Abstract
Administration of combination antiretroviral therapy to human immunodeficiency virus type 1 (HIV-1)–infected pregnant women significantly reduces vertical transmission. In contrast, maternal co-opportunistic infection with primary or reactivated cytomegalovirus (CMV) or other pathogens may facilitate in utero transmission of HIV-1 by activation of cord blood mononuclear cells (CBMCs). Here we examine the targets and mechanisms that affect fetal susceptibility to HIV-1 in utero. Using flow cytometry, we demonstrate that the fraction of CD4+CD45RO+ and CD4+CCR5+ CBMCs is minimal, which may account for the low level of in utero HIV-1 transmission. Unstimulated CD4+ CBMCs that lack CCR5/CD45RO showed reduced levels of HIV-1 infection. However, upon in vitro stimulation with CMV, CBMCs undergo increased proliferation to upregulate the fraction of T central memory cells and expression of CCR5, which enhances susceptibility to HIV-1 infection in vitro. These data suggest that activation induced by CMV in vivo may alter CCR5 expression in CD4+ T central memory cells to promote in utero transmission of HIV-1.
Keywords: mother-to-child transmission, cord blood mononuclear cells, HIV-1, CMV, central memory CD4 cells, CCR5
(See the editorial commentary by Emery on pages 169–71, and the major articles by Lichtner et al on pages 178–86.) 10.1093/infdis/jiu419 10.1093/infdis/jiu417
Despite effective administration of combination antiretroviral therapy to human immunodeficiency virus type 1 (HIV-1)–infected pregnant women, mother-to-child transmission (MTCT) of HIV-1 remains a significant global public health concern. Several copathogens that pose threats to the developing fetus during gestation, such as cytomegalovirus (CMV), malaria parasites, and Mycobacterium tuberculosis, may also facilitate in utero transmission of HIV-1 and contribute to the high incidence of MTCT within specific populations [1–3]. These pathogens can promote transmission through increases in proviral load, immune activation, local inflammation, and maternal stimulation of fetal CD4+cells to increase cellular susceptibility to HIV-1 infection.
CMV, a highly prevalent beta herpesvirus, infects >90% and 50%–80% of reproductive-age women in developing and developed countries, respectively. HIV-1–infected infants who acquire CMV infection in the first 18 months of life have a significantly higher rate of disease progression than those infected with HIV-1 alone [4, 5]. Studies have shown that CMV induces increases in T-cell activation in peripheral blood mononuclear cells (PBMCs) from HIV-1–infected infants [6] and memory cell differentiation [7]. Strong associations between in utero CMV infection and a higher incidence of MTCT of HIV-1 have been documented [8, 9]; however, the mechanisms that increase neonatal HIV-1 transmission remain unknown. Several in vitro studies demonstrate increased viral transcription and replication in phytohemagglutinin (PHA)/interleukin 2 (IL-2)–activated cord blood mononuclear cells (CBMCs), compared with adult PBMCs. However, these conditions do not mimic the in utero environment, which is normally immunoquiescent and associated with a low risk of HIV-1 transmission (7%) in vivo.
In contrast, immune activation may facilitate HIV-1 infection following in utero exposure to CMV antigens. Increased T-cell activation may be associated with CCR5 expression and susceptibility of CBMCs to HIV-1. The goal of the current study is to examine the targets and mechanisms that affect fetal susceptibility to HIV-1 in utero. We demonstrate that the fraction of CD4+CCR5+ and CD4+CD45RO+ T cells in cord blood is significantly lower, compared with adult PBMCs, which may reflect that the low level of in utero HIV-1 transmission is due to a lack of target cells. However, upon in vitro stimulation with CMV antigens or PHA/IL-2, CBMCs undergo increased proliferation and upregulate the fraction of T central memory (TCM) cells and expression of CCR5 among CD4+ T cells. This upregulation of CCR5 was more pronounced in TCM cells, compared with naive CD4+ CBMCs. Unstimulated CD4+ CBMCs that lack CCR5 and CD45RO showed reduced levels of HIV-1 infection and replication in vitro, compared with PBMCs. However, priming CBMCs with CMV antigens influenced the proliferation and activation of CCR5+ TCM cells, rendering these more susceptible to HIV-1 infection in vitro.
METHODS
Ethics Statement
With written informed consent, umbilical cord blood specimens were collected from 15 women (age, >18 years) seronegative for HIV-1, CMV, and hepatitis B virus following cesarean section without labor (gestation, >37 weeks) at Emory Midtown Hospital (Atlanta, GA). Approval of the study was granted from the Emory University Institutional Review Board (IRB). Peripheral blood specimens were obtained from healthy adult volunteer donors according to a protocol approved by the Emory University IRB.
Isolation and Stimulation of Mononuclear Cells
CBMCs and PBMCs were separated from heparinized whole-blood samples by density gradient centrifugation on Ficoll-Hypaque (Sigma Chemical Co., St. Louis, MO) and maintained in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% fetal bovine serum, 1 mM L-glutamine, and 1% pen/strep (Mediatech, Manassas, VA). CBMCs and PBMCs were stimulated with PHA (5 µg/mL) and IL-2 (5%) in the complete medium. CD4+ T cells were purified from isolated mononuclear cells by negative selection, using the CD4 isolation kit (Miltenyi Biotec, Auburn, CA) according to the manufacturer's protocol, yielding an average purity of ≥96%. To isolate macrophages, monocytes were then enriched from PBMCs by use of the MACS Monocyte Isolation Kit II and MACS LS Columns (Miltenyi), yielding an average purity of 98%. Monocytes were suspended in RPMI medium containing pen/strep (1%), glutamine (1%), and heat-inactivated normal human serum (10%; Mediatech); seeded into 24-well plates; and cultivated for 7 additional days to promote full differentiation into macrophages.
HIV-1 Infection of PBMCs and CBMCs
PBMCs and CBMCs were infected at 0.5 50% tissue culture infective doses (TCID50) per cell for 4 hours at 37°C with HIV-1BaL. The TCID50 values were calculated according to the method of Reed and Muench [2]. The HIV-1BaL strain is R5 trophic and was initially isolated from infant lung tissue [10]. To monitor viral production, cell supernatants were collected at various days after infection. Viral replication was measured using enzyme-linked immunosorbent assay to detect p24 released into the supernatant (Advanced BioScience Laboratories, Rockville, MD).
Real-Time Polymerase Chain Reaction (PCR)
Messenger RNA (mRNA) was extracted using the RNAeasy kit (Qiagen, Valencia, CA). The complementary DNA was transcribed using QuantiTect RT kit (Qiagen). The primer sequences were as follows: env, forward primer 5′-GGGGACCAGGGAGAGCATT-3′ and reverse primer 5′-TGGGTCCCCTCCTGAGGA-3′; gag, forward primer 5′-ACATCAAGCAGCCATGCAAAT-3′ and reverse primer 5′-ATCTGGCCTGGTGCAATAGG-3′); cxcr4 forward primer 5′-TGACTCCATGAAGGAACCCTG-3′ and reverse primer 5′-CTTGGCCTCTGACTGTTGGTG-3′; ccr5, forward primer 5′-AATAATTGCAGTAGCTCTAACAGG-3′ and reverse primer 5′-TTGAGTCCGTGTCACAAGCCC-3′; and β-actin forward primer 5′-GGCCCAGTCCTCTCCCAAGTCCAC-3′ and reverse primer 5′-GGTAAGCCCTGGCTGCCTCCACC-3′. Real-time PCR was performed using SYBR Green (Qiagen). All reactions were run in triplicate, using the Applied Biosystems Prism 7500 Sequence Detection System. Delta threshold cycle (Ct) values from the calibrator and experimental groups were measured by subtracting the Ct value of the target from that of the housekeeping transcript, β-actin.
Flow Cytometry
Staining for flow cytometry studies was performed using monoclonal antibodies that are cross-reactive with PBMCs and CBMCs. One million cells were labeled with the following antibodies: anti-CD3 (A700), anti-CD4 (PerCp-Cy5.5), anti-CD8 (PE-Cy7), anti-CD45RO (APC), anti-CD27 (PE), anti-Ki67 (V450), anti-CD38 (PE-Cy5), anti-HLADR (APC-H7), CXCR4 (PE-Cy5), and anti-CCR5 (PE-CF594; BD Biosciences, CA). Detection of intracellular HIV-1 capsid p24 antigen was performed using KC57 monoclonal antibody (Coulter, Barcelona, Spain). Samples were processed on a BD LSR II flow cytometer, and analysis of the data was performed using FlowJo software (Tree Star). Cell frequencies were calculated as the percentage of cells with a fluorescence intensity greater than that of the fluorescence minus one (FMO) gate.
CMV Infection and Coculture Experiments
Human MRC-5 fetal lung fibroblasts (American Type Culture Collection [ATCC], Rockville, MD) were seeded in a T-25 flask and infected with human CMV strain AD169 (ATCC) at a multiplicity of infection (MOI) of 0.1. Macrophages were left untreated or incubated with CMV (MOI, 1). For coculture experiments, CMV-infected macrophages or uninfected macrophages were cultured with CBMCs or purified CD4+ cells in 24-well culture dishes for up to 6 days.
Statistical Analysis
Parametric data were analyzed by using Student t test (2-tailed) to assess whether the mean values for 2 normally distributed groups differed significantly. All nonparametric data were analyzed using the Mann–Whitney U Test. All error bars represent the standard error (±SE).
RESULTS
Unstimulated CBMCs Have Reduced Ability to Replicate HIV-1
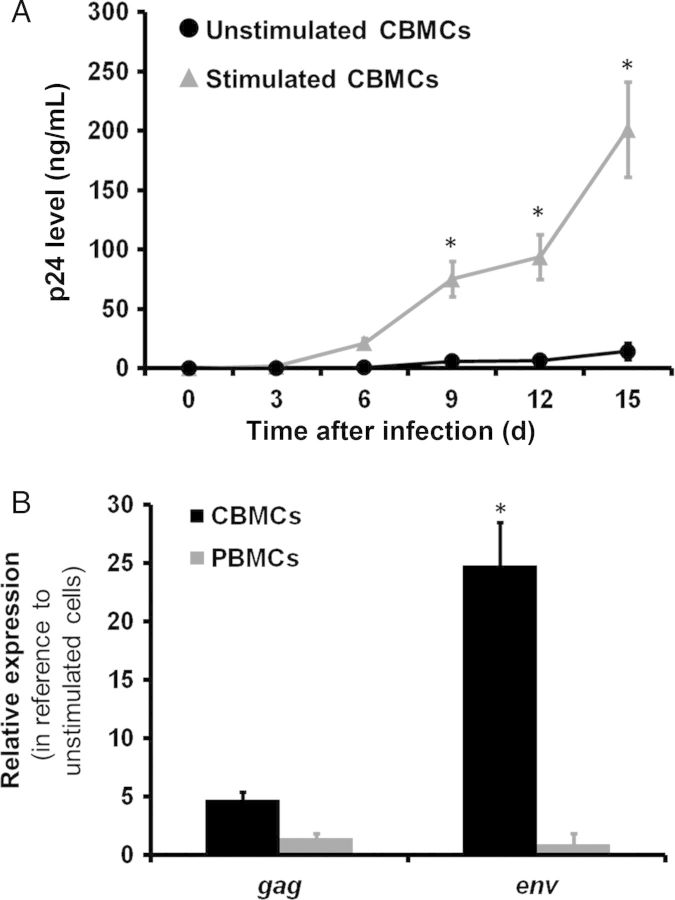
Several studies have demonstrated increased HIV-1 replication in PHA/IL-2–activated CBMCs, compared with adult PBMCs, but conditions in these studies do not mimic the in utero environment, which is typically an immunoquiescent milieu. To determine the ability of fetal blood to replicate HIV-1, we compared viral replication in unstimulated CBMCs to that in PHA/IL-2–stimulated cells, using HIV-1BaL at a TCID50 of 0.5 per cell. p24 was detected over time in supernatant fluid, compared with their stimulated counterparts. Mean production of p24 antigen in unstimulated cells was greatly reduced, compared with that detected in PHA/IL-2–stimulated CBMCs cultured in parallel at the same TCID50 (Figure 1A). To further account for the reduced ability of unstimulated CBMCs to replicate HIV-1, we monitored viral gene transcription 6 days after infection, using real-time PCR. We found that unstimulated CBMCs showed mean fold-decreases (±SE) of 4.7 ± 0.6 in gag mRNA (average Ct values) and 24 ± 4.3 in env mRNA, compared with PHA/IL-2–stimulated cells (Figure 1B). In contrast, stimulation had minimal effects on viral transcription in adult PBMCs. Combined, these results demonstrate that stimulated CBMCs have a markedly increased sensitivity to HIV-1 infection, while unstimulated CBMCs limit viral replication of HIV-1BaL in vitro.
Figure 1.
Unstimulated cord blood mononuclear cells (CBMCs) have reduced ability to replicate human immunodeficiency virus type 1 (HIV-1). Unstimulated and phytohemagglutinin/interleukin 2–stimulated CBMCs infected by HIV-1BaL in vitro showed differences in HIV-1 replication over time. A, HIV-1 replication was measured in the cell supernatants by detection of HIV-1 p24 viral antigen, using enzyme-linked immunosorbent assay. Data shown are expressed as the mean ± standard error (SE) of triplicate samples from 8 cord blood and 8 adult blood donors. B, Six days after HIV-1BaL infection, messenger RNA levels of unstimulated and stimulated CBMC or peripheral blood mononuclear cells (PBMCs) were measured by real-time polymerase chain reaction to determine the relative expression of gag and env. Data shown are expressed as the mean ± SE of triplicate samples from 8 cord blood and 8 adult blood donors. *P < .001, compared with unstimulated CBMCs at the corresponding time point.
CBMCs Have a Lower Fraction of CD45RO+ Memory T Cells, Compared With PBMCs
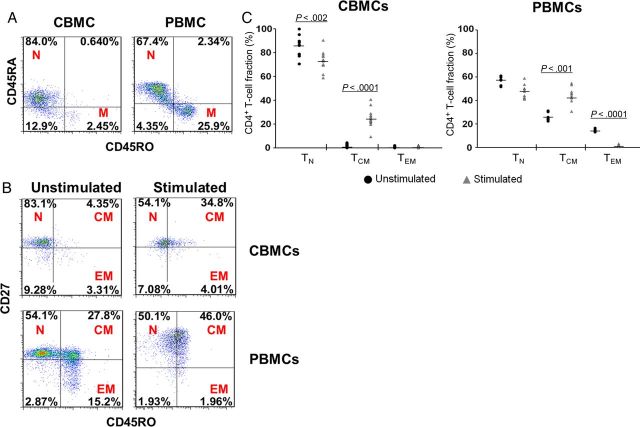
Previous studies have noted a paucity of CD4+ T cells with a memory phenotype in infants and children. In neonates, we investigated this further by first comparing the percentage of naive (TN), TCM, and effector memory (TEM) subsets among CD3+CD4+ T cells in cord blood samples derived from HIV-1–uninfected newborns and peripheral blood samples from HIV-1–uninfected healthy adults. Cell surface markers were used to divide the CD3+CD4+ T-cell populations into memory (CD45RA−CD45RO+), TN (CD45RO−CD27+), TCM (CD45RO+CD27+), and TEM (CD45RO+CD27−) subsets, as previously described [11–13]. The majority of cord blood lymphocytes have a naive phenotype and express minimal levels of CD45RO (Figure 2A). We also noted a significantly lower fraction of both memory subsets in CD4+ CBMCs, compared with PBMCs (Figure 2B). These findings may reflect relatively limited exposure to foreign antigens in utero in uncomplicated pregnancies [14] and corroborate previous data demonstrating a paucity of T cells with the memory phenotype in infants and children [15].
Figure 2.
Cord blood mononuclear cells (CBMCs) have a lower fraction of CD45RO+ memory T (TM) cells, compared with peripheral blood mononuclear cells (PBMCs). A, The fractions of naive T (TN) cells (N; CD45RA+CD45RO−) and TM cells (M; CD45RA−CD45RO+) were determined in cord blood samples derived from human immunodeficiency virus type 1 (HIV-1)–uninfected newborns and peripheral blood samples from HIV-1–uninfected healthy adults. Data shown are dot plots of 1 representative donor of 12 cord blood and 8 adult blood donors. B, Representative dot plots show TN cell (N; CD45RO−CD27+), T central memory (TCM) cell (CM; D45RO+CD27+), and T effector memory (TEM) cell (EM; CD45RO+CD27−) subsets among CD3+CD4+ T cells in unstimulated cells or following 4 days of phytohemagglutinin (PHA)/interleukin 2 (IL-2) stimulation of CBMCs and PBMCs. Data shown are dot plots of 1 representative donor of 12 cord blood and 8 adult blood donors. C, TN, TCM, and TEM fractions of CD3+CD4+ CBMCs or PBMCs without mitogen stimulation or following 4 days of in vitro stimulation with PHA/IL-2. Lines represent median values for each condition. Each data point represents the mean of triplicate samples from 12 cord blood and 8 adult blood donors. Statistical analyses were performed to compare stimulated cells (▴) to unstimulated cells (•) in each subset.
In Vitro Stimulation Upregulates the Fraction of TCM Cells in CD3+CD4+ CBMCs
To investigate how in vitro activation affects the fraction of memory cells in CBMCs, we assessed the percentage of TN and TCM in cells that were stimulated with the mitogens PHA/IL-2. We found that the percentage of TCM cells was significantly upregulated in CBMCs and PBMCs (Figure 2B). No differences were noted in the TEM fraction of the CBMCs, while stimulation induced a significant decrease of the TEM cells in adult PBMCs. In cord blood, the TCM fraction was significantly upregulated in PHA/IL-2–stimulated cells (P < .0001), compared with unstimulated CBMCs (Figure 2C).
CCR5 Expression Upon In Vitro Stimulation and Proliferation Is Increased in CD3+CD4+ CBMCs to Levels Similar to Those for Adult PBMCs
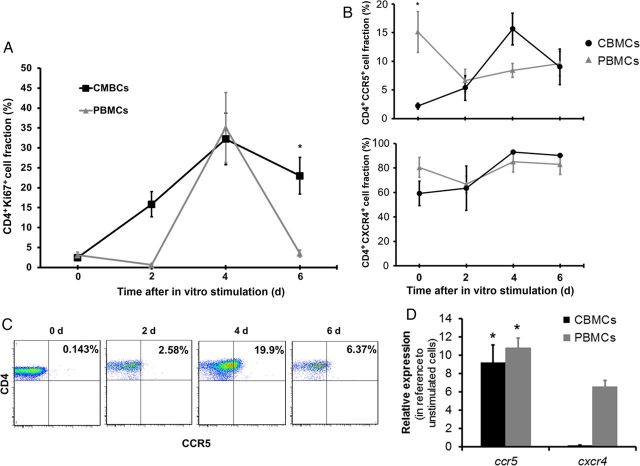
Next, we measured the fraction of CD4+CCR5+ and CD4+CXCR4+ T cells in CBMCs and PBMCs that were stimulated with PHA/IL-2 for 6 days. Over time, CD4+ T cells from cord and adult blood proliferated and became activated to a similar extent after stimulation, as determined by the expression of Ki67 (Figure 3A), a cellular marker for proliferation, and the activation marker CD38 (Figure 6A). In addition, CD4+CXCR4+ T cells were equivalent in CBMCs and PBMCs before and after stimulation (Figure 3B). In contrast, the fraction of CD4+ T cells expressing CCR5 was significantly lower in the CBMCs at baseline, compared with the PBMCs (P < .0001). Following stimulation with PHA/IL-2, the fraction of cord blood CD4+CCR5+ T cells increased such that, by day 4, CCR5 levels were significantly elevated, compared with the unstimulated cells (P < .0001), and that, by day 6, CCR5 levels were similar to those noted in stimulated adult PBMCs (Figure 3B and 3C). To determine whether this low level of CCR5 expression by CBMCs at baseline and following in vitro stimulation was associated with an increase in CCR5 transcription, we assessed CCR5 and CXCR4 mRNA expression in purified CD4+ T cells obtained from cord and adult blood specimens 24 hours after stimulation with PHA/IL-2. Following treatment, we found that stimulated CBMCs showed a mean fold-increase (±SE) of 9.25 ± 1.9 in CCR5 mRNA expression (average Ct values) and a mean fold-decrease of 0.15 in CXCR4 mRNA expression. In contrast, PBMCs showed an increase in mRNA expression of both chemokine receptors. These results suggest that CCR5 expression may be upregulated in neonates in response to stimuli, which could increase susceptibility to HIV-1.
Figure 3.
CCR5 expression upon in vitro stimulation and proliferation is increased in CD3+CD4+ cord blood mononuclear cells (CBMCs) to levels similar to those in adult peripheral blood mononuclear cells (PBMCs). A, Fractions of CD4+Ki67+ cells were determined in CBMCs and PBMCs over time following in vitro stimulation with phytohemagglutinin (PHA)/interleukin 2 (IL-2). Data shown are expressed as the mean ± standard error (SE) of 12 cord blood and 8 adult blood samples. B, The fraction of CD4+CCR5+ and CD4+CXCR4+ after stimulation with PHA/IL-2 in CBMCs and PBMCs. Data shown are expressed as the mean ± SE of 12 cord blood and 8 adult blood samples. C, Representative dot plots showing the fraction of CD4+CCR5+ T cells after in vitro simulation with PHA/IL-2 in CBMCs. Data shown are dot plots of 1 representative donor of 12. D, Unstimulated and stimulated purified CD4+ CBMC and PBMC messenger RNA levels were measured after 24 hours by real-time polymerase chain reaction to determine the relative expression of CCR5 and CXCR4. Data shown are expressed as the mean ± SE of triplicate samples from 6 donors. *P < .001, compared with PBMCs (A and B) or unstimulated cells (D) at the corresponding time point.
Figure 6.
Expression of activation markers on T central memory (TCM) cord blood mononuclear cells (CBMCs) may correlate with human immunodeficiency virus type 1 (HIV-1) susceptibility. A, The fraction of CD4+ naive T (TN) cells and TCM CBMCs that express CD38 with or without HLA-DR was determined on day 4 after coculture with cytomegalovirus (CMV)–infected macrophages, uninfected macrophages (control), or phytohemagglutinin (PHA)/interleukin 2 (IL-2). Data shown are expressed as the mean ± standard error (SE) of samples from 8 individual donors. Purified CD4+ CBMCs were infected by HIV-1BaL after coculture with CMV-infected macrophages, uninfected macrophages (control), or PHA/IL-2. B, HIV-1 replication was measured in the cell supernatants over time by detection of HIV-1 p24 viral antigen, using enzyme-linked immunosorbent assay. Data shown are expressed as the mean ± SE of samples from 8 individual donors. C, Messenger RNA levels were measured 6 days after infection by real-time PCR to determine the relative expression of env and gag. Data shown are expressed as the mean ± SE of samples from 8 individual donors. *P < .001, compared with control CBMCs at the corresponding time point.
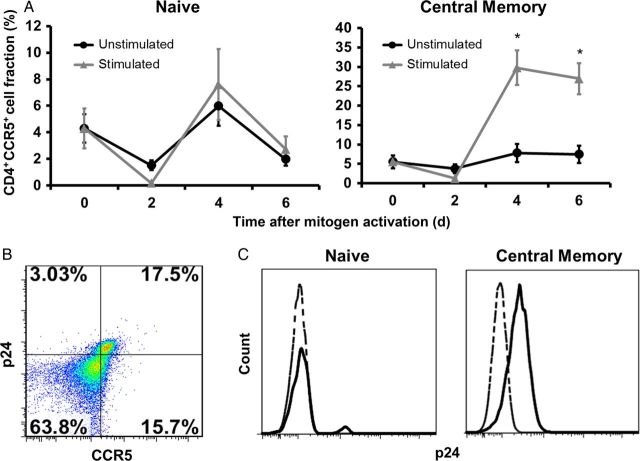
CCR5 Is Upregulated in Activated Cord Blood TCM Cells, Which Greatly Increases Their Susceptibility to HIV-1 Infection
Recent studies have demonstrated that regulation of CCR5 expression on CD4+ TCM cells and protection of these cells from direct virus infection are key factors that favor the lack of progression to AIDS in sooty mangabeys [16]. The low fraction of TCM and CD4+CCR5+ T cells may account for the low rate of MTCT of HIV-1 in utero (7%). To determine the effect of stimulation on CCR5 expression of CD4+ TCM cells, we assessed the fraction of CCR5+ cells in CD4+ TN and TCM cells in cord blood. Whereas CD4+ TN cells from unstimulated and stimulated groups showed similar kinetics of CCR5 expression (Figure 4A), only CD4+ TCM cells consistently upregulated CCR5, which was significantly higher in the stimulated cells, compared with the unstimulated cells (P < .001). To confirm whether increased CCR5 expression is related to an increase in HIV-1 susceptibility, we infected CBMCs and measured the fraction of p24+/CCR5+ in CD4+ T cells or p24+ cells within the CD4+ TN and TCM subsets on day four after infection. Double staining for p24 and CCR5 following stimulation showed preferential infection of CD4+CCR5+ T cells (Figure 4B). We also found that the fraction of p24+ cells was greater in the TCM subset, compared with the naive cells, following stimulation (Figure 4C). Collectively, these findings indicate that stimulation of cord blood T cells leads to upregulation of CCR5 in TCM cells, which greatly increases their susceptibility to HIV-1.
Figure 4.
CCR5 is upregulated in activated cord blood T central memory (TCM) cells, which greatly increases their susceptibility to human immunodeficiency virus type 1 (HIV-1) infection. A, The fractions of CD4+ naive T (TN) cells and TCM cord blood mononuclear cells (CBMCs) that express CCR5 were determined over time after in vitro mitogen activation with phytohemagglutinin (PHA)/interleukin 2 (IL-2). Data shown are expressed as the mean ± standard error of samples from 12 individual donors. B, Flow cytometry dot plots showing p24 and CCR5 double staining in a representative PHA/IL-2–stimulated cord blood sample 4 days after infection with HIV-1BaL. Data shown are dot plots of 1 representative donor of 8. C, Representative histograms show p24 expression in TN and TCM CBMCs 4 days after infection with HIV-1BaL. Unstimulated cells are represented with a dashed lined, and stimulated cells are represented with a solid line. Relative cell number is on the y-axis, and fluorescence intensity is on the x-axis. Data shown are dot plots of 1 representative donor of 8. *P < .001, compared with unstimulated CBMCs at the corresponding time point.
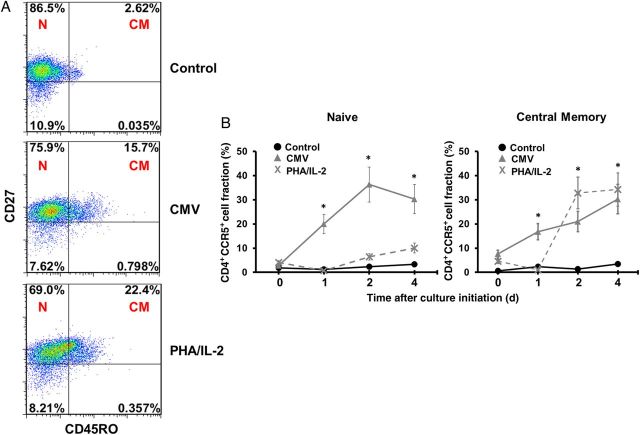
CBMCs Primed With CMV Upregulate CCR5 Expression on TCM CD4+ Cells
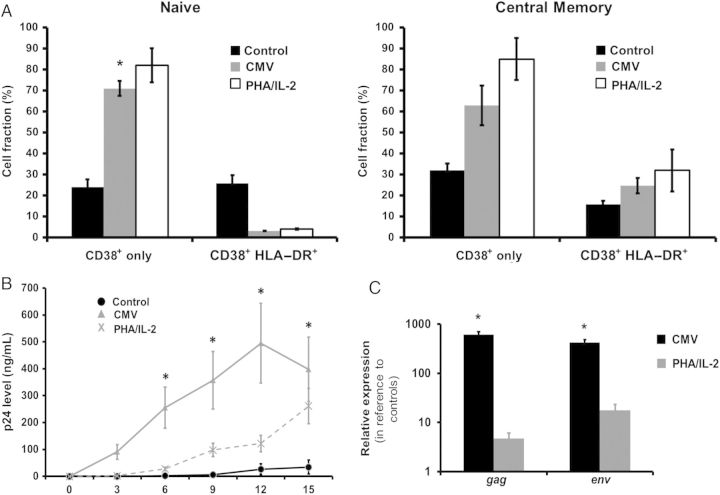
Next we determined how CMV affects CCR5 expression of CD4+ T cells isolated from cord and adult blood specimens. Cells were cocultured with CMV-infected adult macrophages for 4 days in the absence of other mitogenic stimulation. Treatment of CBMCs with CMV antigens resulted in a moderate increase of CD4+ proliferation in both cord and adult cells, but a dramatic increase in the TCM population was noted, compared with control CBMCs cocultured with uninfected macrophages (increase, approximately 8-fold; Figure 5A). In addition, the TCM subset in the CMV-experienced CBMCs expressed high percentages of CCR5 after in vitro stimulation. Unlike the PHA/IL-2 group, CMV treatment appears to upregulate CCR5 expression on both naive and memory subsets (Figure 5B).
Figure 5.
Cord blood mononuclear cells (CBMCs) primed with cytomegalovirus (CMV) upregulate CCR5 expression on CD4+ T central memory (TCM) cells. A, Naive T (TN) cell (N; CD45RO−CD27+) and TCM cell (CM; CD45RO+CD27+) subsets were analyzed among CD3+CD4+ CBMCs cocultured for 4 days with CMV-infected macrophages, uninfected macrophages (control), or phytohemagglutinin (PHA)/interleukin 2 (IL-2). Data shown are dot plots of 1 representative donor of 8. B, The fraction of CD4+ TN and TCM CBMCs that express CCR5 was determined over time after coculture with CMV-infected macrophages (▴), uninfected macrophages (control; •), or PHA/IL-2 (X). Data shown are expressed as the mean ± standard error of samples from 8 individual donors. *P < .001, compared with control CBMCs at the corresponding time point.
Expression of Activation Markers on TCM CBMCs May Correlate With HIV-1 Susceptibility
We further examined the role of CMV priming on immune activation by evaluating whether TN and TCM from CMV-primed and/or PHA/IL-2–treated CBMCs showed increased activation as measured by CD38 and HLA-DR expression. We found that the control groups maintained consistently lower levels of CD38 in both groups. In contrast, stimulation with CMV and PHA/IL-2 induced upregulation of CD38+ cells in TN and TCM subsets. Coexpression of CD38 and HLA-DR was lower for the TN cells, compared with control, and was slightly increased in the TCM subset (Figure 6A). Taken together, these findings indicate that CBMCs become activated following stimulation of CMV antigen.
To investigate whether CMV priming is associated with greater susceptibility to HIV-1 infection, we compared viral replication in purified CD4+ CBMCs cocultured with CMV-infected macrophages, uninfected macrophages, or PHA/IL-2, using HIV-1BaL at a TCID50 of 0.5 per cell. p24 fluid was detected in supernatant over time. Mean production of p24 antigen in CBMCs cocultured with CMV-infected macrophages was significantly elevated over time, compared with production in control CBMCs cultured in parallel (Figure 6B). Interestingly, the replication kinetics of CBMCs primed with CMV was more robust than that for the PHA-IL-2–treated cells. In addition, we monitored viral gene transcription 6 days after infection, using real-time PCR. We found that CMV antigen–experienced cells exhibited mean fold-increases (±SE) of 607.57 ± 125.63 in gag mRNA (average Ct values) and 421.4 ± 117.75in env mRNA, compared with unstimulated cells (Figure 6C). Collectively, these results demonstrate that priming CBMCs with CMV may render these cells more susceptible to HIV-1 infection in vitro.
DISCUSSION
The risk of in utero transmission is <7%. Therefore, even in the absence of virologic suppression with maternal antiretroviral therapy, >90% of HIV-1–exposed newborns are naturally protected from infection in utero. In addition, cases of transient HIV-1 infection in infants, defined as detection of HIV-1 in 1 or more peripheral blood specimen from an exposed infant followed by no detection of HIV-1 in a subsequent specimen, have been reported [17–19]. These observations suggest that the developing fetus and neonate may restrict establishment of lentiviral infection and viral latency in resting CD4+CD45RO+ memory cells, which may reflect a lack of antigenic exposure in utero and during infancy. In peripheral blood, CD4+CD45RO+ memory cells were found to be major in vivo targets of infection by R5-trophic HIV-1 [20, 21]. Here we showed a paucity of memory cells in CBMCs and reduced expression of CCR5. Furthermore, in vitro studies show that CD4+ T cells from cord blood must be activated prior to infection with HIV-1. This suggests that the neonatal immune system is immunoquiescent and that a reduced number of target cells may limit vertical transmission of HIV-1.
Neonates have higher rates of morbidity and mortality from infectious diseases than adults, owing to their increased susceptibility to infection and their increased risk of disease progression. This intrinsic susceptibility to pathogens has been related to the immaturity of the neonatal immune system. Several studies have shown that neonatal T cells have reduced proliferative capacity in response to stimulation and limited cytotoxic activity [22]. However, in the presence of strong costimulatory signals, neonatal CD4+ and CD8+ T cells capable of adult-level responses have been reported [23–25]. A potential mechanism of fetal activation leading to increased viral susceptibility is by upregulation of CCR5, thereby facilitating viral entry [26]. Preferential R5 virus replication in memory cells was associated with higher CCR5 expression than in naive cells. Here we showed that stimulation of CBMCs with PHA/IL-2 induced a dramatic increase in CCR5 expression, particularly in CD4+ TCM CBMCs. In addition, stimulated CD4+ CBMCs showed high levels of viral replication, while HIV-1 replication was relatively restricted in unstimulated CD4+ CBMCs. Lack of CCR5 expression in cord blood CD4+ cells in vivo appears to be protective for neonates; however, induction of this receptor by appropriate stimuli may render fetal lymphocytes more susceptible to HIV-1 infection and could potentially facilitate transmission in utero.
Previous studies have shown that cord blood T cells can respond to endogenous antigens, minor histocompatibility antigens that are disparate between mother and fetus, and viral antigens if the mother is seropositive or vaccinated [27–29]. Therefore, antigens in the maternal environment may prime fetal cells transplacentally to elicit antigen-specific T-cell responses. Maternal infections with potential pathogens such as CMV, malaria parasites, and M. tuberculosis may influence the risk of MTCT of HIV-1. Previous studies documented that CBMCs from 50% of malaria-sensitized Kenyan infants were capable of being productively infected with HIV-1 in vitro without prior exogenous stimulation [1]. Other studies demonstrated that maternal tuberculosis is associated with increased immune activation and HIV-1 replication in infant T cells [3]. Initial reports dating back to 1989 demonstrated correlations between CMV infection and rapid progression to AIDS [30]. More recently, a strong association has been noted between maternal HIV-1 and congenital CMV infection in populations in sub-Saharan Africa where the seroprevalence of HIV-1 and CMV are high [9]. In addition, congenital and postnatal CMV acquisition are independent correlates of vertical transmission of HIV-1 [31]. Despite the immaturity of the neonatal immune system, fetuses infected in utero with CMV develop a mature and functional CD8+ T-cell response, similar to that detected in adults [23, 32]. During the acute phase of infection, these differentiated cells have a central memory phenotype. Several studies that noted preponderant CD8+ T-cell responses in antigen-primed cord blood also detected CMV-specific CD4+ responses in CBMCs from congenitally infected newborns.
The goal of the current study was to examine the targets and mechanisms that affect fetal susceptibility to HIV-1 in utero. We developed an in vitro model to examine how naive cord blood T cells might respond to CMV antigens through allopriming with CMV-infected macrophages. Macrophages have been shown to play an important role in initiating primary T-cell responses and are involved in the regulation of CMV latency and reactivation [33, 34]. We found that stimulation with CMV antigens led to an approximately 10-fold increase in TCM cells. These cells showed evidence of T-cell activation, along with steady increases in CCR5 expression over time. Activated T lymphocytes with increased CCR5 expression are strongly implicated in HIV-1 acquisition and disease progression [35, 36]. Other studies have noted that stimulated CD4+CD45RO+ CBMCs showed increased HIV-1 replication and gene expression, compared with adult PBMCs [37]. CD3+CD4+ CBMCs are the only cellular compartment that HIV-1BaL enters, and while TCM cells are capable of sustaining detectable viral replication following exposure, naive CD4+ T cells do not exhibit similar characteristics [11]. Interestingly, even though CD45RO+ T cells are minimal in neonates and infants, in cases where MTCT of HIV-1 has occurred, the majority of HIV-1–infected CD4+ T cells in infants and children are of the CD45RO+ phenotype [38]. Furthermore, recent studies have demonstrated that infected memory T cells that survive the apoptotic effects of the virus may return to a resting state and serve as a latent reservoir of HIV-1 in vivo [39]. In this regard, novel studies have reported that when R5-tropic HIV-1 abortively infects nonpermissive, quiescent CD4+ T cells from lymphoid tissue, these cells die by pyroptosis, an intensely inflammatory form of programmed cell death [40, 41]. This was shown to be particularly prominent within the CD4+CCR5+ T cells, and the chronic inflammation may stimulate proliferation of memory CD4+ T cells. Pyroptosis has not been defined in HIV-1–infected fetal/neonatal CD4+ T cells and warrants further investigation.
Maintaining low or negligible fractions of CD4+CD45RO+CCR5+ T cells by the fetus and infant may serve as an important mechanism of protection and account for the reduced rates of MTCT observed in utero. However, infants born to mothers coinfected with CMV may be primed by viral antigens so that subsequent activation and upregulation of CCR5 in TCM CBMCs could increase the risk of vertical transmission of HIV-1. Understanding host factors that increase HIV-1 susceptibility is essential to devise novel therapies for viral eradication.
Notes
Acknowledgment. We thank all of the donors who provided informed consent.
Financial support. This work is supported by the Emory Center for AIDS Research (grant P30AI050409) and the Emory Medical Care Foundation.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Steiner K, Myrie L, Malhotra I, et al. Fetal immune activation to malaria antigens enhances susceptibility to in vitro HIV infection in cord blood mononuclear cells. J Infect Dis. 2010;202:899–907. doi: 10.1086/655783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayisi JG, van Eijk AM, Newman RD, et al. Maternal malaria and perinatal HIV transmission, western Kenya. Emerg Infect Dis. 2004;10:643–52. doi: 10.3201/eid1004.030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Bhosale R, Kinikar A, et al. Maternal tuberculosis: a risk factor for mother-to-child transmission of human immunodeficiency virus. J Infect Dis. 2011;203:358–63. doi: 10.1093/jinfdis/jiq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovacs A, Schluchter M, Easley K, et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. N Engl J Med. 1999;341:77–84. doi: 10.1056/NEJM199907083410203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle M, Atkins JT, Rivera-Matos IR. Congenital cytomegalovirus infection in infants infected with human immunodeficiency virus type 1. Pediatr Infect Dis J. 1996;15:1102–6. doi: 10.1097/00006454-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Slyker JA, Rowland-Jones SL, Dong T, et al. Acute cytomegalovirus infection is associated with increased frequencies of activated and apoptosis-vulnerable T cells in HIV-1-infected infants. J Virol. 2012;86:11373–9. doi: 10.1128/JVI.00790-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lissauer D, Choudhary M, Pachnio A, Goodyear O, Moss PA, Kilby MD. Cytomegalovirus sero positivity dramatically alters the maternal CD8+ T cell repertoire and leads to the accumulation of highly differentiated memory cells during human pregnancy. Hum Reprod. 2011;26:3355–65. doi: 10.1093/humrep/der327. [DOI] [PubMed] [Google Scholar]
- 8.Slyker JA, Lohman-Payne BL, John-Stewart GC, et al. Acute cytomegalovirus infection in Kenyan HIV-infected infants. AIDS. 2009;23:2173–81. doi: 10.1097/QAD.0b013e32833016e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mwaanza N, Chilukutu L, Tembo J, et al. High rates of congenital cytomegalovirus infection linked with maternal HIV infection among neonatal admissions at a large referral center in sub-Saharan Africa. Clin Infect Dis. 2013;58:728–35. doi: 10.1093/cid/cit766. [DOI] [PubMed] [Google Scholar]
- 10.Gartner S, Markovits P, Markovitz D, Kaplan M, Gallo R, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–9. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 11.Steiner KL, Malhotra I, Mungai PL, Muchiri EM, Dent AE, King CL. In utero activation of fetal memory T cells alters host regulatory gene expression and affects HIV susceptibility. Virology. 2012;425:23–30. doi: 10.1016/j.virol.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrando-Martinez S, Ruiz-Mateos E, Leal M. CD27 and CCR7 expression on naive T cells, are both necessary? Immunol Lett. 2010;127:157–8. doi: 10.1016/j.imlet.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 14.Trivedi HN, HayGlass KT, Gangur V, Allardice JG, Embree JE, Plummer FA. Analysis of neonatal T cell and antigen presenting cell functions. Hum Immunol. 1997;57:69–79. doi: 10.1016/s0198-8859(97)00202-4. [DOI] [PubMed] [Google Scholar]
- 15.Prescott SL, Jones CA. Cord blood memory responses: are we being naive? Clin Exp Allergy. 2001;31:1653–6. doi: 10.1046/j.1365-2222.2001.01258.x. [DOI] [PubMed] [Google Scholar]
- 16.Paiardini M, Cervasi B, Reyes-Aviles E, et al. Low levels of SIV infection in sooty mangabey central memory CD(4)(+) T cells are associated with limited CCR5 expression. Nat Med. 2011;17:830–6. doi: 10.1038/nm.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryson YJ, Pang S, Wei LS, Dickover R, Diagne A, Chen IS. Clearance of HIV infection in a perinatally infected infant. N Engl J Med. 1995;332:833–8. doi: 10.1056/NEJM199503303321301. [DOI] [PubMed] [Google Scholar]
- 18.Newell ML, Dunn D, De Maria A, et al. Detection of virus in vertically exposed HIV-antibody-negative children. Lancet. 1996;347:213–5. doi: 10.1016/s0140-6736(96)90401-8. [DOI] [PubMed] [Google Scholar]
- 19.Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369:1828–35. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaak H, van't Wout AB, Brouwer M, Hooibrink B, Hovenkamp E, Schuitemaker H. In vivo HIV-1 infection of CD45RA(+)CD4(+) T cells is established primarily by syncytium-inducing variants and correlates with the rate of CD4(+) T cell decline. Proc Natl Acad Sci U S A. 2000;97:1269–74. doi: 10.1073/pnas.97.3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnittman SM, Lane HC, Greenhouse J, Justement JS, Baseler M, Fauci AS. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci U S A. 1990;87:6058–62. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adkins B. T-cell function in newborn mice and humans. Immunol Today. 1999;20:330–5. doi: 10.1016/s0167-5699(99)01473-5. [DOI] [PubMed] [Google Scholar]
- 23.Hermann E, Truyens C, Alonso-Vega C, et al. Human fetuses are able to mount an adultlike CD8 T-cell response. Blood. 2002;100:2153–8. [PubMed] [Google Scholar]
- 24.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–6. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 25.Franchini M, Abril C, Schwerdel C, Ruedl C, Ackermann M, Suter M. Protective T-cell-based immunity induced in neonatal mice by a single replicative cycle of herpes simplex virus. J Virol. 2001;75:83–9. doi: 10.1128/JVI.75.1.83-89.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu L, Paxton WA, Kassam N, et al. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–91. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornton CA, Upham JW, Wikstrom ME, et al. Functional maturation of CD4+CD25+CTLA4+CD45RA+ T regulatory cells in human neonatal T cell responses to environmental antigens/allergens. J Immunol. 2004;173:3084–92. doi: 10.4049/jimmunol.173.5.3084. [DOI] [PubMed] [Google Scholar]
- 28.Mommaas B, Stegehuis-Kamp JA, van Halteren AG, et al. Cord blood comprises antigen-experienced T cells specific for maternal minor histocompatibility antigen HA-1. Blood. 2005;105:1823–7. doi: 10.1182/blood-2004-07-2832. [DOI] [PubMed] [Google Scholar]
- 29.Pedron B, Guerin V, Jacquemard F, et al. Comparison of CD8+ T Cell responses to cytomegalovirus between human fetuses and their transmitter mothers. J Infect Dis. 2007;196:1033–43. doi: 10.1086/521196. [DOI] [PubMed] [Google Scholar]
- 30.Webster A, Lee CA, Cook DG, et al. Cytomegalovirus infection and progression to aids. Lancet. 1989;334:681. doi: 10.1016/s0140-6736(89)90928-8. [DOI] [PubMed] [Google Scholar]
- 31.Khamduang W, Jourdain G, Sirirungsi W, et al. The interrelated transmission of HIV-1 and cytomegalovirus during gestation and delivery in the offspring of HIV-infected mothers. J Acquir Immune Defic Syndr. 2011;58:188–92. doi: 10.1097/QAI.0b013e31822d0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchant A, Appay V, Van Der Sande M, et al. Mature CD8(+) T lymphocyte response to viral infection during fetal life. J Clin Invest. 2003;111:1747–55. doi: 10.1172/JCI17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michelson S. Interaction of human cytomegalovirus with monocytes/macrophages: a love-hate relationship. Pathol Biol (Paris) 1997;45:146–58. [PubMed] [Google Scholar]
- 34.Sinclair J. Human cytomegalovirus: Latency and reactivation in the myeloid lineage. J Clin Virol. 2008;41:180–5. doi: 10.1016/j.jcv.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Begaud E, Chartier L, Marechal V, et al. Reduced CD4 T cell activation and in vitro susceptibility to HIV-1 infection in exposed uninfected Central Africans. Retrovirology. 2006;3:35. doi: 10.1186/1742-4690-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koning FA, Otto SA, Hazenberg MD, et al. Low-level CD4+ T cell activation is associated with low susceptibility to HIV-1 infection. J Immunol. 2005;175:6117–22. doi: 10.4049/jimmunol.175.9.6117. [DOI] [PubMed] [Google Scholar]
- 37.Ahmad N, Mehta R, Harris DT. HIV-1 replication and gene expression occur at higher levels in neonatal blood naive and memory T-lymphocytes compared with adult blood cells. Virology. 2011;413:39–46. doi: 10.1016/j.virol.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 38.Ullum H, Lepri AC, Victor J, Skinhoj P, Phillips AN, Pedersen BK. Increased losses of CD4+CD45RA+ cells in late stages of HIV infection is related to increased risk of death: evidence from a cohort of 347 HIV-infected individuals. AIDS. 1997;11:1479–85. doi: 10.1097/00002030-199712000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Finzi D, Blankson J, Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–7. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 40.Doitsh G, Galloway NL, Geng X, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–14. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monroe KM, Yang Z, Johnson JR, et al. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science. 2014;343:428–32. doi: 10.1126/science.1243640. [DOI] [PMC free article] [PubMed] [Google Scholar]