | |
| Stenotrophomonas maltophilia | Treatment | Pre-ivacaftor | … | … | … |
| Ivacaftor | 0.71 | (.31–1.61) | .42 | ||
| Culture type | OP swab | … | … | … | |
| Sputum | 3.01 | (.98–9.21) | .05 | ||
| Culture counta | Each additional | 0.99 | (.72–1.38) | .98 | |
| Haemophilus influenzae | Treatment | Pre-ivacaftor | … | … | … |
| Ivacaftor | 2.13 | (1.15–3.96) | .016 | ||
| Culture type | OP swab | … | … | … | |
| Sputum | 0.69 | (.35–1.36) | .28 | ||
| Culture counta | Each additional | 0.81 | (.66–.99) | .036 | |
| Aspergillus | Treatment | Pre-ivacaftor | … | … | … |
| Ivacaftor | 0.47 | (.23–.96) | .039 | ||
| Culture type | OP swab | … | … | … | |
| Sputum | 6.3 | (2.2–18.0) | <.001 | ||
| Culture counta | Each additional | 1.17 | (.96–1.45) | .12 |
Total of 1013 respiratory cultures on 150 participants.
Abbreviations: CI, confidence interval; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; OP, oropharyngeal; OR, odds ratio.
a The total number of cultures during the year treatment interval.
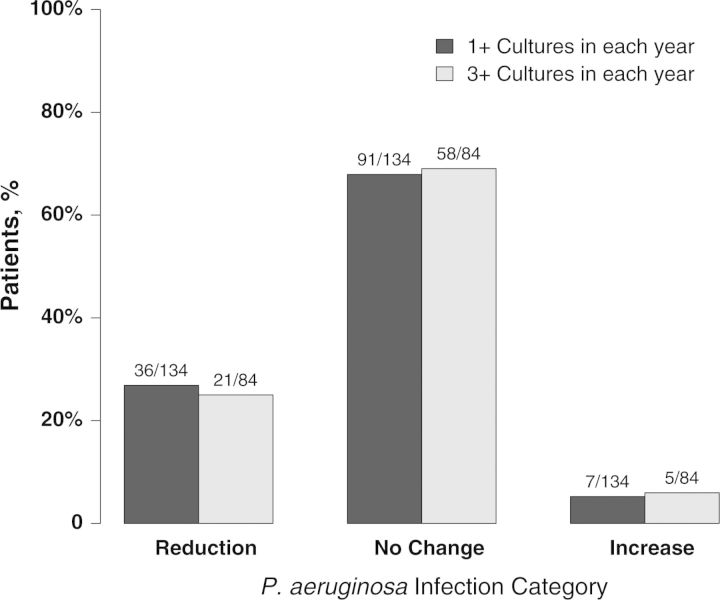
Change in P. aeruginosa Infection Category
In the year after ivacaftor, there was a significant shift in the distribution of P. aeruginosa infection categories (Table 3; P < .001): 27% (36/134) had less frequent isolation of P. aeruginosa (ie, reduction of infection category), 5% (7/134) had more frequent isolation, and 68% (91/134) did not change (Figure 2). Seventy percent (21/30) of those with intermittent infection were P. aeruginosa infection free after initiation of ivacaftor, compared with only 10% (5/48) of those with persistent infection (P < .001). Findings remained significant with a sensitivity analysis that assumed everyone without respiratory cultures during a year had P. aeruginosa culture positivity. In contrast to the change observed with ivacaftor, P. aeruginosa infection category shift between the 2 years before ivacaftor are shown in Supplementary Table 2; there was no significant change in the distribution of P. aeruginosa frequency (P = .95) in the absence of ivacaftor: 15% (21/139) had less frequent P. aeruginosa, 14% (20/139) had more frequent isolation, and 71% (98/139) did not change.
Table 3.
Shift in Pseudomonas aeruginosa Infection Category Post-Ivacaftor Initiation
| Pa Infection Category in Year Prior to Ivacaftor | No. (%) of Participants (N = 151) |
Pa Infection Category in Year After Ivacaftora |
|||
|---|---|---|---|---|---|
| Free |
Intermittent |
Persistent |
No Cultures |
||
| No. (%) of Row | No. (%) of Row | No. (%) of Row | No. (%) of Row | ||
| Free | 59 (39.1) | 52 (88.1) | 2 (3.4) | 2 (3.4) | 3 (5.1) |
| Intermittent | 30 (19.9) | 21 (70.0) | 6 (20.0) | 3 (10.0) | 0 (0) |
| Persistent | 59 (39.1) | 5 (8.5) | 10 (17.0) | 33 (55.9) | 11 (18.6) |
| No cultures | 3 (2.0) | 0 (0) | 1 (33.3) | 1 (33.3) | 1 (33.3) |
Abbreviation: Pa, Pseudomonas aeruginosa.
a Free: 0% of respiratory cultures Pa positive; intermittent: 1%–50% Pa positive; persistent: >50% Pa positive.
Figure 2.
Change in Pseudomonas aeruginosa frequency from year before to after ivacaftor initiation, stratified by number of respiratory cultures per year. Reduction includes transition from persistent infection (>50%) to intermittent infection (1%–50%) or infection free (0%), and intermittent infection to infection free. Increase includes transition from infection free to intermittent infection or persistent infection, and intermittent infection to persistent infection.
To further evaluate the robustness of these findings, we limited the analysis to those participants with ≥3 respiratory cultures in the year prior to ivacaftor initiation. Seventy-eight percent (118/151) of participants met this criteria and had a similar distribution of change in P. aeruginosa status (Supplementary Table 3). Among participants with ≥3 cultures in both years, there was a significant reduction in the distribution of P. aeruginosa isolation frequency category (P = .014; Figure 2). Again, among those with ≥3 cultures in both years, participants with intermittent infection were more likely to be infection free after ivacaftor than the participants with persistent infection (70% [14/20] vs 7% [2/27], respectively; P < .001). A sensitivity analysis assuming that participants with <3 respiratory cultures in a year were persistently infected also showed a significant (P = .017) reduction in P. aeruginosa status, driven by the high proportion of intermittent participants who were P. aeruginosa free in the year following ivacaftor.
Pseudomonas aeruginosa infection category and other clinical characteristics are presented by their change in infection status to assess possible associations (Table 4). Higher FEV1 at baseline was the only other baseline clinical characteristic shown to be associated with reduction in P. aeruginosa frequency after ivacaftor initiation (92.5 vs 81.3% predicted; P = .024).
Table 4.
Characteristics of Cohort by Pseudomonas aeruginosa Infection Category Change
| Characteristic | Change in Pa Infection Category Post-ivacaftor |
||||
|---|---|---|---|---|---|
| No Change or Increase (n = 98) | Reductiona (n = 36) | NAb(n = 17) | P Valuec | ||
| Female, No. (%) | 43 (43.9) | 19 (52.8) | 8 (47.1) | .36 | |
| Age, mean (SD) | 20.8 (11.4) | 18.6 (11.4) | 28.0 (8.5) | .32 | |
| 6–11 y, No. (%) | 25 (25.5) | 13 (36.1) | 0 (0) | .63 | |
| 12–17 y, No. (%) | 26 (26.5) | 6 (16.7) | 0 (0) | ||
| ≥18 y, No. (%) | 47 (48.0) | 17 (47.2) | 17 (100) | ||
| FEV1 % predicted at baseline | 81.3 (25.7) | 92.5 (22.6) | 69.4 (25.0) | .024 | |
| FEV1 predicted at baseline <90%, No. (%) | 58 (59.2) | 12 (33.3) | 14 (82.4) | .008 | |
| Genotype class of other allele, No. (%) | |||||
| I–II | 79 (80.6) | 32 (88.9) | 13 (76.5) | .10 | |
| III–V | 11 (11.2) | 0 (0) | 3 (17.7) | ||
| Unknown | 8 (8.2) | 4 (11.1) | 1 (5.9) | ||
| BMI, kg/m2 | 21.3 (5.0) | 20.2 (3.3) | 23.2 (3.1) | .14 | |
| Hospitalization rate, count/person/year | 0.65 (1.1) | 0.72 (1.0) | 0.86 (1.4) | .75 | |
| Pa infection category in year prior to ivacaftor, No. (%) | |||||
| Free | 56 (57.1) | … | 3 (17.7) | <.001 | |
| Intermittent | 9 (9.2) | 21 (58.3) | 0 (0) | ||
| Persistent | 33 (33.7) | 15 (41.7) | 11 (64.7) | ||
| No respiratory cultures | 0 (0) | 0 (0) | 3 (17.7) | ||
| Change after ivacaftor | |||||
| FEV1 % predictedd | 4.5 (11.4) | 6.6 (9.6) | 10.7 (13.0) | .31 | |
| BMId, kg/m2 | 1.1 (1.5) | 0.9 (1.4) | 0.7 (1.9) | .52 | |
| Hospitalization rate, count/person/year | −0.36 (0.92) | −0.58 (0.81) | −0.77 (1.3) | .20 | |
| Pulmonary exacerbation rate, count/person/year | −0.46 (1.18) | −0.64 (1.05) | −1.23 (2.24) | .43 | |
| Sweat chloride, mmol/Le | −57.9 (19.7) | −64.1 (16.0) | −52.2 (17.4) | .12 | |
Data are presented as mean (SD) unless otherwise indicated.
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 second; NA, not available; Pa, Pseudomonas aeruginosa; SD, standard deviation.
a Reduction in Pa infection category with ivacaftor (eg, intermittent to free, or persistent to intermittent or free) vs no change or increase in Pa infection category.
b Seventeen participants did not have recorded respiratory culture results in year prior to and after ivacaftor.
c P value test of no change or increase vs reduction; NA not included.
d Change per subject from baseline through 12 months after initiation of ivacaftor (data from Cystic Fibrosis Foundation's National Patient Registry).
e Maximal change per subject from baseline through 6 months after initiation of ivacaftor.
Associations With Clinical Outcomes
Overall for the entire cohort, there were significant improvements in FEV1 percentage predicted (mean change, 5.7% [95% CI, 3.8%–7.5%]; P < .001), BMI (1.0 kg/m2 [95% CI, .76–1.24 kg/m2]; P < .001), hospitalization rate (−0.45 [95% CI,−0.60 to −0.30]) admissions/participant/year; P < .001), and pulmonary exacerbations/participant/year (−0.57 [95% CI,−0.78 to −0.36]; P < .001) 1 year after ivacaftor initiation. Although there were differences in age, baseline FEV1, and hospitalization rate with P. aeruginosa infection category in the year before ivacaftor (Table 1), reduction in P. aeruginosa frequency with ivacaftor was not significantly associated with improvement in FEV1, BMI, hospitalization, or exacerbation rate alone (Table 4), or after adjustment for year-prior P. aeruginosa infection category, sex, age, baseline FEV1, or sweat chloride (Supplementary Table 4). However, when P. aeruginosa culture positivity was analyzed as a continuous variable based on the proportion of positive cultures, we detected a weak but statistically significant association with FEV1 improvement: a 10% reduction in P. aeruginosa positivity corresponds with a 0.765% increase in FEV1 percentage predicted (95% CI, .04%–1.5%; P = .030; Supplementary Table 5).
DISCUSSION
This is the first report on CF respiratory pathogens after clinical exposure to the CFTR modulator ivacaftor. Significant reductions in P. aeruginosa culture positivity (both overall and mucoid isolates) were observed in CF patients with at least 1 copy of the G551D mutation who were prescribed ivacaftor as part of clinical care. Reductions were not observed in the same cohort over the preceding 2-year period in the absence of ivacaftor. Participants with intermittent infection in the year prior to ivacaftor as well as those with higher FEV1 at the time of ivacaftor initiation had greater likelihoods of experiencing reduced frequency of P. aeruginosa culture positivity compared to those with persistent infection (>50% cultures positive). To address variation in clinical care among centers, a number of sensitivity analyses were performed to account for differential follow-up, specimen type, respiratory culture frequency, changes in concomitant chronic CF medications, and missing data. All of these substantiate the overall findings that there was a decrease in the frequency of P. aeruginosa–positive cultures associated with ivacaftor among CF patients with G551D.
The underlying mechanisms responsible for the susceptibility of the CF lung to P. aeruginosa infection are challenging to define and cannot be answered by this observational study [16]. However, several hypotheses relate directly to CFTR function, including P. aeruginosa adherence to airway epithelial cells [17], CFTR as a receptor of P. aeruginosa lipopolysaccharide [18], and alteration of CFTR expression by P. aeruginosa cytotoxins [19]. Changes in CF-specific pathogens may be attributed to increased mucociliary clearance [9]; sloughing of bacteria-laden epithelial cells [20] and biofilms due to improved chloride transport could also contribute. CFTR modulation can also lead to increased bicarbonate secretion, elevating pH, which may produce an antagonistic airway environment for P. aeruginosa by augmenting defense-related bacterial killing [21]. There is evidence that ivacaftor raises the pH in the gastrointestinal tract in CF [9]; however, its effects on airway pH are not yet known. Although in vitro data have shown that ivacaftor may have quinolone-like anti-infective properties against S. aureus, but not P. aeruginosa [22], no change in S. aureus (methicillin-sensitive or -resistant) was exhibited.
Although less prevalent overall, there was a significant reduction of culture positivity for Aspergillus species, a pathogen associated with both absent CFTR function [23] and worsened clinical outcomes, particularly when it is corresponds with allergic bronchopulmonary aspergillosis [24]. In contrast, we also observed an increase in H. influenzae culture positivity in this population. Interestingly, this is an organism generally seen early in the pathogenesis of CF [25, 26]. Complete PCR-based microbiome analysis in a subset of these GOAL participants found modest reductions in the relative abundance of CF pathogens and an increase in the anaerobe Prevotella [27], consistent with these findings that there may be a shift toward “normal” or less severe respiratory flora with efficacious CFTR modulation. Longer observation and more in-depth examination of the complex interaction between CF microbiota might elucidate these and other more subtle effects of ivacaftor.
Reduction of P. aeruginosa frequency category was not shown to be associated with clinical responses in FEV1, BMI, or hospitalization rate; however, in a secondary analysis, we detected a weak association between P. aeruginosa culture positivity rate and FEV1, suggesting that clinical benefit may be conferred by ivacaftor-associated bacterial clearance, even at this early stage. Longer follow-up will be needed to determine whether reduced bacterial burden is sustained and its independent effect on long-term prognosis. Studies among participants receiving ivacaftor demonstrated poor correlation between clinical responses (ie, lung function and weight gain) and sweat chloride, the principal biomarker of biologic effect [9, 28]. While the one-to-one relationship between early indicators of CFTR recovery and clinical outcomes is complex, further evaluation of sweat chloride, mucociliary clearance, pH, inflammation, and CF respiratory pathogens as possible predictors of clinical response may reveal an important prognostic biomarker or panel of markers.
This observational study is subject to the potential biases one might expect from the epidemiologic collection of patient data [29]. There was differential follow-up between the year before and the year after ivacaftor initiation with regard to duration, and number and type of respiratory cultures. This observation may be due to improved patient health, reducing expectoration or opportunities to collect samples; chance; or another unobservable reason. Because of the potential sampling bias this might introduce [30], logistic regression was performed to adjust for number of cultures during each year and type of respiratory culture, which showed substantial reduction in culture positivity for both P. aeruginosa and mucoid P. aeruginosa. Similarly, sensitivity analyses of only participants with at least 3 respiratory cultures and of a “worst case” where those missing data were treated as persistently infected produced results nearly identical to those using the primary data. The reported changes in microbiology are unlikely attributed to the unblinded nature of the study, as cultures are a relatively objective respiratory biologic measure. Although there is the possibility of confounding by concomitant medications or other latent exposures, significant reductions in respiratory bacterial burden were observed when adjusted for factors known to impact P. aeruginosa in CF. Less restrictive cohort studies are often more generalizable than data obtained in randomized controlled studies; nevertheless, the G551D CF patients who enrolled in this study may be unique in that some individuals may not have qualified or chose to enroll in concurrent clinical trials. Nevertheless, the clinical response in FEV1 and BMI among the GOAL participants was consistent with that observed in controlled trials [2, 4, 9], indicating that these participants are likely representative. Further analyses of CFFNPR could be used to determine whether these microbiologic findings persist in all CF patients with G551D or over a longer observation period; however, we showed that these reductions were not observed in the same cohort 2 years prior to ivacaftor in the absence of CFTR modulation.
Reducing the burden of P. aeruginosa in CF patients is likely to be highly significant over the long term, and could alter the natural history of the disease [31]. There is reason to surmise that early eradication could positively impact pulmonary exacerbations, lung function, and even survival [5–8, 32]. The 25%–35% reduction in P. aeruginosa culture positivity observed within the GOAL cohort is greater than the 10%–20% reductions observed in ecologic studies spanning 1990 to 2005—a period associated with numerous therapeutic improvements, including the introduction of aerosolized antibiotics, early eradication routines, dornase alfa, and azithromycin [33, 34], and is more consistent with reductions seen due to eradication strategies [35, 36]. Although spontaneous clearance can occur [37, 38], aggressive antipseudomonal therapy may not only eradicate the organism, improving outcomes [39], but could also prolong time to chronic infection [40, 41] or development of mucoidy [31]. The mucoid phenotype is more resistant to antibiotics and immunologic defense [31] and is associated with poorer prognosis [8, 42]. Mucoid P. aeruginosa is thought to have limited treatment options [43, 44]; however, recent data indicate that eradication may be possible [45–47]. Ivacaftor or other CFTR modulators in development may be a surprisingly useful tool for early eradication or prevention of CF respiratory infections, even in those with mucoid P. aeruginosa, and warrants prospective evaluations. It is possible that CFTR modulators will supplement or augment certain anti-infectives' activity as shown in vitro [22] without promoting antibiotic resistance, while also improving lung function and growth beyond what has been seen from antibiotic regimens alone. Further examination of the potential interaction between ivacaftor and other CF therapies such as mucolytics, antibiotics, or specific eradication regimens in vivo is warranted and may reveal long-term efficacy beyond the immediate improvements in weight, lung function, and exacerbation rate.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. This study was conducted with the participation of the substudy investigators (Drs Drucy Borowitz, Scott H. Donaldson, Daniel Gelfond, Tanja Gonska, and Scott D. Sagel) and study site principal investigators and coordinators listed in the Supplementary Data. The authors acknowledge assistance from the Cystic Fibrosis Foundation National Patient Registry team including Dr Bruce Marshall, and support for study planning and execution provided by Dr Preston W. Campbell III, Dr Elizabeth Joseloff, Jill M. Van Dalfsen, and the Therapeutics Development Network Coordinating Center.
Financial support. This work was supported by the Cystic Fibrosis Foundation Therapeutics (CFFT) (grant numbers GOAL11K1 and GOAL13K1) and the National Institutes of Health (NIH) (grant numbers P30 DK089507 and P30 DK072482).
Potential conflicts of interest. S. L. H. receives grant support from the CFFT and NIH and her institution receives payment for consulting with Vertex. N. M. H. receives grant support from the CFFT, NIH, Novartis, and serves on an advisory committee for Insmed for which her institution receives payment. SMR's institution receives grant support from the CFFT, NIH, Novartis, Vertex, PTC Therapeutics, Bayer, N30 Pharmaceuticals, and Forest Research Institute. J. L. B.'s microbiology laboratory holds contracts with Gilead, Novartis, PharmaCaribe, and Grifols. B. R. receives grant support from the CFFT and NIH, and consults with Novartis, and Vertex. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 2.Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibbs GE. Sweat tests for diagnosis of cystic fibrosis. Q Rev Pediatr. 1958;13:188–91. [PubMed] [Google Scholar]
- 4.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–72. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerem E, Corey M, Gold R, Levison H. Pulmonary function and clinical course in patients with cystic fibrosis after pulmonary colonization with Pseudomonas aeruginosa. J Pediatr. 1990;116:714–9. doi: 10.1016/s0022-3476(05)82653-8. [DOI] [PubMed] [Google Scholar]
- 6.Pamukcu A, Bush A, Buchdahl R. Effects of Pseudomonas aeruginosa colonization on lung function and anthropometric variables in children with cystic fibrosis. Pediatr Pulmonol. 1995;19:10–5. doi: 10.1002/ppul.1950190103. [DOI] [PubMed] [Google Scholar]
- 7.Kosorok MR, Zeng L, West SE, et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol. 2001;32:277–87. doi: 10.1002/ppul.2009.abs. [DOI] [PubMed] [Google Scholar]
- 8.Henry RL, Mellis CM, Petrovic L. Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr Pulmonol. 1992;12:158–61. doi: 10.1002/ppul.1950120306. [DOI] [PubMed] [Google Scholar]
- 9.Rowe SM, Heltshe SL, Gonska T, et al. Clinical mechanism of the CFTR potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190:175–84. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bethesda, MD: Cystic Fibrosis Foundation; 2012. Cystic Fibrosis Foundation Patient Registry: Annual data report 2012. [Google Scholar]
- 11.Flume PA, O'Sullivan BP, Robinson KA, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957–69. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 12.Saiman L, Siegel J. Infection control recommendations for patients with cystic fibrosis: microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Infect Control Hosp Epidemiol. 2003;24(5 suppl):S6–52. doi: 10.1086/503485. [DOI] [PubMed] [Google Scholar]
- 13.Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros. 2003;2:29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Dockery DW, Wypij D, et al. Pulmonary function growth velocity in children 6 to 18 years of age. Am Rev Respir Dis. 1993;148(6 pt 1):1502–8. doi: 10.1164/ajrccm/148.6_Pt_1.1502. [DOI] [PubMed] [Google Scholar]
- 15.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 16.Murray TS, Egan M, Kazmierczak BI. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr Opin Pediatr. 2007;19:83–8. doi: 10.1097/MOP.0b013e3280123a5d. [DOI] [PubMed] [Google Scholar]
- 17.Imundo L, Barasch J, Prince A, Al-Awqati Q. Cystic fibrosis epithelial cells have a receptor for pathogenic bacteria on their apical surface. Proc Natl Acad Sci U S A. 1995;92:3019–23. doi: 10.1073/pnas.92.7.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pier GB. Role of the cystic fibrosis transmembrane conductance regulator in innate immunity to Pseudomonas aeruginosa infections. Proc Natl Acad Sci U S A. 2000;97:8822–8. doi: 10.1073/pnas.97.16.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong F, Young L, Chen Y, et al. Pseudomonas aeruginosa pyocyanin inactivates lung epithelial vacuolar ATPase-dependent cystic fibrosis transmembrane conductance regulator expression and localization. Cell Microbiol. 2006;8:1121–33. doi: 10.1111/j.1462-5822.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder TH, Lee MM, Yacono PW, et al. CFTR is a pattern recognition molecule that extracts Pseudomonas aeruginosa LPS from the outer membrane into epithelial cells and activates NF-kappa B translocation. Proc Natl Acad Sci U S A. 2002;99:6907–12. doi: 10.1073/pnas.092160899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pezzulo AA, Tang XX, Hoegger MJ, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487:109–13. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reznikov LR, Abou Alaiwa MH, Dohrn CL, et al. Antibacterial properties of the CFTR potentiator ivacaftor. J Cyst Fibros. 2014;13:515–9. doi: 10.1016/j.jcf.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di A, Brown ME, Deriy LV, et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol. 2006;8:933–44. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 24.Kraemer R, Delosea N, Ballinari P, Gallati S, Crameri R. Effect of allergic bronchopulmonary aspergillosis on lung function in children with cystic fibrosis. Am J Respir Crit Care Med. 2006;174:1211–20. doi: 10.1164/rccm.200603-423OC. [DOI] [PubMed] [Google Scholar]
- 25.Lipuma JJ. The changing microbial epidemiology in cystic fibrosis. Clin Microbiol Rev. 2010;23:299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pihet M, Carrere J, Cimon B, et al. Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis—a review. Med Mycol. 2009;47:387–97. doi: 10.1080/13693780802609604. [DOI] [PubMed] [Google Scholar]
- 27.Sagel SD, Harris JK, Wagner BD, et al. Effects of ivacaftor on airway microbiome and inflammation in G551D patients. Pediatr Pulmonol. 2013;48(S36):285. [Google Scholar]
- 28.Seliger VI, Rodman D, Van Goor F, Schmelz A, Mueller P. The predictive potential of the sweat chloride test in cystic fibrosis patients with the G551D mutation. J Cyst Fibros. 2013;12:706–13. doi: 10.1016/j.jcf.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359:248–52. doi: 10.1016/S0140-6736(02)07451-2. [DOI] [PubMed] [Google Scholar]
- 30.Burns JL, Gibson RL, McNamara S, et al. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J Infect Dis. 2001;183:444–52. doi: 10.1086/318075. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Kosorok MR, Farrell PM, et al. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA. 2005;293:581–8. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 32.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 33.Lee TW, Brownlee KG, Denton M, Littlewood JM, Conway SP. Reduction in prevalence of chronic Pseudomonas aeruginosa infection at a regional pediatric cystic fibrosis center. Pediatr Pulmonol. 2004;37:104–10. doi: 10.1002/ppul.10401. [DOI] [PubMed] [Google Scholar]
- 34.Razvi S, Quittell L, Sewall A, Quinton H, Marshall B, Saiman L. Respiratory microbiology of patients with cystic fibrosis in the United States, 1995 to 2005. Chest. 2009;136:1554–60. doi: 10.1378/chest.09-0132. [DOI] [PubMed] [Google Scholar]
- 35.Treggiari MM, Retsch-Bogart G, Mayer-Hamblett N, et al. Comparative efficacy and safety of 4 randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Arch Pediatr Adolesc Med. 2011;165:847–56. doi: 10.1001/archpediatrics.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen CR, Pressler T, Hoiby N. Early aggressive eradication therapy for intermittent Pseudomonas aeruginosa airway colonization in cystic fibrosis patients: 15 years experience. J Cyst Fibros. 2008;7:523–30. doi: 10.1016/j.jcf.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Wiesemann HG, Steinkamp G, Ratjen F, et al. Placebo-controlled, double-blind, randomized study of aerosolized tobramycin for early treatment of Pseudomonas aeruginosa colonization in cystic fibrosis. Pediatr Pulmonol. 1998;25:88–92. doi: 10.1002/(sici)1099-0496(199802)25:2<88::aid-ppul3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 38.Gibson RL, Emerson J, McNamara S, et al. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. Am J Respir Crit Care Med. 2003;167:841–9. doi: 10.1164/rccm.200208-855OC. [DOI] [PubMed] [Google Scholar]
- 39.Mayer-Hamblett N, Kronmal RA, Gibson RL, et al. Initial Pseudomonas aeruginosa treatment failure is associated with exacerbations in cystic fibrosis. Pediatr Pulmonol. 2012;47:125–34. doi: 10.1002/ppul.21525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valerius NH, Koch C, Hoiby N. Prevention of chronic Pseudomonas aeruginosa colonisation in cystic fibrosis by early treatment. Lancet. 1991;338:725–6. doi: 10.1016/0140-6736(91)91446-2. [DOI] [PubMed] [Google Scholar]
- 41.Frederiksen B, Koch C, Hoiby N. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr Pulmonol. 1997;23:330–5. doi: 10.1002/(sici)1099-0496(199705)23:5<330::aid-ppul4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 42.Parad RB, Gerard CJ, Zurakowski D, Nichols DP, Pier GB. Pulmonary outcome in cystic fibrosis is influenced primarily by mucoid Pseudomonas aeruginosa infection and immune status and only modestly by genotype. Infect Immun. 1999;67:4744–50. doi: 10.1128/iai.67.9.4744-4750.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koch C. Early infection and progression of cystic fibrosis lung disease. Pediatr Pulmonol. 2002;34:232–6. doi: 10.1002/ppul.10135. [DOI] [PubMed] [Google Scholar]
- 44.West SE, Zeng L, Lee BL, et al. Respiratory infections with Pseudomonas aeruginosa in children with cystic fibrosis: early detection by serology and assessment of risk factors. JAMA. 2002;287:2958–67. doi: 10.1001/jama.287.22.2958. [DOI] [PubMed] [Google Scholar]
- 45.Douglas TA, Brennan S, Gard S, et al. Acquisition and eradication of P. aeruginosa in young children with cystic fibrosis. Eur Respir J. 2009;33:305–11. doi: 10.1183/09031936.00043108. [DOI] [PubMed] [Google Scholar]
- 46.McPherson H, Rosenthal M, Bush A. Can mucoid Pseudomonas aeruginosa be eradicated in children with cystic fibrosis? Pediatr Pulmonol. 2010;45:566–8. doi: 10.1002/ppul.21220. [DOI] [PubMed] [Google Scholar]
- 47.Troxler RB, Hoover WC, Britton LJ, Gerwin AM, Rowe SM. Clearance of initial mucoid Pseudomonas aeruginosa in patients with cystic fibrosis. Pediatr Pulmonol. 2012;47:1113–22. doi: 10.1002/ppul.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.