Abstract
Background
Human papillomavirus (HPV) vaccines are ideally administered before HPV exposure; therefore, catch-up programs for girls past adolescence have not been readily funded. We evaluated the benefits and cost-effectiveness of a delayed, 1-year female catch-up vaccination program in Norway.
Methods
We calibrated a dynamic HPV transmission model to Norwegian data and projected the costs and benefits associated with 8 HPV-related conditions while varying the upper vaccination age limit to 20, 22, 24, or 26 years. We explored the impact of vaccine protection in women with prior vaccine-targeted HPV infections, vaccine cost, coverage, and natural- and vaccine-induced immunity.
Results
The incremental benefits and cost-effectiveness decreased as the upper age limit for catch-up increased. Assuming a vaccine cost of $150/dose, vaccination up to age 20 years remained below Norway's willingness-to-pay threshold (approximately $83 000/quality-adjusted life year gained); extension to age 22 years was cost-effective at a lower cost per dose ($50–$75). At high levels of vaccine protection in women with prior HPV exposure, vaccinating up to age 26 years was cost-effective. Results were stable with lower coverage.
Conclusions
HPV vaccination catch-up programs, 5 years after routine implementation, may be warranted; however, even at low vaccine cost per dose, the cost-effectiveness of vaccinating beyond age 22 years remains uncertain.
Keywords: human papillomavirus, vaccine, cost-effectiveness analysis, disease transmission models, herd immunity
High-risk HPV infection is associated with invasive cancer in a variety of organ systems in both women (ie, cervix, vulva, vagina, anus, and oropharynx) and men (ie, penis, anus, and oropharynx) [1]. In addition, low-risk HPV infections are linked to the development of genital warts and recurrent respiratory papillomatosis (RRP) [2].
The prophylactic quadrivalent (4-valent) HPV vaccine, which protects against 2 high-risk types (HPV-16 and -18) and 2 low-risk types (HPV-6 and -11), is most effective when administered before HPV exposure and is, therefore, ideally targeted to young individuals before sexual initiation. Several countries elected to fund temporary catch-up programs for females up to ages 16 or 18 years (eg, United Kingdom) and, in fewer countries, up to age 26 years (eg, Australia) [3]. While few girls will be exposed to vaccine-type infections by age 12 years (ie, the target age of many routine HPV vaccination programs), exposure to HPV increases as sexual activity increases, resulting in decreased effectiveness of the vaccine program. In 2014, girls from the first Norwegian 12-year-old cohort vaccinated against HPV (in 2009) are 17 years old (born in 1997). Females aged ≥18 years (born before 1997) will not have had the opportunity to be vaccinated during the routine vaccination program yet still may benefit from direct protection because vaccine trials have demonstrated that acute infection with one HPV type does not impede protection against incident infection with another HPV type [4]. In addition, a recent analysis from the 4-valent clinical trial demonstrated that individuals with a history of a vaccine-type infection may receive some protection against future type-specific infections [5]; however, the magnitude of this protection is uncertain. A recent cost-effectiveness analysis of the 2-valent HPV vaccine [6] varied assumptions about vaccine efficacy among women with a prior history of infection and found that this assumption had a decisive impact on the cost-effectiveness of a catch-up program (cervical cancer end points only were assessed in this analysis). Moreover, herd immunity, propagated by expanding coverage in the population, may benefit unvaccinated individuals (both women and men).
Given the emerging evidence of the effectiveness of HPV vaccination in surveillance studies [7–9], coupled with declining vaccine prices, Norwegian health authorities are currently debating whether implementation of a temporary catch-up program for cohorts of women born between 1988 and 1996 (aged 18–26 years in 2014) is warranted, even 5 years after the start of the routine vaccination program. The decision to implement a temporary catch-up program is time sensitive because the initially vaccinated cohorts are approaching the upper vaccination age limit of 26 years. Decision-analytic models, used to project the expected benefits and costs of alternative vaccination strategies, can help inform health policy in the absence of long-term health outcomes. Our primary objective was to estimate the incremental benefits and cost-effectiveness of a delayed, 1-year catch-up HPV vaccination program for females up to age 26 years by explicitly considering the vaccine impact on multiple HPV-related conditions in women, as well as the indirect herd immunity benefits provided to both men and women. Our secondary objective was to explore the impact of varying vaccine protection in women with a history of vaccine-targeted infections on the cost-effectiveness.
METHODS
Analytic Approach
Under various scenarios of catch-up vaccination in females, we used a dynamic transmission model to project reductions in cumulative HPV incidence in multiple birth cohorts, capturing both direct and indirect benefits. To project long-term cost and health outcomes, we applied these reductions to a microsimulation model of cervical cancer and incidence-based models of noncervical HPV-related diseases. We conducted a cost-effectiveness analysis consistent with Norwegian guidelines [10], adopted a societal perspective, and discounted costs and quality-adjusted life years (QALYs) by 4% per year over the lifetime of each simulated cohort. We calculated the incremental cost-effectiveness ratio (ICER), defined as the incremental costs divided by the incremental benefits of one strategy, compared with the next less costly strategy. We used a commonly cited Norwegian threshold of Norwegian Kroner (NOK) 500 000, or approximately $83 000, per QALY gained to represent a cost-effective intervention but explored a range of threshold values to reflect a lack of consensus in Norway.
Models
First, we used a previously published [11–13] dynamic model of HPV-16 and -18 infections that simulates sexual mixing and HPV transmission among females and males. Females can acquire an HPV-16 or -18 infection and develop precancerous cervical lesions and, over time, may develop invasive cancer. Women who clear their infection or lesion develop natural immunity, which effectively reduces their future risk of being infected with that same type but keeps them fully susceptible to the other type. The model has a similar structure for males but only reflects HPV infections.
We used a microsimulation model of cervical carcinogenesis to capture cervical cancer outcomes associated with all HPV types and Norwegian screening strategies [13–15]. The model is individual based and tracks the history of each woman, including screening schedule, treatment history, and healthcare expenditures. We leveraged the strengths of both models by applying the reductions in sex- and type-specific HPV incidence from vaccination from the dynamic model to the corresponding inputs in the microsimulation model. This linkage between the 2 models allowed us to reflect herd immunity and to assess the joint impact of vaccination and screening on cervical outcomes. Finally, we developed separate Markov models to simulate the incidence of 7 additional HPV-related conditions (ie, vaginal, vulvar, anal, oropharyngeal, and penile cancer; genital warts; and RRP). The dynamic model outcomes were linked with these other disease models to estimate the additional benefits of the vaccine on noncervical HPV-related health outcomes in both women and men.
Epidemiologic Data
Baseline inputs for the dynamic and microsimulation models were based on data from epidemiological and clinical studies and have been described previously [11, 12, 14, 16] (Table 1). Likelihood-based calibration methods were used to adjust baseline inputs and identify parameters that achieved good fit to Norwegian epidemiologic outcomes, such as HPV prevalence [13] and cervical cancer incidence [17]. Details of the Norwegian calibration process for both the microsimulation and dynamic models have been previously published [13, 15]. For individuals naive to vaccine-targeted HPV types, we assumed high, lifelong vaccine efficacy of 100% against HPV-16/18 disease. For individuals who cleared HPV-16 or -18 infection, either before or after vaccination (ie, nonnaive individuals), we assumed complete protection against infection or disease caused by the other type [18] but varied the amount of vaccine protection against the type to which they were previously exposed. Trial data from the intention-to-treat arm of the 4-valent HPV randomized trial among adult women aged 24–45 years [5] guided the calibration of a model parameter that varied the level of protection for women previously infected. Before analyses, the model parameter was fit to generate a lower bound (ie, 41.6%), using the observed vaccine protection against all HPV-16/18 end points reported in the intention-to-treat population, and an upper bound (ie, 84.7%), using the observed vaccine protection against HPV-16/18 end points within the per-protocol population. In addition to varying vaccine efficacy for individuals with a prior infection, other vaccine characteristics (eg, vaccine efficacy in HPV-naive individuals) and type-specific natural immunity were explored in sensitivity analysis. The natural immunity parameters were calibrated in the natural history cervical cancer model and used as an input into the dynamic model before calibration [13]. The dynamic model was then recalibrated using lower natural immunity values of 55% and 62% for HPV-16 and -18, respectively.
Table 1.
Selected Input Parameters
| Parameter | Incidence, Cases/100 000a,c | 5-year Survival, %b | Cases Due to HPV-16/18, %c | Utilityd | Cost, $e |
|---|---|---|---|---|---|
| HPV-related condition | |||||
| Anal cancer, women | 1.9 (0–9.1) | 70.4 | 82 | 0.57 | 37 500 |
| Anal cancer, men | 0.9 (0–5.7) | 51.3 | 82 | 0.57 | 37 500 |
| Cervical cancer | 24.0 (0–32.0) | 19.9–91.0 | 72 | 0.48–0.76 | 25 800–59 600 |
| Oropharyngeal cancer, women | 1.5 (0–6.5) | 57.6 | 54 | 0.58 | 49 000 |
| Oropharyngeal cancer, men | 3.8 (0–14.1) | 60.3 | 54 | 0.58 | 49 000 |
| Penile cancer | 2.0 (0–11.4) | 81 | 46 | 0.79 | 17 500 |
| Vaginal cancer | 0.6 (0–4.3) | 48.6 | 66 | 0.59 | 26 400 |
| Vulvar cancer | 3.4 (0–26.5) | 72.8 | 44 | 0.65 | 27 900 |
| Genital warts, women | 0.0–7.14 | … | 90 | 0.9277 | 400 |
| Genital warts, men | 0.0–8.85 | … | 90 | 0.9277 | 400 |
| Juvenile RRP | 0.17 | … | 100 | 0.69 | 133 800 |
| HPV vaccine, per dose | |||||
| Vaccine | … | … | … | … | 50–150 |
| Supplies and administration, ≤19 y | … | … | … | … | 14 |
| Supplies, administration, and transport, >19 ye | … | … | … | … | 117 |
Abbreviations: HPV, human papillomavirus; RRP, recurrent respiratory papillomatosis.
a Data are from the Cancer Registry of Norway [17] (2008–2010) for all noncervical HPV-related cancers. For invasive cervical cancer incidence, prescreening rates (1953–1969) reported by the Norwegian Cancer Registry were used to calibrate the natural history microsimulation model. See Methods for details. The incidence of genital warts is per 1000.
b Data are estimated on the basis of information from the Cancer Registry of Norway [17], using calendar-period observations for 2006–2010. For cervical cancer, the range represents stage-specific estimates for local (91%), regional (66%), and distant (19.9%) cancers.
c The noncervical incidence-based models were based on data from the Cancer Registry of Norway (cancer-specific incidence and survival [17]), published studies (eg, incidence of genital warts and RRP [33–35]), and the World Health Organization HPV database (proportion of cases attributable HPV-16 and -18, by site) [36, 37].
d Quality-of-life adjustment range from a health state utility weight of 0 (death) to 1 (perfect health). For cancer-specific conditions, we conservatively assumed that individuals would remain in a reduced quality of life for 5 years, after which they would return to their age- and sex-specific utility values elicited from another Scandinavian country [27]. Disease-specific utility weights were multiplied by baseline age-specific utility weights to estimate overall utility. Weights for cervical cancer varied according to stage (local, 0.76 for 5 years; regional, 0.67 for 5 years; distant, 0.48 for lifetime with disease); utility weights for other noncervical HPV-related cancers were applied for 5 years [20]. For genital warts, a mean quality-of-life loss of 6.6 days was assumed [21], which is approximately a utility weight of 0.9277 over 3 months; for RRP, a health state utility weight of 0.68 over 4 years was assumed.
e The cost per case is expressed in 2010 US dollars ($1 = 6.05 Norwegian Krone) and represents discounted (4% per year) costs for diagnosis and 5-year follow-up, inclusive of direct costs (procedures, inpatient stays, and general practitioner visits), direct nonmedical costs (transport), and patient time costs. The proportion of direct nonmedical and patient-time costs for all noncervical conditions was estimated from cervical cancer (15%) applied to baseline direct medical costs. The cost of treatment of cervical cancer varies according to stage of detection (local, $25 800; regional, $51 600; distant, $59 600).
f Data include the cost of an office visit (adjusted according to Norwegian economic evaluation guidelines), co-pay, and time and transport (using the average 2010 monthly earning plus fringe benefits for females <25 years old [http://www.ssb.no/en]) associated with vaccine administration outside the school-based program. The office visit, office wait time, and travel time (to/from) the appointment was assumed to take 1.5 hours.
Economic and Quality-of-Life Data
Screening and treatment-related costs included direct medical costs (procedures, inpatients stays, and general practitioner visits), nondirect medical costs (transport), and patient time costs, and the methods to estimate these costs have been reported previously (Table 1) [13, 15]. We assumed that vaccination of females aged >19 years would incur higher delivery costs (ie, through their family physician), compared with school-based vaccine administration for each of the 3 required doses. For the base case results, we assumed a vaccine cost per dose of $75 [13] but varied the assumption widely to account for the higher market cost, as well as future (lower) tender negotiations. All costs were measured in 2010 NOK and converted to US dollars, using the average annual 2010 exchange rate ($1 = NOK6.05) [19]. To reflect diminished quality of life due to morbidity of disease (eg, cancer and genital warts), we applied health-state utility weights for each condition [20, 21]. Sensitivity analysis explored the impact of only accounting for disease-specific mortality, reported here as life-years saved (LYS).
Strategies
We compared the current program of routine vaccination of 12-year-old girls (starting in 2009), assuming 3-dose coverage of 71%, to different catch-up scenarios in 2014, assuming 50% coverage of women aged 18 years up to ages 20, 22, 24, or 26 years (Figure 1). All strategies included cervical cancer screening starting at age 25 years, based on current practice in Norway [22]. Our primary analysis included the vaccine effects on all 8 HPV-related disease outcomes and assumed the lower bound of vaccine protection for women with a history of infection across 3 alternative vaccine costs assumptions. A secondary analysis assessed the impact of the upper bound of vaccine protection for women with a prior history of vaccine-targeted infections. For the primary analysis, 1-way sensitivity analysis also explored the impact of differential coverage among women targeted by the catch-up program (30% vs 50%) and the discount rate (0% and 3%).
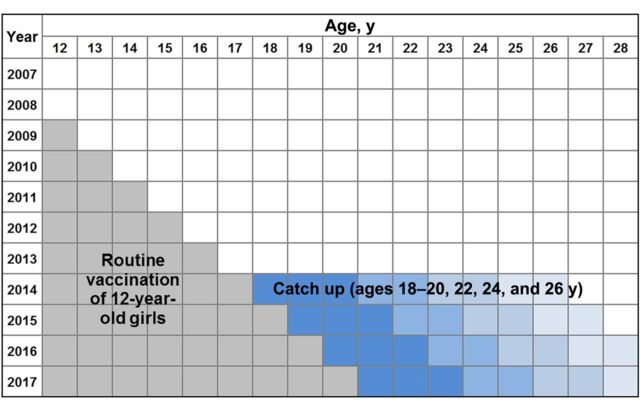
Figure 1.
Lexis diagram of the delayed temporary catch-up program for human papillomavirus vaccination. Gray boxes designate the current strategy of vaccinating incoming cohorts of 12-year-old girls (initiated in 2009). Blue boxes signify the proposed delayed, 1-year catch-up vaccination of females aged 18–20 years (dark blue) or 22, 24, or 26 years (lightest blue) in 2014.
RESULTS
The incremental benefits from vaccination decreased as the upper age limit of the catch-up program increased. For example, at 50% coverage and assuming the lower bound of vaccine protection in women previously exposed to vaccine-type infections, the cohort of women aged 20 years at the time of catch-up vaccination (in 2014) experience an absolute 22% higher cumulative reduction in HPV-16/18 incidence, compared with routine vaccination of 12-year-old girls over their lifetime. For the cohort of girls vaccinated at age 26 years in 2014, this gain was only 4.2% (data not shown). When we assumed the upper bound of vaccine protection in those who were previous exposed, these benefits increased to 26% and 7.3% among females aged 20 years and 26 years, respectively. Importantly, increasing the vaccination age limit in the catch-up program expedited the overall declines in HPV-16/18 prevalence (Figure 2). The projected impact of the vaccine on the average lifetime risk of cervical cancer also diminished as additional catch-up cohorts were vaccinated and varied considerably by the amount of vaccine protection assumed for women with a prior vaccine-type infection (Figure 3). For example, when we applied the lower bound vaccine protection in nonnaive women, the lifetime risk of cervical cancer for the 26-year-old cohort was reduced from a baseline of 1.10% (screening only) to 0.93% (with routine vaccination program only) and 0.88% (with a temporary catch-up program achieving 50% coverage for women aged 18–26 years; Figure 3). However, when we assumed the upper bound of vaccine protection, the lifetime risk of cervical cancer was projected to be closer to that of the 18-year-old cohort (Figure 3).
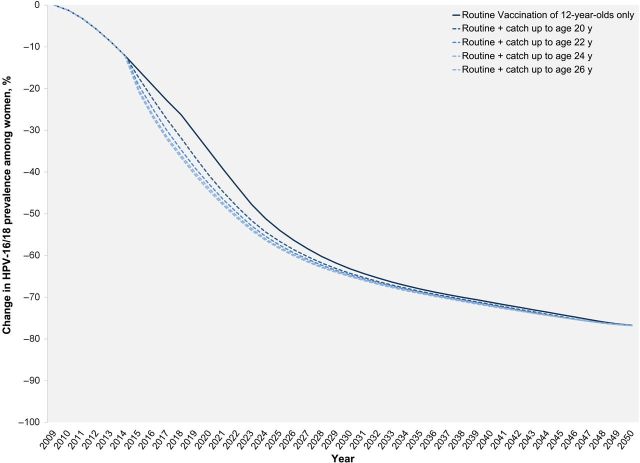
Figure 2.
Projected change in human papillomavirus (HPV)–16/18 prevalence, by year, for the primary analysis (assuming 71% coverage for routine vaccination of 12-year-old girls, 50% coverage for the catch-up cohorts, and the lower-bound vaccine protection in women previously exposed to a vaccine-targeted HPV types). Among males, the expected declines in HPV prevalence (due to herd immunity) follow a similar trend, although at a slower pace and with less overall benefit (data not shown).
Figure 3.
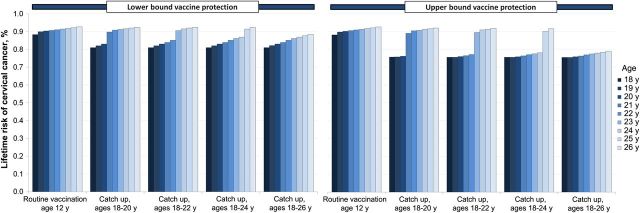
Lifetime cervical cancer risk, by age of cohort in 2014, by catch-up vaccination strategy (assuming 50% coverage), and by vaccine protection in women previously exposed to a vaccine-targeted human papillomavirus (HPV) type. In the absence of any HPV vaccination program (ie, status quo cervical cancer screening only), the model projects a lifetime cervical cancer risk of 1.1%.
The results of our cost-effectiveness analysis followed a similar trend at different levels of vaccine cost and HPV-related outcomes (Table 2 and Supplementary Table 1). For example, when considering only cervical cancer outcomes, a market price of $150 per dose, all catch-up programs yielded incremental cost-effectiveness ratios of greater than $83 000 per QALY gained, the common benchmark for cost-effectiveness in Norway. However, when we considered vaccine benefits for all male and female HPV-related diseases, the incremental cost-effectiveness ratio of a 1-year catch-up program up to age 20 years fell below the Norwegian threshold (ie, $53 500 per QALY gained). At more realistic vaccine tender prices (ie, $50 or $75 per dose), a 1-year catch-up program could be extended to include women up to age 22 years while still remaining cost-effective in Norway. However, for settings with cost-effectiveness thresholds less than $50 000 per QALY gained, vaccinating beyond age 20 years would not be considered cost-effective unless the vaccine cost per dose was $10 or less (data not shown).
Table 2.
Incremental Cost-effectiveness Ratios (ICER) for Alternative Costs Per Human Papillomavirus (HPV) Vaccine Dose, Assuming the Lower or Upper Bound of Vaccine Protection for Women With a Previous HPV-16 or -18 Infection
| Outcome, Age Group | Cost Per Dosea |
|||||
|---|---|---|---|---|---|---|
| $50 |
$75 |
$150 |
$50 |
$75 |
$150 |
|
| Lower Bound Protectionb | Upper Bound Protectionb | |||||
| Cervical cancer outcomes onlyc | ||||||
| Catch up, ages 18–20 yd | $44 000 | $57 400 | $96 000 | $24 800 | $33 100 | $56 800 |
| Catch up, ages 18–22 y | $116 300 | $135 400 | $190 200 | $53 100 | $62 300 | $88 700 |
| Catch up, ages 18–24 y | $169 300 | $196 700 | $275 100 | $59 800 | $70 100 | $99 500 |
| Catch up, ages 18–26 y | $204 200 | $236 800 | $330 500 | $60 100 | $70 300 | $99 800 |
| Oncogenic outcomes only | ||||||
| Catch up, ages 18–20 yd | $32 200 | $42 400 | $71 600 | $17 400 | $23 500 | $41 300 |
| Catch up, ages 18–22 y | $90 900 | $106 000 | $149 300 | $41 100 | $48 400 | $69 300 |
| Catch up, ages 18–24 y | $151 100 | $175 600 | $245 700 | $50 000 | $58 700 | $83 600 |
| Catch up, ages 18–26 y | $196 200 | $227 500 | $317 500 | $52 700 | $61 800 | $87 800 |
| All HPV-related outcomes | ||||||
| Catch up, ages 18–20 yd | $22 500 | $30 500 | $53 500 | $12 900 | $18 700 | $33 300 |
| Catch up, ages 18–22 y | $67 800 | $79 600 | $113 200 | $34 300 | $40 700 | $59 100 |
| Catch up, ages 18–24 y | $117 700 | $137 200 | $193 100 | $44 500 | $52 400 | $75 200 |
| Catch up, ages 18–26 y | $167 200 | $194 000 | $271 700 | $49 500 | $58 100 | $82 900 |
ICER values in bold signify a cost-effective strategy given a cost-effectiveness threshold of $83 000 per quality-adjusted life-year gained.
Abbreviation: HPV, Human papillomavirus.
a Exclusive of administration (all ages), as well as time and transport, for females >19 years-old.
b Vaccine protection in those with prior history of vaccine-targeted HPV types. See Methods for definitions of upper and lower bounds. Vaccine efficacy was assumed to be 100% among individuals with no prior history.
c All strategies assume status quo cervical cancer screening in Norway.
d This strategy is compared with routine vaccination of 12-year-old girls only, assuming 71% coverage for all 3 required doses.
Our secondary analysis, which considered a high level of vaccine protection for women with a history of prior infection, yielded similar trends but generally more-attractive ratios. For example, when all HPV-related outcomes were considered, vaccinating up to age 26 years fell below common benchmarks of cost-effectiveness in Norway (Table 2 and Supplementary Table 1). At a vaccine price of $50 per dose, the incremental cost-effectiveness ratio dropped below $50 000 per QALY gained. However, even if the vaccine price were $25 per dose, vaccinating up to ages 24 and 26 years would remain unattractive strategies at a cost-effectiveness threshold of $30 000 per QALY gained, despite assuming optimistic and comprehensive vaccine benefit to multiple HPV-related diseases (data not shown).
Sensitivity Analysis
When we varied the assumptions made in our primary analysis (50% coverage, $75 per dose, and lower bound of vaccine protection in women with prior vaccine-targeted HPV types), we found that, apart from a 0% discount rate, it was never attractive to vaccinate beyond age 22 years, as the incremental cost-effectiveness ratios consistently remained above the common benchmark for cost-effectiveness in Norway (Figure 4). In addition, expressing the incremental cost-effectiveness ratios in terms of life-years (not QALYs) gained was the only situation in which vaccinating up to age 22 years was not attractive. At $50 per dose (data not shown), vaccinating up to age 24 years yielded ratios below $83 000 per QALY gained either when the vaccine efficacy for those naive to vaccine-targeted HPV types was reduced from 100% to 90% ($80 400 per QALY gained) or when only direct medical costs (ie, costs excluding travel and time costs) were considered ($81 000 per QALY gained).
Figure 4.
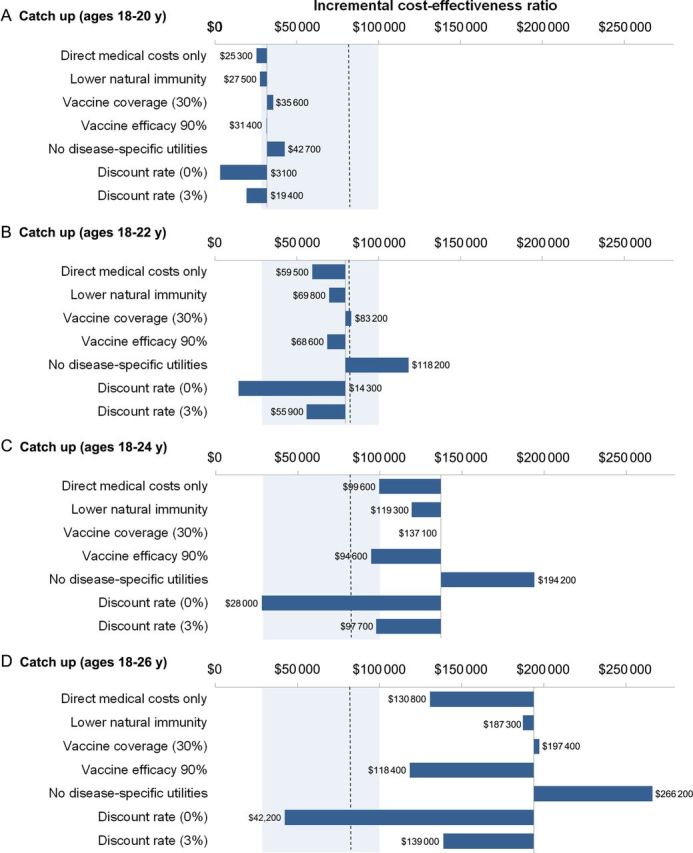
Impact of parameter assumptions on the incremental cost-effectiveness ratio. Dashed line represents the commonly cited cost-effectiveness threshold in Norway (ie, $83 000 per quality-adjusted life-year gained), and the shaded region represents the upper and lower bounds of commonly cited cost-effectiveness thresholds. The base scenario assumes comprehensive vaccine benefit to multiple human papillomavirus (HPV)–related conditions (cervical, vaginal, vulvar, anal, penile, and oropharyngeal cancer; genital warts; and juvenile-onset recurrent respiratory papillomatosis), 50% coverage for catch-up vaccination (ages 18–26 years), $75 per vaccine dose, and the lower bound of vaccine protection among women previous exposed to vaccine-targeted HPV types.
DISCUSSION
A temporary HPV vaccination program to catch-up cohorts of women not initially offered the 4-valent HPV vaccine may still be warranted 5 years after successfully introducing routine HPV vaccination of preadolescent girls. From a cost-effectiveness perspective, the upper vaccine age limit is influenced by several decisive factors, including (1) the amount of vaccine protection provided to women previously exposed to vaccine-targeted infections, (2) the number of HPV-related conditions considered, and (3) the vaccine cost. Even under the most favorable assumptions, vaccinating up to age 26 years would only be considered cost-effective if the threshold willingness-to-pay per QALY gained is not less than $50 000.
To our knowledge, this is the first analysis to assess the value of a delayed temporary HPV vaccination program (ie, 5 years after initiating routine vaccination of 12-year-old girls) that incorporates transmission dynamics, includes multiple male and female HPV-related end points, and reports results in an incremental fashion. A recent health technology assessment commissioned by the Norwegian Institute of Public Health [23] found that a catch-up program for ages 18–26 years, compared with no catch-up, was cost-effective for a willingness-to-pay threshold of NOK265 327 per QALY gained (approximately $43 900), assuming a vaccine cost per dose of approximately $82. We found similarly attractive results ($36 000 per QALY gained) if we compared vaccination of women up to age 26 years directly to routine vaccination only (ie, no other age limits for the temporary catch-up vaccination program). As noted in a recent review [24], however, HPV vaccination catch-up programs may appear artificially more attractive if the catch-up age groups are not disaggregated, and therefore, the additional benefits and costs should be presented incrementally by age. More generally, several studies that have assessed catch-up and routine HPV vaccination programs have found that vaccinating beyond age 22 years is not cost-effective [11, 25], although considerable variation in the optimal upper age limit exists. For example, some studies [26–28] found that vaccinating up to age 26 years is attractive, while another study found vaccinating beyond age 15 years [29] was unattractive. Explanation for the differences between model analyses have been discussed in a recent review [24] and are generally attributed to model assumptions, such as the amount of prior exposure to HPV, natural immunity assumptions, transmission dynamics, and vaccine characteristics (eg, protection among those with prior exposure and cost per dose). Reported differences may also stem from analysis presentation, as noted earlier.
Our study accounted for the additional indirect benefits (ie, herd immunity) that may be conferred to unvaccinated individuals, including men. Transmission dynamics are particularly important for determining whether to implement a delayed catch-up program, as multiple cohorts of 12-year-old girls have already achieved high coverage and are likely generating herd immunity benefits among those cohorts not initially vaccinated. For example, in our analysis, the 18-year-old cohort was projected to experience a 20% reduction in the lifetime risk of cervical cancer in the absence of a catch-up program, because of the herd immunity benefits generated from adjacent vaccinated cohorts (Figure 3). Analyses that fail to consider transmission dynamics are likely to overestimate the incremental benefits generated by a catch-up program. We also included vaccine impact on other noncervical HPV-related conditions; accounting for these additional conditions reduced the incremental cost-effectiveness ratios by as much as 42% and had more impact on the primary analysis (which assumed a lower bound of vaccine protection), compared with the secondary analysis (which assumed an upper bound of vaccine protection). Benefits to noncervical HPV-related conditions are seldom included in other studies but are clearly important.
We assumed a base case coverage rate of 50%, which is higher than what is currently observed in temporary catch-up programs in both Denmark and Australia for individuals aged >19 years (ie, 35% and 39%, respectively) [30, 31]; however, our results remained stable for coverage rates of 30% in the catch-up program. Similar to another study [6], we found that considering a lower natural immunity yielded more attractive cost-effectiveness ratios for catch-up campaigns; however, this assumption did not change the optimal strategy of vaccination up to age 22 years in the primary analysis. Across parameter variations, the incremental cost-effectiveness ratio associated with vaccinating up to age 20 years remained below $50 000 per QALY gained, vaccinating up to age 22 years remained above $50 000 per QALY gained, and vaccinating up to ages 24 or 26 years yielded a ratio above $95 000 per QALY gained across key parameter variations.
Similar to our analysis, a study that evaluated the 2-valent HPV vaccine [6] and considered cervical cancer end points also found that assumptions surrounding vaccine protection among nonnaive women played a pivotal role in determining the optimal upper vaccine age limit. As an alternative to assuming an uninformed prior (between 0 and full benefit) for vaccine protection in women with previous vaccine-type HPV exposure, we chose to calibrate a model parameter to fit the 4-year reductions observed in the clinical trial for adult women [5]. The model-projected impact of the HPV vaccine on reducing HPV-related disease among those previously exposed to vaccine-type infections corresponded with the lower bound of the range reported from posttrial evaluations for women >24 years old [5]. The calibrated upper bound of vaccine protection represented an optimistic scenario because it was defined using the observed protection among women who completed all 3 vaccine doses and tested negative for HPV (across multiple genital sites) by both DNA and serologic analysis at study entry (ie, the per protocol population). Although unrealistic in population-based implementation, this upper bound analysis provides a best-case estimate.
Our study has several limitations, particularly surrounding the natural history of infection and behavioral trends that may influence expected benefits. For example, preliminary insights from women in Denmark suggest there may be a correlation between opting to participate in the national catch-up program and having a lower average number of sex partnerships and, possibly, having a higher likelihood of participating in screening (M. Nygård, unpublished data). Under this assumption, we would expect the proposed catch-up program to be less cost-effective because the overall risk of disease in these individuals is lower. Conversely, these women may be less likely to have had previous exposure to HPV, and the vaccination may yield greater benefits resulting in more attractive scenarios for older catch-up cohorts.
We also did not include the impact of vaccine efficacy in reducing the incidence of infection due to nonvaccine HPV types (ie, cross-protection). This additional benefit would impact our results only to the extent to which these other cross-protected types play a causal role in the cancers: for cervical cancer, those other types are important, but for most other cancers, HPV-16 is of primary importance. The 4-valent vaccine (on tender in Norway) has been suggested to be partially efficacious against HPV-31, yet the vaccine's cross-protective effects are uncertain with respect to durability [32]. We expect that including these additional benefits may make the program more attractive, although previous analyses [11, 25] have found that including cross-protection provides small additional benefits. Last, our understanding of the natural history and HPV type attribution of noncervical HPV-related diseases is limited but growing; analyses should be revisited as new information on the burden of HPV on these other diseases emerges.
Policies surrounding the implementation of a delayed temporary catch-up program are time sensitive, because initially vaccinated cohorts will soon reach the upper age limit of 26 years. Moreover, as we demonstrate in this analysis, the incremental benefit of vaccinating older women decreases with age. For countries assessing whether to implement a temporary catch-up program, the maximum value achieved by these decisions will only decrease as decisions are delayed.
In summary, under reasonable vaccine price assumptions and comprehensive vaccine benefit to multiple HPV-related conditions, the Norwegian HPV vaccination program may be temporarily extended to age 22 years while remaining cost-effective. The cost-effectiveness of vaccinating beyond this age becomes more uncertain, because the upper vaccine age limit is influenced by several decisive factors, most notably, the level of vaccine protection among women with previous exposure to vaccine-targeted HPV types.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. E. A. B., M. N., I. S. K., and J. J. K. contributed to the conception and design of the study. E. A. B. and S. S. performed the analysis. E. A. B., S. S., I. S. K., and J. J. K. assisted with interpretation of data. E. A. B. drafted the manuscript. All authors assisted in critically revising the manuscript for important intellectual content and approved the final version to be published. E. A. B. is the guarantor for the study.
Disclaimer. All work by the authors was independent of the funders, and the funding sources had no involvement in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the manuscript for publication.
Financial support. This work was supported by the Norwegian Cancer Society (634201-2012 to E. A. B.) and the US National Cancer Institute of the National Institutes of Health (R01CA160744 to J. J. K.).
Potential conflicts of interest. I. S. K. and M. N. were members of the Norwegian Directorate of Health national advisory board for cervical cancer screening. M. N. has received a research grant through her affiliated institution, the Cancer Registry of Norway, from MSD Norge (a subsidiary of Merck) to perform registry linkage studies. Specifically, and in order to adhere to regulatory commitment, the Nordic Cancer Registries were invited by Merck to perform 2 analyses: (1) a long-term follow-up study that evaluates the long-term effectiveness, safety, and immunogenicity of Gardasil (HPV-6, -11, -16, and -18 recombinant vaccine) over 14 years among vaccinated subjects who were enrolled in the clinical phase 3 study and residing in one of the 4 Nordic countries (Denmark, Iceland, Norway, and Sweden), and (2) a study to evaluate the impact of Gardasil in the general female population. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.zur Hausen H. Papillomaviruses in the causation of human cancers—a brief historical account. Virology. 2009;384:260–5. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 2.Lacey CJN, Lowndes CM, Shah KV. Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24:35–41. doi: 10.1016/j.vaccine.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Markowitz LE, Tsu V, Deeks SL, et al. Human papillomavirus vaccine introduction—the first five years. Vaccine. 2012;30:F139–48. doi: 10.1016/j.vaccine.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 4.Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30:F123–38. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castellsague X, Munoz N, Pitisuttithum P, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24-45 years of age. Br J Cancer. 2011;105:28–37. doi: 10.1038/bjc.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner HC, Baussano I, Garnett GP. Vaccinating women previously exposed to human papillomavirus: a cost-effectiveness analysis of the bivalent vaccine. PLoS One. 2013;8:e75552. doi: 10.1371/journal.pone.0075552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldur-Felskov B, Dehlendorff C, Munk C, Kjaer SK. Early impact of human papillomavirus vaccination on cervical neoplasia—nationwide follow-up of young Danish women. J Natl Cancer Inst. 2014;106:djt460. doi: 10.1093/jnci/djt460. [DOI] [PubMed] [Google Scholar]
- 8.Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ. 2013;346:f2032. doi: 10.1136/bmj.f2032. [DOI] [PubMed] [Google Scholar]
- 9.Markowitz LE, Hariri S, Lin C, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis. 2013;208:385–93. doi: 10.1093/infdis/jit192. [DOI] [PubMed] [Google Scholar]
- 10.Norwegian Directorate of Health. Economic evaluation of healthcare—a guide. Oslo, Norway: Norwegian Directorate of Health. 2012.
- 11.Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359:821–32. doi: 10.1056/NEJMsa0707052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JJ, Goldie SJ. Cost effectiveness analysis of including boys in a human papillomavirus vaccination programme in the United States. BMJ. 2009;339:b3884. doi: 10.1136/bmj.b3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burger E, Sy S, Nygår M, Kristiansen I, Kim J. Prevention of HPV-related cancers in Norway: Cost-effectiveness of expanding the HPV vaccination programme to include pre-adolescent boys. PLoS One. 2014;9:e89974. doi: 10.1371/journal.pone.0089974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JJ, Kuntz KM, Stout NK, et al. Multiparameter calibration of a natural history model of cervical cancer. Am J Epidemiol. 2007;166:137–50. doi: 10.1093/aje/kwm086. [DOI] [PubMed] [Google Scholar]
- 15.Burger EA, Ortendahl JD, Sy S, Kristiansen IS, Kim JJ. Cost-effectiveness of cervical cancer screening with primary human papillomavirus testing in Norway. Br J Cancer. 2012;106:1571–8. doi: 10.1038/bjc.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldhaber-Fiebert JD, Stout NK, Ortendahl J, Kuntz KM, Goldie SJ, Salomon JA. Modeling human papillomavirus and cervical cancer in the United States for analyses of screening and vaccination. Popul Health Metr. 2007;5:11. doi: 10.1186/1478-7954-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancer Registry of Norway. Institute of Population-Based Cancer Research. http://www.kreftregisteret.no . Accessed 1 February 2013.
- 18.Villa LL, Perez G, Kjaer SK, et al. Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection. J Infect Dis. 2007;196:1438–46. doi: 10.1086/522864. [DOI] [PubMed] [Google Scholar]
- 19.Federal Reserve. Historical rates for the Norwegian Krone. 2011. http://www.federalreserve.gov/RELEASES/H10/Hist/dat00_no.htm. Accessed 13 June 2011.
- 20.Conway EL, Farmer KC, Lynch WJ, Rees GL, Wain G, Adams J. Quality of life valuations of HPV-associated cancer health states by the general population. Sex Transm Infect. 2012;88:517–21. doi: 10.1136/sextrans-2011-050161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodhall SC, Jit M, Soldan K, et al. The impact of genital warts: loss of quality of life and cost of treatment in eight sexual health clinics in the UK. Sex Transm Infect. 2011;87:458–63. doi: 10.1136/sextrans-2011-050073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer Registry of Norway. Oslo: Cancer Registry of Norway: 2009. 2008 annual report population-based screening against cervical cancer. [Google Scholar]
- 23.Jimenez E, Wisløff T, Klemp M. Oslo: Norwegian Knowledge Centre for the Health Services; 2014. Cost-effectiveness of a HPV-vaccination catch-up program for females aged 26 years or younger in a Norwegian setting. Report from Kunnskapssenteret no. 5-2014. [PubMed] [Google Scholar]
- 24.Canfell K, Chesson H, Kulasingam SL, Berkhof J, Diaz M, Kim JJ. Modeling preventative strategies against human papillomavirus-related disease in developed countries. Vaccine. 2012;30:F157–67. doi: 10.1016/j.vaccine.2012.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jit M, Choi YH, Edmunds WJ. Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ. 2008;337:a769. doi: 10.1136/bmj.a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dasbach EJ, Insinga RP, Elbasha EH. The epidemiological and economic impact of a quadrivalent human papillomavirus vaccine (6/11/16/18) in the UK. Bjog-An Int J Obstet Gynaecol. 2008;115:947–56. doi: 10.1111/j.1471-0528.2008.01743.x. [DOI] [PubMed] [Google Scholar]
- 27.Olsen J, Jepsen MR. Human papillomavirus transmission and cost-effectiveness of introducing quadrivalent HPV vaccination in Denmark. Int J Technol Assess Health Care. 2010;26:183–91. doi: 10.1017/S0266462310000085. [DOI] [PubMed] [Google Scholar]
- 28.Elbasha EH, Dasbach EJ, Insinga RP, Haupt RM, Barr E. Age-based programs for vaccination against HPV. Value Health. 2009;12:697–707. doi: 10.1111/j.1524-4733.2009.00512.x. [DOI] [PubMed] [Google Scholar]
- 29.Usher C, Tilson L, Olsen J, Jepsen M, Walsh C, Barry M. Cost-effectiveness of human papillomavirus vaccine in reducing the risk of cervical cancer in Ireland due to HPV types 16 and 18 using a transmission dynamic model. Vaccine. 2008;26:5654–61. doi: 10.1016/j.vaccine.2008.07.098. [DOI] [PubMed] [Google Scholar]
- 30.Statens Serum Institute. EPI-NEWS. http://www.ssi.dk/English/News/EPI-NEWS/2013/No%2020%20-%202013.aspx. Accessed 20 December 2013.
- 31.Australian Government Department of Health. Immunise human papillomavirus (HPV) http://www.health.gov.au/internet/immunise/publishing.nsf/content/immunise-hpv. Accessed 20 December 2013.
- 32.Malagón T, Drolet M, Boily M, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:781–9. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- 33.Leval A, Herweijer E, Ploner A, et al. Quadrivalent human papillomavirus vaccine effectiveness: a Swedish national cohort study. J Natl Cancer Inst. 2013;105:469–74. doi: 10.1093/jnci/djt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Health Protection Agency. Number & rates of anogenital warts diagnosed in England, 2002-2011. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1296688631209. Accessed 11 January 2013.
- 35.Omland T, Akre H, Vardal M, Brondbo K. Epidemiological aspects of recurrent respiratory papillomatosis: A population-based study. Laryngoscope. 2012;122:1595–9. doi: 10.1002/lary.23327. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. ICO (Institut Català d'Oncologia). Information Centre on Human Papilloma Virus (HPV) and Cervical Cancer: data query system. http://www.who.int/hpvcentre/en/ Accessed 15 November 2012.
- 37.Mork J. Prevalence of HPV in oropharyngeal carcinomas in patients diagnosed at Oslo University Hospital 2010-1011 [conference proceeding]. Presented at: MD Anderson GAP Conference; 2012. http://webtv.medinfo.no/Mediasite/Play/99a9cb5b7412477c8a92e0413%20db5321b1d?catalog=54190625-deb6-433e-869c-b4fbf3b58b6c. Accessed 2 February 2013. [Google Scholar]