Abstract
Context.
The exact relationship between the bed rest-induced loss of skeletal muscle and reductions in muscle strength and physical performance in the older individuals is still unclear.
Objective.
We examined the effect of 10 days of bed rest on changes in regional body composition, muscle strength, and functional status, and the relationship between these variables in older individuals.
Design, Participants, and Intervention.
Regional body composition was measured using dual energy x-ray absorptiometry. We also determined changes in leg strength and several indices of functional status, including walking speed.
Results.
Body weight, body mass index, and total and lower extremity lean mass decreased with bed rest. There were also significant reductions in knee extension one repetition maximum, isometric knee extension, knee extension 60° concentric, stair ascent time, stair ascent power, stair descent time, VO2 max, floor transfer test, 5-minute walk time, and chair stand. The overall change in total and lower extremity lean mass was also directly related to bed rest-induced reductions in one repetition maximum knee extension.
Conclusions.
Bed rest promoted overall declines in muscle mass, muscle strength, and physical function in older individuals. The changes in lean tissue were closely correlated with the bed rest-induced decline of muscle strength.
Key Words: Inactivity, Hospitalization and metabolic disease
Several studies have highlighted the detrimental influence of bed rest on metabolism and muscle protein synthesis (1–4). Additional studies have demonstrated that chronic bed rest may result in the loss of postural muscles, leading to increased risk of falls and disability (5). Realizing the high incidence of anorexia in the geriatric population (6), our group has studied the influence of bed rest in the elderly under eucaloric conditions and still observed reductions in strength in healthy older individuals despite the lack of deficiencies in macronutrient intake or caloric balance (7).
Walking speed has been confirmed as a clear predictor for adverse health-related events (8). In addition, the findings from Health Aging and Body Composition study demonstrated that age-related reductions in thigh and grip strength were closely correlated with increased mortality (9). These changes in lean mass were consistent in earlier reports, and changes in physical function were not evaluated in these studies.
Recently, Drummond and coworkers (10) demonstrated significant bed rest-induced reductions in total lean mass that were linked to impairment in the response to amino acids. Even small reductions in physical activity in older adults have also resulted in changes altered muscle protein synthesis associated with reductions in insulin sensitivity (11). In addition, we recently demonstrated significant reductions in hepatic and peripheral insulin sensitivity with short-term bed rest in older adults (12). Therefore, bed rest might promote tissue lipid accumulation and aggravated insulin resistance in the elderly that has been linked to detrimental reductions in muscle mass and strength (13).
Therefore, we hypothesized that short-term bed rest would promote reductions in muscle strength and physical function, and that these changes would be related to the reduction of lean mass. We also sought to examine the relationships between changes in lean mass and muscle strength and physical function.
Materials and Methods
Research Volunteers
We recruited a total of 19 volunteers between the ages of 60 and 85 years (14 females/5 males) within a body mass index (BMI) range of 20–35kg/m2 (Table 1). All participants were weight stable and free of chronic disease prior to enrollment. Participants underwent a physical examination, which included a medical history, baseline, and exercise electrocardiogram, and routine blood chemistries. A maximal exercise test was utilized for the determination of VO2 peak pre- and postbed rest. The exclusion criteria included: (i) body mass index (20 < BMI > 40kg/m2), (ii) diabetes (determined by history and physical and/or glucose greater than 200mg/dL at 2 hours of an oral glucose tolerance test), (iii) current smoking, (iv) active malignancy, (v) uncontrolled hypertension, (vi) positive history of cardiovascular disease including stroke, (vii) history of deep vein thrombosis/pulmonary embolism or hypercoagulability disorder, (viii) history of hepatic or renal disease, (ix) history of chronic inflammatory disease (eg, rheumatoid arthritis), and a (x) short physical performance battery score less than 9. Potential volunteers were also excluded if they were taking any medications known to affect protein metabolism (eg, corticosteroids and anabolic steroids). Given that this study was powered to study changes in the metabolism in an aging population, there was no attempt to match or stratify groups based on gender or outcomes variables. As such, the interpretation of the study is limited by these constraints. Each volunteer provided written informed consent. All study procedures were approved by the Institutional Review Board of the University of Arkansas for Medical Sciences (UAMS).
Table 1.
Age, Gender, Body Composition, Strength, and Physical Function
| Total Group—Pre | Total Group—Post | |
|---|---|---|
| Age | 69.9±1.0 | |
| Gender (females/males) | 14/5 | |
| Height | 162.8±1.6 | |
| Weight | 75.2±3.4 | 73.9±3.4* |
| Body mass index (kg/m2) | 28.0±1.0 | 27.5±1.0* |
| Total lean mass (kg) | 45.4±2.4 | 44.6±2.3* |
| Lean mass lower extremity (kg) | 14.4±0.8 | 14.0±0.8* |
| Total fat mass (kg) | 27.2±2.0 | 27.1±2.0 |
| Body fat (%) | 35.9±1.8 | 36.5±1.7* |
| Muscle quality (nM/kg) | 15.9±1.4 | 14.6±1.0* |
| Muscle attenuation (HU) | n/a | n/a |
| 1 RM knee extension (N) | 85.9±8.0 | 75.2±6.6* |
| Isometric knee extension (N) | 133.6±10.1 | 122.5±8.9* |
| Concentric knee extension (60°; Nm/s) | 115.4±10.4 | 102.7±7.8* |
| Concentric knee extension (180°; Nm/s) | 69.1±6.7 | 54.2±5.0 |
| Stair ascent time (s) | 4.37±0.24 | 5.29±0.38* |
| Stair ascent power (Nm/s) | 302.1±19.5 | 264.8±17.4* |
| Stair descent time (s) | 4.00±0.24 | 4.94±0.41* |
| Stair descent power (Nm/s) | 302.1±19.5 | 292.1±27.8* |
| Maximal VO2 peak (l/min) | 21.5±1.3 | 18.6±1.0* |
| Floor transfer (s) | 6.86±0.72 | 9.68±1.46* |
| Five step test (s) | 9.50±0.47 | 9.58±0.54 |
| Five-minute walk (m) | 438.1±19.1 | 405.5±19.6* |
| Four-meter walk (s) | 2.69±0.24 | 2.70±0.26 |
| Walking speed (m/s) | 1.46±0.06 | 1.35±0.06* |
| Chair stand (s) | 8.41±0.58 | 9.43±0.57* |
Notes: HU = Hounsfield units; RM = repetition maximum. Values are mean ± SEM.
*Denotes significant difference between pre- and postbed rest values (p < .05).
Experimental Protocol
The experimental protocol has been published earlier (7). All volunteers consumed a lacto-ovo vegetarian diet providing the recommended daily allowance for protein (0.8g/kg of protein per day), and the nonprotein component of the diet consisted of 60% energy from carbohydrate and 40% from fat during diet stabilization and bed rest. The Nutritional Services component of the Clinical Research Services Core at UAMS provided a 3-day rotation diet based on the Harris–Benedict equation designed to maintain body weight throughout the study. An activity factor (AF) of 1.6 during diet stabilization (Study Days 1–8) and 1.3 during bed rest (Study Days 9–19) was used to estimate the total daily energy requirement according to the following equation: = [66 + (13.7 × weight in kg) + (5 × height in cm) − (608 × age in years)] × AF. As described earlier, all individuals participated in a 3-month rehabilitation program that enabled them to return or exceed their baseline strength and physical performance values (7). There were no adverse events. We administered prophylactic measures to prevent deep vein thrombosis. All ultrasound results were negative at the conclusion of the bed rest period.
Body Composition
Dual energy x-ray absorptiometry (DXA) using a Hologic QDR 2000 densitometer (Hologic Inc., Bedford, MA) was utilized to determine lean tissue, adipose tissue, and bone composition (14).
Functional Parameters
The familiarization of strength and functional measures were performed on Study Day 1 and again on Study Day 3 for actual testing purposes to diminish the influence of a learning effect. For the measurement of stair ascent/descent power, volunteers were asked to start from a standing position and walk up 10 steps in an expeditious and safe manner for the stair ascent test. They were also instructed to place one hand close to the handrail for balance if necessary, but not on the handrail. The technician assigned to the study measured the time to walk up 10 steps as determined by stopwatch. After a short rest period, the volunteer was repositioned at the top of the stairs, and the stair descent test was performed in a similar fashion. Pre- and postbed rest stair tests were conducted on the same flight of stairs. Power was calculated as work (force [participants’ body mass in kg × g (9.8 m/s2)] × the vertical height of the stairs in meters) divided by time in seconds (15). As published earlier by Wang and coworkers (16), floor transfer time was calculated according to the time required for a subject to move from a complete standing to a long-sitting position on a mat (ie, legs extended in front of the body) and then back up to complete standing was measured using a stop-watch. A chair was placed in close proximity for support, if needed, during this test. The 5-minute walk test was administered using a stopwatch and the participant was instructed to walk as swiftly as possible without assistance for the entire period. Two separate trials were completed. Walking speed was calculated from this test in meters/second (m/s). For the 4-m walk, the participant walked in a straight line at a normal ambulatory pace. The chair stand test was administered such that the participant was asked to stand up five times as quickly as possible without the use of their arms for assistance (16).
Aerobic Capacity Test
An exercise stress test was performed pre- and postbed rest to determine VO2peak on a bicycle ergometer (Lode, Groningen, Netherlands) (17). Respiratory gases were collected and analyzed using indirect calorimetry (Vmax 29N, Sensormedics, Yorba Linda, CA).
Statistical Analyses
Statistical comparisons for all variables including anthropometrics, regional body composition, muscle attenuation, VO2 peak were made using paired t tests. To characterize the relationships between changes in body composition and strength/functional parameters, linear regression analyses were performed. All data are reported as means ± SEM.
Results
Human Participants
We enrolled a total of 14 females and 5 males (66 ± 1 years) volunteer(s), and all individuals were able to comply with the study protocol (Table 1). There were no drop-outs.
Anthropometrics
There was a significant reduction in body weight (p = .01) and BMI (p = .01; Table 1). Baseline BMI did not influence the results of the study (p > .05). Total lean mass and lower extremity (LE) lean mass was also decreased (ie, p = .006 and p = .02, respectively; Table 1) with bed rest. Overall, there was an increase in the percentage of body fat (p = .03), presumably due to the decrease in lean tissue.
Strength Measurements
There was a significant reduction in knee extension 1 repetition maximum (RM; p =.0006), knee extension isometric (p = .04), and concentric knee extension (p = .01; Table 1). Knee extension 1 RM was lower (p = .02) prior to bed rest in the subset of nine volunteers.
Physical Function Tests
There were significant reductions in stair ascent time (p = .009), stair ascent power (p = .004), stair descent time (p = .002), floor transfer test (p = .003), 5-minute walk time (p = .03), chair stand (p = .001; Table 1). There was a strong trend towards a reduction in stair descent power (p = .051) and 5-minute walk (p = .079; Table 1).
Maximal Exercise Test
Bed rest promoted a decrease in the VO2 peak (p = .002) during maximal exercise (Table 1).
Body Composition and Muscle Strength/Physical Function
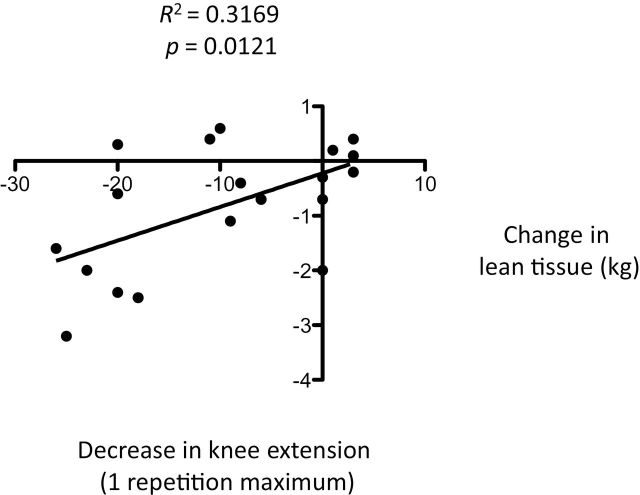
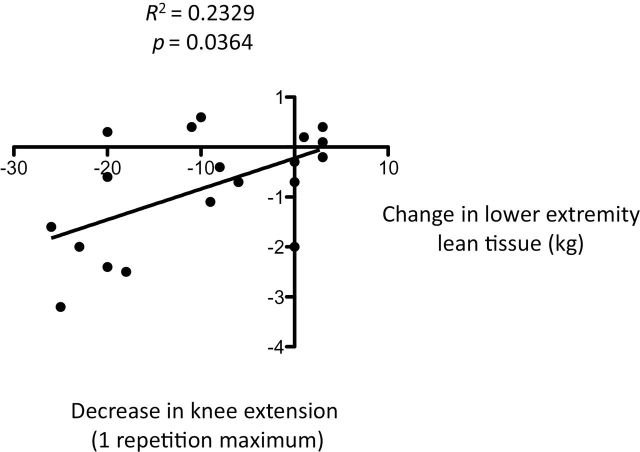
There was a significant relationship between bed rest-induced reductions in total lean mass and LE lean mass, and knee extension 1 RM (p = .012 and .0364, respectively; Figures 1 and 2, respectively). No significant relationships existed between total lean mass or LE lean mass and changes in physical function.
Figure 1.
Relationship between bed rest-induced loss of lean tissue and decrease in knee extension one repetition maximum.
Figure 2.
Relationship between bed rest-induced loss of lower extremity lean tissue and decrease in knee extension one repetition maximum.
Discussion
It has been recognized that unanticipated bed rest produces a plethora of undesirable consequences (18). Despite the recognition that bed rest may accelerate muscle atrophy, the exact relationship between loss of skeletal muscle and physical performance during unanticipated hospitalization in the elderly is still unclear. Therefore, we hypothesized that unanticipated bed rest would result in muscle loss that was closely related to decreased muscle strength and physical function. Similar to our expectations, there was a significant reduction in body weight, BMI, total lean mass, LE lean mass, muscle quality, and a significant increase in the percentage of body fat with bed rest. These changes occurred in concert with decreased strength (ie, 1 RM, isometric, and concentric knee extension), physical function (ie, stair ascent/descent time and power, floor transfer, walking speed, and chair stand), and fitness level (ie, VO2 peak).
As outlined by Janssen (19), a significant percentage of older adults may be either sarcopenic or severely sarcopenic. As a consequence of unanticipated bed rest, it has been suggested that some of the individuals may be closer to a threshold whereby they might not recover from prolonged bed rest (20,21), especially when additionally challenged by a chronic inflammatory condition that may exacerbate deleterious changes in the anabolic response to nutrition with advancing age (22). In the present study, muscle mass was reduced and there was a concomitant increase in fat which may have also been indirectly responsible for the loss of muscle strength and physical function. Moreover, there was a significant relationship between the loss of lean mass and decreased muscle strength. The bed rest-induced loss of muscle has been linked earlier to the reduction in the fractional synthetic rate of muscle protein (7). We now report bed rest-induced reductions in muscular strength and VO2 peak that are comingled with reductions in several parameters of physical function in older adults, including almost every variable designed to ascertain changes in functional status. It might be suggested that physical mobility programs and/or medically formulated foods should be utilized to offset these detrimental consequences (22,23). Indeed, the efficacy of these strategies towards the preservation of lean tissue have now been demonstrated (22,24).
The infiltration of lipids into skeletal muscle has been suggested to play a role in precipitating or affecting the decline of strength and/or functional capacity (25). The current data highlight the important associations between increased fat mass and reduced lean tissue, and their relationships to impairments in muscle strength and reduced walking speed (26). Therefore, the predictive importance of lean mass when evaluating muscle strength and/or walking speed should not necessarily be discounted in favor of the “imaging-specific” classification of sarcopenia. This is not to say that longitudinal studies performed over the course of years, instead of days, may shed light on the etiological background responsible for declines in gait speed as we age (28). These types of associations should be considered in light of the multiple physiological systems required for efficacious motor control and energy metabolism, and thus make it difficult to completely elucidate the underlying etiology of a clinical condition.
Although we recognize the value of multiple physiological assessments, it should be recognized that walking speed has been confirmed as a clear predictor for adverse health-related events (29). In our particular study, we set out to define the functional consequences of unanticipated bed rest and found that bed rest accentuates the deleterious decline in walking speed across wide BMI range (ie, 21–36kg/m2). The decline in walking speed occurred along with a reduction in VO2 peak, which is interesting because this variable is strongly associated from a cross-sectional standpoint in older adults (30). Other authors have also demonstrated the relationship between slow walking speed and the increased incidence of frailty (9). Several recent studies have now, in fact, demonstrated the strong relationship between walking speed and mortality (31–34), and walking speed is clearly an important simple variable to evaluate that can be negatively affected even in the short-term context of bed rest in older adults (35).
In conclusion, we confirm our initial hypothesis and demonstrate short-term bed rest promotes detrimental changes in muscle strength and physical function. Simultaneously, these individuals experienced significant reductions in muscle mass (determined by DXA) and aerobic exercise capacity (assessed by maximal VO2 peak). Bed rest promoted a reduction in walking speed, stair ascent power, floor transfer, and chair stand in all volunteers. It could be argued that these particular parameters would have a consistently more negative impact on a person’s ability to return to the activities of daily living than changes in lean tissue or muscle strength alone. Therefore, the consequences of bed rest without appropriate countermeasures may be closely linked to inability to perform activities of daily living, increased hospitalizations, and possibly lead to increased morbidity/mortality (36).
Funding
Supported by National Institutes of Health grant PO1 AG023591-01 (W.J.E.), KO1 DK 64716-01 (R.H.C.) and American Heart Association grant SDA 0335172N (R.H.C.), and grant M01 RR14288.
Acknowledgments
The authors extend their gratitude to Oumitana Kajkenova and Leizleigh Robinette for their clinical support. The authors also extend their gratefulness to our volunteers for their efforts.
References
- 1. Brower P, Hicks D. Maintaining muscle function in patients on bed rest. Am J Nurs. 1972;72:1250. [PubMed] [Google Scholar]
- 2. Cress RH, Burrell F, Fleming WC. A review of the dangers of prolonged bed rest. Ala J Med Sci. 1968;5:434–440. [PubMed] [Google Scholar]
- 3. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223. [DOI] [PubMed] [Google Scholar]
- 4. Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Phys. 1996;270(4 Pt 1):E627–E633. [DOI] [PubMed] [Google Scholar]
- 5. Ikezoe T, Mori N, Nakamura M, Ichihashi N. Effects of age and inactivity due to prolonged bed rest on atrophy of trunk muscles. Eur J Appl Physiol. 2012;112:43–48. :10.1007/s00421-011-1952-x [DOI] [PubMed] [Google Scholar]
- 6. Donini LM, Poggiogalle E, Piredda M, et al. Anorexia and eating patterns in the elderly. PLoS One. 2013;8:e63539. :10.1371/journal.pone.0063539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kortebein P, Symons TB, Ferrando A, et al. Functional impact of ten days bed rest in healthy older adults. J Gerontol Med Sci. 2008;63:1076–1081. [DOI] [PubMed] [Google Scholar]
- 8. Cesari M, Pahor M, Laurentani F, et al. Skeletal muscle and mortality results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2009;64:377–384. :10.1093/gerona/gln031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Newman AB, Kupelin V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. [DOI] [PubMed] [Google Scholar]
- 10. Drummond MJ, Dickinson JM, Fry CS, et al. Bed rest impairs skeletal amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab. 2012;302:E1113–E1122. :10.1152/ajpendo.00603.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Breen L, Stokes KA, Churchward-Venne TA, et al. Two weeks of reduced activity decreases lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab. 2013;98:2604–2612. :10.1210/jc.2013-1502 [DOI] [PubMed] [Google Scholar]
- 12. Coker RH, Hays NP, Williams RH, Xu L, Wolfe RR, Evans WJ. Bed rest worsens impairments in fat and glucose metabolism in older, overweight adults. J Gerontol A Biol Sci Med Sci. 2014;69:363–370. :10.1093/gerona/glt100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koster A, Stenholm S, Alley DE, et al. ; for the Health ABC study. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring). 2010;18:2354–2361. :10.1038/oby.2010.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yeo SE, Hays NP, Dennis RA, et al. Fat distribution and glucose metabaolism in older, obese men and women. J Gerontol A Biol Sci Med Sci. 2007;62:1393–1401. [DOI] [PubMed] [Google Scholar]
- 15. Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. [DOI] [PubMed] [Google Scholar]
- 16. Wang CY, Olson SL, Protas EJ. Physical-performance tests to evaluate mobility disability in community-dwelling elders. J Aging Phys Act. 2005;13:184–197. [DOI] [PubMed] [Google Scholar]
- 17. Coker RH, Williams RH, Yeo SE, et al. The impact of exercise training compared to caloric restriction on hepatic and peripheral insulin resistance in obesity. J Clin Endocrinol Metab. 2009;94:4258–4266. :10.1210/jc.2008-2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cress RH, Burrell F, Fleming WC. A review of the dangers of prolonged bed rest. Ala J Med Sci. 1968;5:434–440. [PubMed] [Google Scholar]
- 19. Janssen I. Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J Am Geriatr Soc. 2006;54:56–62. [DOI] [PubMed] [Google Scholar]
- 20. Hirsch CH, Sommers L, Olsen A, Mullen L, Winograd CH. The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38:1296–1303. [DOI] [PubMed] [Google Scholar]
- 21. Visser M, Harris TB, Fox KM, et al. Change in muscle mass and muscle strength after a hip fracture: relationship to mobility recovery. J Gerontol A Biol Sci Med Sci. 2000;55:M434–M440 [DOI] [PubMed] [Google Scholar]
- 22. Deutz NE, Safar A, Schutzler S, et al. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr. 2011;30:759–760. :10.1016/j.clnu.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dang SL. ABCDEs of ICU: early mobility. Crit Care Nurs Q. 2013;36:163–168. :10.1097/CNQ.0b013e318283cf45 [DOI] [PubMed] [Google Scholar]
- 24. Ferrando AA, Paddon-Jones D, Hays NP, et al. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr. 2010;29:18–23. :10.1016/j.clnu.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 25. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well functioning older persons. J Gerontol A Biol Sci Med Sci. 2006;60A:324–333. [DOI] [PubMed] [Google Scholar]
- 26. Volpato S, Bianchi L, Lauretani F, et al. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care. 2012;35:1672–1679. :10.2337/dc11-2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koster A, Stenholm S, Alley DE, et al. ; Health ABC study. Body fat distribution and inflammation among older adults with or without metabolic syndrome. Obesity (Silver Spring). 2010;18:2354–2361. :10.1038/oby.2010.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beavers KM, Beavers DP, Houston DK, et al. Associations between body composition and gait-speed decline: results from the Health, Aging and Body Composition study. Am J Clin Nutr. 2013;97:552–560. :10.3945/ajcn.112.047860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cesari M, Pahor M, Lauretani F, et al. Skeletal muscle and mortality results from the InCHIANTI study. J Gerontol Med Sci. 2009;64A:377–384. :10.1093/gerona/gln031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fiser WM, Hays NP, Rogers SC, et al. Energetics of walking in elderly people: factors related to gait speed. J Gerontol. Med Sci. 2010;65:1332–1337. :10.1093/gerona/glq137 [DOI] [PubMed] [Google Scholar]
- 31. Castell MV, Sánchez M, Julián R, Queipo R, Sagrario M, Otero A. Frailty prevalence and slow walking speed in persons age 65 and older: implications for primary care. BMC Fam Pract. 2013;14:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vasunilashorn S, Coppin AK, Patel KV, et al. Use of the short physical performance battery score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2009;64:223–229. :10.1093/gerona/gln022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. [DOI] [PubMed] [Google Scholar]
- 34. Elbaz A, Sabia A, Brunner E, et al. Association of walking speed in late midlife with mortality: results from the Whitehall II cohort study. Age (Dordr). 2013;35:943–952. :10.1007/s11357-012-9387-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coker RH, Wolfe RR. Bedrest and sarcopenia. Curr Opin Clin Nutr Metab Care. 2012;15:7–11. :10.1007/s11357-012-9387-9 [DOI] [PMC free article] [PubMed] [Google Scholar]