Abstract
A model of cerebral ischemia and reperfusion was established in mice. Mice were treated with ketamine via intraperitoneal injection immediately following ischemia or ischemia/reperfusion. Ketamine did not remarkably change infarct volume in mice immediately following ischemia, but injection immediately following ischemia/reperfusion significantly decreased infarct volume. Ketamine injection immediately after ischemia or ischemia/reperfusion inhibited c-Jun protein expression in mouse hippocampus, but nuclear factor kappa B expression was unaltered. In addition, the Longa scale score for neural impairment was not reduced in mice following cerebral ischemia/reperfusion. These results indicate that ketamine can protect mice against cerebral ischemia and reperfusion injury by modulating c-Jun protein expression in mouse hippocampus.
Keywords: ketamine, ischemia/reperfusion, c-Jun, nuclear factor kappa B, infarct volume
Abbreviations:
NF-κB, nuclear factor kappa B
INTRODUCTION
The proto-oncogene c-Jun is an important component of the transcription factor activator protein-1, and plays a major role in cell proliferation and differentiation[1]. Under ischemic and hypoxic stress conditions, cells can activate the c-Jun pathway by stimulating mitogen-activated protein kinase, which strongly activates c-Jun N-terminal kinase[2]. Increased c-Jun protein expression and N-terminal phosphorylation lead to activation of the transcription factor activator protein-1, which regulates the expression of genes controlling stress-induced neuronal necrosis and apoptosis[3].
Nuclear factor kappa B (NF-κB) is an oxidative stress response transcription factor, and neuronal ischemia and hypoxia can activate the protein[4]. Ketamine, a clinical anesthetic drug, protects the heart and brain against ischemia/reperfusion injury[5]. However, there is very little research on the effects of ketamine on hippocampal c-Jun or NF-κB protein expression following cerebral ischemia/reperfusion injury.
In this study, we investigated the influence of ketamine on hippocampal intracellular c-Jun and NF-κB protein expression in a model of cerebral ischemia/reperfusion injury in mice, established by internal carotid artery suture and ipsilateral common carotid artery occlusion.
RESULTS
Quantitative analysis of experimental animals
Thirty Kunming mice were randomly divided into four groups: sham-surgery (n = 7), model (n = 8), treatment after ischemia (n = 8) and treatment after ischemia/reperfusion (n = 7) groups. Cerebral ischemia/reperfusion was performed on the mice, except for those in the sham-surgery group. The ischemia group was treated with ketamine via intraperitoneal injection after the middle cerebral artery was occluded. The ischemia/reperfusion group was treated with ketamine after the blood supply was restored. All mice were included in the final analysis, with no loss.
Ketamine had no impact on nerve function following cerebral ischemia or ischemia/reperfusion
Nerve function in mice was determined using the Longa method[6]. The sham-surgery group was scored 0, and there was no significant difference in nerve impairment scores between the model, ischemia and ischemia/reperfusion groups (Table 1).
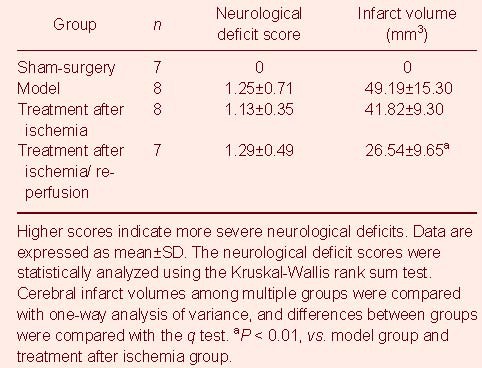
Table 1.
Neurological deficit scores and infarct volumes in mice
Ketamine reduced infarct volume following cerebral ischemia/reperfusion
Cerebral infarction mainly occurred in the frontoparietal dorsolateral cortex, hippocampi and dentate gyrus. The lesion was white colored in mice after ischemia/reperfusion. Ketamine injection immediately after ischemia did not induce a remarkable change in infarct volume in mice, while ketamine injection immediately following ischemia and reperfusion significantly decreased infarct volume (P < 0.01; Figure 1, Table 1).
Figure 1.
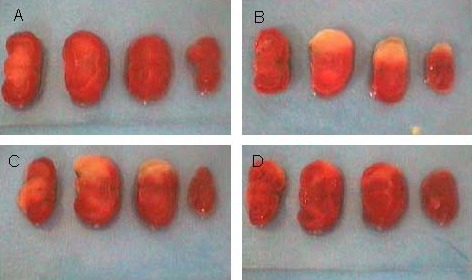
Morphology of brain coronal slices in mice. White part is the cerebral infarction lesion.
(A) In the sham-surgery group, brain tissue was red colored.
(B) In the model group, cerebral infarction was obviously visible.
(C) After ischemia, ketamine treatment failed to induce a significant change in infarct volume.
(D) After ischemia and reperfusion, ketamine treatment significantly decreased infarct volume.
Ketamine reduced hippocampal c-Jun protein expression following cerebral ischemia/reperfusion
Hippocampal c-Jun protein expression was significantly increased in mice following cerebral ischemia/reperfusion. Ketamine treatment after ischemia and ischemia/reperfusion significantly decreased hippocampal c-Jun protein expression (P < 0.01; Figure 2).
Figure 2.
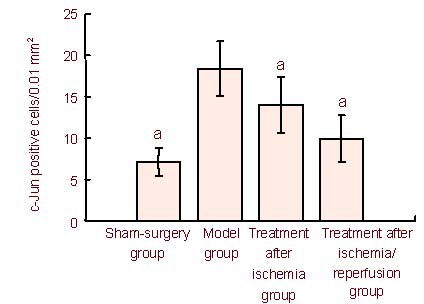
Number of c-Jun-positive cells in the mouse hippocampus. Data are expressed as mean ± SD. Differences among multiple groups were compared with one-way analysis of variance, and differences between groups were further compared with the q test. aP < 0.01, vs. model group.
Ketamine had no impact on hippocampal NF-κB expression following cerebral ischemia or ischemia/reperfusion
The absorbance of hippocampal NF-κB was 0.144 ± 0.046 in the sham-surgery group, but only 0.069 ± 0.014 following ischemia/reperfusion, indicating that hippocampal NF-κB expression was significantly reduced following ischemia/reperfusion (P < 0.05). After ischemia or ischemia/reperfusion, ketamine did not significantly change hippocampal NF-κB expression, with absorbances of 0.081 ± 0.027 and 0.103 ± 0.018, respectively (P > 0.05; Figure 3).
Figure 3.
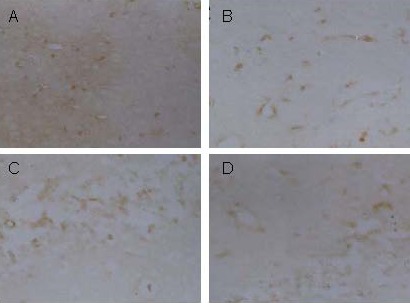
Nuclear factor kappa B (NF-κB) expression in the mouse hippocampus (immunohistochemical staining, × 200). Yellow staining represents protein expression.
(A) In the sham-surgery group, NF-κB was widely expressed.
(B) In the model group, NF-κB expression was lower than in the sham-surgery group.
Ketamine treatment after cerebral ischemia (C) or after ischemia/reperfusion (D) did not significantly affect NF-κB expression, which was similar to that in the model group.
DISCUSSION
After mice were subjected to focal ischemia for 2 hours and then to reperfusion for 22 hours, hippocampal c-Jun expression was increased, the infarction lesion was apparent, and significant neurological deficits were visible. This result is consistent with those of Dai et al[7]. These researchers observed that the sharp increase in c-Jun protein in cortical cells induced by focal cerebral ischemia was associated with neurological deficits, suggesting that c-Jun may induce damage to the brain following ischemia/reperfusion.
In this study, infarct volume in mice treated with ketamine following ischemia was significantly larger compared with mice treated with ketamine following ischemia/reperfusion. The reason for this may be that the use of ketamine soon after cerebral ischemia may increase cerebral blood flow, exacerbating injury, thereby limiting the protective effect of ketamine on the brain[8]. Seegers et al[9] suggested that 7 hours after permanent middle cerebral artery infarction, ischemic stress activated NF-κB, and NF-κB expression was significantly apparent in dying neurons in the ischemic penumbra. Irving et al[10] demonstrated NF-κB activation 3 hours after cerebral infarction. Mattson et al[11] showed that NF-κB signaling is neuroprotective following cerebral ischemia and NF-κB activation reduced neuronal necrosis following stroke.
In our present study, hippocampal NF-κB expression following 2 hours of ischemia and 22 hours of reperfusion was lower than in the sham-surgery group. This may be due to transient activation of NF-κB in living nerve cells[12]. NF-κB expression peaked 6 hours following ischemia, while it was dramatically reduced at 24 hours[10]. In this study, hippocampal NF-κB expression was not significantly changed in mice treated with ketamine following ischemia/reperfusion.
In summary, ketamine reduced hippocampal c-Jun expression and cerebral infarct volume in mice with ischemia/reperfusion injury, but there was no impact on hippocampal NF-κB protein expression.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
Experiments were performed in Yunyang Medical College, China in 2009.
Materials
Animals
A total of 30 clean, Kunming mice, irrespective of gender and age, weighing 32-40 g, were provided by the Animal Experiment Center of Yunyang Medical College, China (license No. SCXK (E) 2005-2008). Experimental procedures complied with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[13].
Drugs
Ketamine was purchased from Henry Pharmaceutical Company Limited, Jiangsu Province, China, with the National Medicine Permit No. H32022820.
Methods
Establishment of the cerebral artery ischemia and reperfusion model
Mice were anesthetized by intraperitoneal injection of 10% chloral hydrate and fixed in a supine position. The right common carotid artery, external carotid artery and internal carotid artery were exposed. The middle cerebral artery was occluded with a 3/0 nylon thread (Hangzhou Warwick Medical Supplies Company, Hangzhou, China), in which the head had been heated into a smooth spherical surface. Nylon thread was inserted ± 11 mm through the bifurcation in the common carotid artery, from the external carotid and internal carotid arteries to the middle cerebral artery. The thread line was maintained for 2 hours and then withdrawn to restore blood flow to the middle cerebral artery. In the sham-surgery group, the common carotid artery bifurcation was distally ligated and the external carotid artery was cut.
Intraperitoneal injection of ketamine
In the ischemia group, mice were intraperitoneally injected with ketamine (100 mg/kg) immediately after successful modeling, and middle cerebral artery blood flow was restored 2 hours later. In the treatment after ischemia/reperfusion group, mice were treated with ketamine (100 mg/kg) immediately following recovery of middle cerebral artery blood flow.
Neurological deficit scoring
Following 2-hour ischemia and 22-hour reperfusion periods, the Longa scale[6] was used to assess neurological deficits: 0 point, no nerve injury symptoms; 1 point, mice cannot completely extend the contralateral forepaw; 2 points, mice circle to the lateral side; 3 points, mice inclined to the lateral side; 4 points, mice cannot spontaneously walk and exhibit loss of consciousness.
Determination of infarct volume
Following 2-hour ischemia and 22-hour reperfusion periods, mice were anesthetized with an overdose of chloral hydrate and subjected to craniotomy immediately after perfusion with 4% paraformaldehyde phosphate buffer via the left ventricle. The brain tissue was completely removed and four coronal slices were obtained from the frontal pole[14] at intervals of 2 mm. The ischemic infarction loci were pale colored. Infarct volumes were measured using a Leica Q500 color image analysis system (Solms, Germany).
Immunohistochemical detection of c-Jun and NF-κB protein expression at the infarct loci
The third coronal slice from the frontal pole was fixed with 4% paraformaldehyde phosphate buffer for 48 hours, then dehydrated, cleared, embedded in paraffin, and microwaved for antigen recovery. 5-μm sections were incubated with rabbit anti c-Jun and NF-κB polyclonal antibodies (SC-1694; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and goat anti-rabbit IgG (Beijing Zhongshan), followed by diaminobenzidine color development. The negative control was treated with phosphate-buffered saline instead of antibodies. The number of c-Jun-positive cells and NF-κB absorbance in the hippocampus were measured under a Leica Q500 color image analysis system (400 × magnification).
Statistical analysis
Data were expressed as mean ± SD. SPSS 10.0 software (SPSS, Chicago, IL, USA) was used to compare neurological deficit scores with the Kruskal-Wallis rank sum test. Cerebral infarct volumes, hippocampal c-Jun and NF-κB protein expression were compared with one-way analysis of variance and the q test. P < 0.05 was considered a statistically significant difference.
Footnotes
Funding: This study was supported by the Medical and Health Research Guidance Program of the Hubei Provincial Department of Health, No. 2001WZ01514.
Conflicts of interest: None declared.
Ethical approval: The project was approved by the Animal Ethics Committee of Yunyang Medical College in China.
(Edited by Shi XQ, Hu WL/Yang Y/Song LP)
REFERENCES
- [1].Schlingensiepen KH, Wollnik F, Kunst M, et al. The role of Jun transcription factor expression and phosphorylation in neuronal differentiation, neuronal cell death, and plastic adaptations in vivo. Cell Mol Neurobiol. 1994;14(5):487–505. doi: 10.1007/BF02088833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab. 2002;22(6):631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- [3].Gillardon F, Spranger M, Tiesler C, et al. Expression of cell death-associated phospho-c-Jun and p53-activated gene 608 in hippocampal CA1 neurons following global ischemia. Brain Res Mol Brain Res. 1999;73(1-2):138–143. doi: 10.1016/s0169-328x(99)00251-x. [DOI] [PubMed] [Google Scholar]
- [4].Cárdenas A, Moro MA, Hurtado O, et al. Implication of glutamate in the expression of inducible nitric oxide synthase after oxygen and glucose deprivation in rat forebrain slices. J Neurochem. 2000;74(5):2041–2048. doi: 10.1046/j.1471-4159.2000.0742041.x. [DOI] [PubMed] [Google Scholar]
- [5].Xiao F, Zhou L, Xiong LZ, et al. The effect of ketamine on IL-1β in rats following ischemic brain damage. Shanxi Yixue Zazhi. 2009;38(9):1118–1120. [Google Scholar]
- [6].Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- [7].Dai WJ, Funk A, Herdegen T, et al. Blockade of central angiotensin AT(1) receptors improves neurological outcome and reduces expression of AP-1 transcription factors after focal brain ischemia in rats. Stroke. 1999;30(11):2391–2399. doi: 10.1161/01.str.30.11.2391. [DOI] [PubMed] [Google Scholar]
- [8].An G, Lin TN, Liu JS, et al. Expression of c-fos and c-jun family genes after focal cerebral ischemia. Ann Neurol. 1993;33(5):457–464. doi: 10.1002/ana.410330508. [DOI] [PubMed] [Google Scholar]
- [9].Seegers H, Grillon E, Trioullier Y, et al. Nuclear factor-kappa B activation in permanent intraluminal focal cerebral ischemia in the rat. Neurosci Lett. 2000;288(3):241–245. doi: 10.1016/s0304-3940(00)01245-3. [DOI] [PubMed] [Google Scholar]
- [10].Irving EA, Hadingham SJ, Roberts J, et al. Decreased nuclear factor-kappaB DNA binding activity following permanent focal cerebral ischaemia in the rat. Neurosci Lett. 2000;288(1):45–48. doi: 10.1016/s0304-3940(00)01203-9. [DOI] [PubMed] [Google Scholar]
- [11].Mattson MP, Culmsee C, Yu ZF. Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res. 2000;301(1):173–187. doi: 10.1007/s004419900154. [DOI] [PubMed] [Google Scholar]
- [12].Clemens JA. Cerebral ischemia: gene activation, neuronal injury, and the protective role of antioxidants. Free Radic Biol Med. 2000;28(10):1526–1531. doi: 10.1016/s0891-5849(00)00258-6. [DOI] [PubMed] [Google Scholar]
- [13].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [14].Paxinos G, Watson C. 5th ed. London: Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]


