Abstract
Foeniculum vulgare Mill. (fennel) is used to flavor food, in cosmetics, as an antioxidant, and to treat microbial, diabetic and common inflammation. No study to date, however, has assessed the anti-inflammatory effects of fennel in experimental models of inflammation. The aims of this study were to investigate the anti-inflammatory effects of fennel in model of lipopolysaccharide (LPS)-induced acute lung injury. Mice were randomly assigned to seven groups (n=7~10). In five groups, the mice were intraperitoneally injected with 1% Tween 80-saline (vehicle), fennel (125, 250, 500µl/kg), or dexamethasone (1 mg/kg), followed 1 h later by intratracheal instillation of LPS (1.5 mg/kg). In two groups, the mice were intraperitoneally injected with vehicle or fennel (250µl/kg), followed 1 h later by intratracheal instillation of sterile saline. Mice were sacrificed 4 h later, and bronchoalveolar lavage fluid (BALF) and lung tissues were obtained. Fennel significantly and dose-dependently reduced LDH activity and immune cell numbers in LPS treated mice. In addition fennel effectively suppressed the LPS-induced increases in the production of the inflammatory cytokines interleukin-6 and tumor necrosis factor-alpha, with 500µl/kg fennel showing maximal reduction. Fennel also significantly and dose-dependently reduced the activity of the proinflammatory mediator matrix metalloproteinase 9 and the immune modulator nitric oxide (NO). Assessments of the involvement of the MAPK signaling pathway showed that fennel significantly decreased the LPS-induced phosphorylation of ERK. Fennel effectively blocked the inflammatory processes induced by LPS, by regulating pro-inflammatory cytokine production, transcription factors, and NO.
Keywords: ERK, Foeniculum vulgare Mill., LPS, TNF-α
INTRODUCTION
Acute lung injury (ALI), characterized by unbalanced inflammatory responses, is a leading cause of acute respiratory failure and multiple organ dysfunctions [1,2]. ALI is associated with neutrophilic inflammation, which can be accelerated by endotoxins such as lipopolysaccharide (LPS) from Gram-negative bacteria [3]. Experimental models of LPS-induced ALI have therefore been used to explore inflammatory responses in the lung. LPS-induced ALI has been associated with the production of reactive oxygen species (ROS) in alveolar macrophages and to involve NF-κB signaling pathways, including the MAPK/JNK/p38/ERK pathways [4].
Foeniculum vulgare Mill. (fennel) is used to flavor foods, in cosmetics, and to treat microbial, diabetic and common inflammation. Evaluation of fennel seed extracts using a DPPH radical scavenging assay also indicated that fennel may have antioxidant activity effect [5]. Oral administration of a methanolic extract of F. vulgare fruit decreased malondialdehyde (MDA) level, suggesting that this extract has inflammation-relieving effects in experimental animals [6]. Moreover, fennel decreased ROS and MDA in mouse tumor tissue [7]. Trans-anethole, the major component of fennel, was found to reduce paw edema and inflammatory pain [8], with the anti-inflammatory effects of trans-anethole reported to derive from its regulation of NF-κB signaling pathways [9].
Because fennel contains several components, which can affect each other, there is a need to confirm that this essential oil shows consistent effects. To date, however, no study has analyzed the anti-inflammatory effects of fennel in a mouse model of LPS-induced ALI. This study therefore explored the anti-inflammatory effects of fennel in LPS-induced ALI in mice, and investigated the signaling pathways involved.
METHODS
Animals and Materials
Male BALB/C mice, aged five weeks and weighing 19 to 21 g, were obtained from Orient Bio (Sungnam, Korea) and acclimatized to standard laboratory conditions for 3 to 5 days. All experimental procedures were conducted in accordance with guidelines relevant to the care of experimental animals, as approved by the Animal Research Committee of Korea University (approval no. KUIACUC-2012-181), informed by the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23; revised 1996). Mice were randomly assigned to seven groups (n=7~10) and were anesthetized by intraperitoneal injection of a mixture of 0.3 mg/kg tiletamine-zolazepam (Zoletil 50, Virbac Laboratories, Carros, France) and 0.2 mg/kg xylazine (Rompun, Bayer Korea, Ansan, Korea). In five groups, the mice were intraperitoneally injected with 1% Tween 80-saline (vehicle), fennel (125, 250, 500µl/kg), or dexamethasone (DEX) (1 mg/kg), followed 1 h later by intratracheal instillation of LPS (1.5 mg/kg). In the remaining two groups, the mice were intraperitoneally injected with 1% Tween 80-saline (vehicle) or fennel (250µl/kg), followed 1 h later by intratracheal instillation of sterile saline. The dose of fennel was based on a previous study of trans-anethole, the main component of fennel [9]. The mice were sacrificed 4 h later, and their bronchoalveolar lavage fluid (BALF) and lung tissues were obtained. Lipopolysaccharide (LPS, from E. coli 0.55:B5), Tween 80 and DEX were obtained from Sigma-Aldrich (St. Louis, MO, USA). Pure fennel essential oil was purchased from Aromarant Co. Ltd., Rottingen, Germany and came from locally cultivated plants. The fennel essential oil that we used (batch No. 091119; Aromarant Co. Ltd) was analyzed by gas chromatography/mass spectrometry (GC/MS). The main components of fennel essential oil detected by GC/MS analysis were 75.81% trans-anethole, 5.93% fenchonem, 5.82% limonene, 4.30% methyl chavicol, 3.52% α-pinene and 0.39% α-phellandrene.
Lactate dehydrogenase (LDH) assay
The activity of LDH, an enzyme used as a marker for cytotoxicity, was measured using a commercial LDH assay, according to the manufacturer's instructions (Takara Bio Inc., Otsu, Japan). BALF samples were mixed 1:1 with freshly prepared reaction mixture and incubated in the dark for 30 min at room temperature. Absorbance was measured at 490 nm and at a reference wavelength of 620 nm using a microplate reader (BMG Labtech, Ortenberg, Germany).
Cell counting
BALF samples were centrifuged at 500×g for 10 min at 4℃, and the sedimented cells were resuspended in PBS. The cells were stained with Diff-Quick (International Reagents Co., Kobe, Japan), and total and differential leukocyte counts were determined using a Countess automated cell counter (Invitrogen Life Technologies, Carlsbad, CA, USA). Results are expressed as the number of each cell type per milliliter of BALF.
Histopathology
Lung tissues were fixed in 10% paraformaldehyde, embedded in paraffin, and cut into 4µm thick sections. The sections were stained with hematoxylin and eosin (H&E), and viewed under a light microscope (200×).
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of the inflammatory cytokines IL-6 and TNF-α in BALF were measured using commercially available ELISA kits, in accordance to the manufacturer's instructions (PeproTech, London, UK).
Measurement of nitric oxide (NO)
Since NO has a short half-life, we measured nitrite level, an indirect measure of NO production [10]. The amount of nitrite in BALF was measured using the Griess reaction. Griess reagent included 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride and 1% sulfanilamide. Briefly, Griess reagent was added to 100µl of BALF supernatant, and the solutions were mixed and incubated for 10 min at room temperature. Optical density at 540 nm was measured in a microplate reader (BMG Labtech, Ortenberg, Germany).
Zymographic analysis
The secretion of matrix metalloproteinase-9 (MMP-9) protein was measured by gelatin zymography. A volume of BALF sample was mixed with an equal volume of nonreducing sample buffer, and the samples were electrophoresed in 8% sodium dodecyl sulfate polyacrylamide electrophoresis gels (SDS-PAGE) containing 1 mg/ml gelatin. The gels were washed with 2.5% Triton X-100 for 2 h and subsequently incubated for 20 h at 37℃ in 50 mM Tris-Cl buffer (pH 7.4) containing 10 mM CaCl2 and 0.02% NaN3. The gels were subsequently stained for 1 h with 0.5% Coomassie Brilliant Blue G250 in 7.5% acetic acid/10% propanol-2 and destained to visualize the protein bands. Relative densities of MMP-9 were analyzed with Bio-Rad Quantity One software (Bio-Rad, Hercules, CA, USA).
Extraction of lung nuclear proteins
Lung tissues obtained at sacrifice were immediately frozen in liquid nitrogen, and 50 mg samples of frozen lung tissue were subsequently homogenized with a Precellys 24 bead-based tissue homogenizer in 0.5 ml ice-cold buffer A (10 mM HEPES with pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.1 mM Na2EDTA, 0.5 mM DTT, 1% Nonidet P-40, 0.5 mM PMSF, 0.5µg/ml leupeptin, 125µg/ml aprotinin, 25µg/ml pepstatin A). Cell debris was removed by centrifugation at 2,000 rpm for 30 sec; the supernatants were incubated on ice for 5 min and again centrifuged for 10 min at 5,000 rpm. Cytoplasmic proteins in the supernatant were collected and the pellet was resuspended in 50µl of cold buffer B (20 mM HEPES with pH 7.9, 1.5 mM MgCl2, 0.42 M NaCl, 20% glycerol, 0.5 mM DTT, 0.5 mM PMSF, 0.5µg/ml leupeptin, 125µg/ml aprotinin, 25µg/ml pepstatin A) and incubated on ice for 30 min. The nuclear fraction was collected by centrifugation at 12,000 rpm for 2 min.
Western blot analysis
Lung tissue homogenate samples were separated on 10% SDS-PAGE. The proteins were electrophoretically transferred onto nitrocellulose membranes, which were blocked for 30 min at room temperature. The membranes were incubated overnight at 4℃ with primary antibodies to NF-κB p65, Lamin B, IκB-α, GAPDH, p-ERK, ERK, p-p38, p38, p-JNK, and JNK, followed by incubation with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Bands were visualized by enhanced chemiluminescence (ECL) reagents according to the manufacturer's protocol. Relative densities were analyzed using Bio-Rad Quantity One software (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All data were expressed as mean±S.E.M. and compared using one-way analysis of variance, followed by the Tukey HSD post hoc test. All statistical analyses were performed using SPSS 20 software, with results considered statistically significant at p<0.05.
RESULTS
Effect of fennel on LDH activity in BALF of mice with LPS-induced ALI
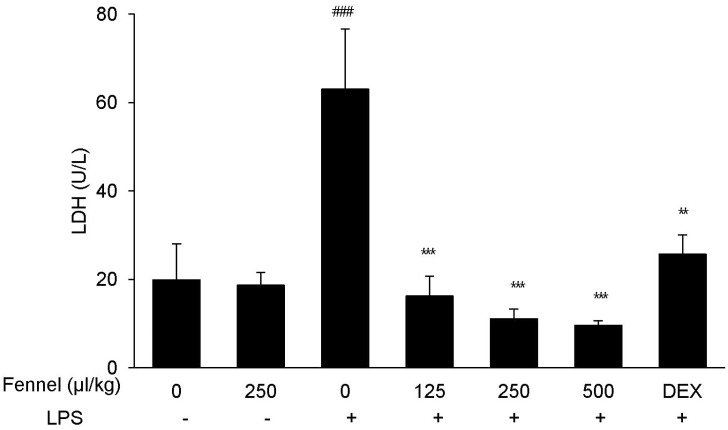
The activity of LDH was significantly higher in BALF of mice with LPS-induced ALI than in mice treated with vehicle alone (63.02±27.28 U/L vs. 19.90±8.13 U/L, p<0.001) (Fig. 1), whereas pretreatment with DEX, which has been shown to protect against LPS-induced ALI, significantly reduced LDH activity (25.66±4.35, p=0.004). Mice pretreated with 125 (16.24±4.43, p<0.001), 250 (11.07±2.21, p<0.001), and 500 (9.57±1.05, p<0.001)µl/kg fennel, followed by LPS, showed significantly decreased LDH activity compared with mice treated with vehicle plus LPS. LDH level was similar in mice treated with fennel (250µl/kg) and vehicle without LPS.
Fig. 1. Effect of fennel on lactate dehydrogenase (LDH) activity in BALF of LPS-treated mice. Mice were intratracheally administered LPS (1.5 mg/kg) 1 h after intraperitoneal injection of 1% Tween 80-saline (vehicle), fennel (125, 250, 500µl/kg), or DEX (1 mg/kg). The activity of LDH in BALF was measured to evaluate cell damage. Data are reported as mean±S.E.M. (n=7~10 per group). ###p<0.001 compared with vehicle group; **p<0.01, ***p<0.001 compared with the vehicle+LPS group.
Effect of fennel on inflammatory cell count in BALF
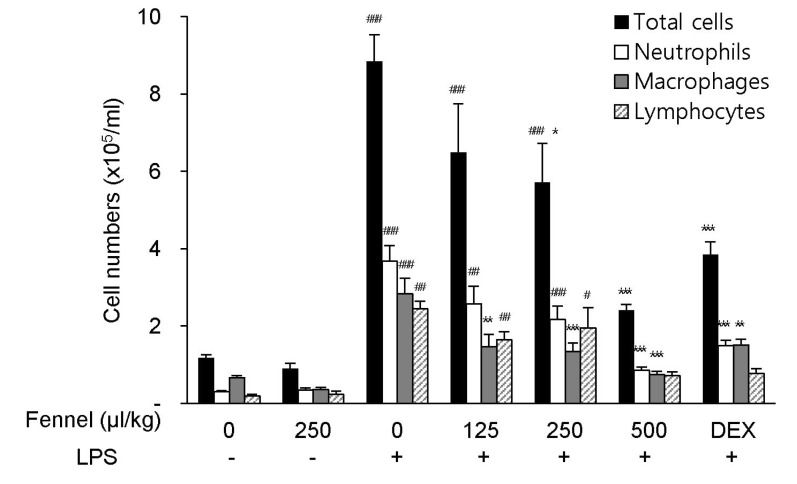
Recruitment of excess numbers of inflammatory cells is necessary for the pathogenesis of ALI. Compared with vehicle alone, treatment with vehicle+LPS significantly increased the numbers of total cells (8.84×105 cells/ml, p<0.001), neutrophils (3.67×105 cells/ml, p<0.001), macrophages (2.84×105 cells/ml, p<0.001), and lymphocytes (2.44×105 cells/ml, p=0.005) in BALF (Fig. 2). In LPS-treated mice, pretreatment with fennel 125µl/kg (total cells, 6.49×105 cells/ml, p=0.204; neutrophils, 2.57×105 cells/ml, p=0.099; macrophages, 1.47×105 cells/ml, p=0.001; lymphocytes, 1.64×105 cells/ml, p=0.236), 250µl/kg (total cells, 5.71×105 cells/ml, p=0.032; neutrophils, 2.16×105 cells/ml, p=0.007; macrophages, 1.34×105 cells/ml, p<0.001; lymphocytes; 1.95×105 cells/ml, p=0.769), and 500µl/kg (total cells, 2.41×105 cells/ml, p<0.001; neutrophils, 0.85×105 cells/ml, p<0.001; macrophages, 0.74×105 cells/ml, p<0.001; lymphocytes, 0.72×105 cells/ml, p<0.001) significantly and dose-dependently reduced the total numbers of cells, similar to DEX, as well as decreasing the numbers of neutrophils, macrophages, and lymphocytes (Fig. 2).
Fig. 2. Effects of fennel on cell numbers in BALF of LPS-treated mice. The numbers of total cells, neutrophils, macrophages, and lymphocytes in BALF were analyzed. Data are reported as mean±S.E.M. (n=7~10 per group). #p<0.05, ##p<0.01, ###p<0.001 compared with the vehicle group; *p<0.05, **p<0.01, ***p<0.001 compared with the vehicle+LPS group.
Effect of fennel on lung histopathology of LPS-treated mice
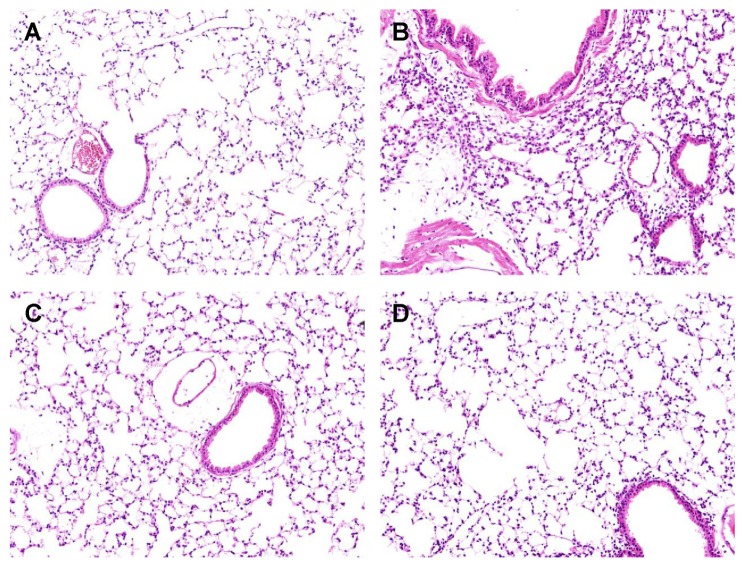
Hematoxylin and eosin (H&E) staining showed that LPS treatment (Fig. 3B) was characterized by neutrophil sequestration, infiltration around the pulmonary vessels, and alveolar wall thickening in lung tissue compared with vehicle (Fig. 3A). However neutrophil sequestration, infiltration around the pulmonary vessels, and alveolar wall thickening were significantly alleviated by pretreatment with 500µl/kg fennel (Fig. 3C), as well as by DEX (Fig. 3D).
Fig. 3. Effect of fennel on the histopathology of lung tissues in LPS-treated mice. Fennel (500µl/kg) or DEX (1 mg/kg) was administered intraperitoneally to mice 1 h prior to LPS treatment. Lung sections from each group were stained with hematoxylin and eosin (H&E) (×200). (A) Vehicle group, (B) Vehicle+LPS group, (C) Fennel+LPS group, (D) DEX+ LPS group.
Effect of fennel on IL-6 and TNF-α in BALF
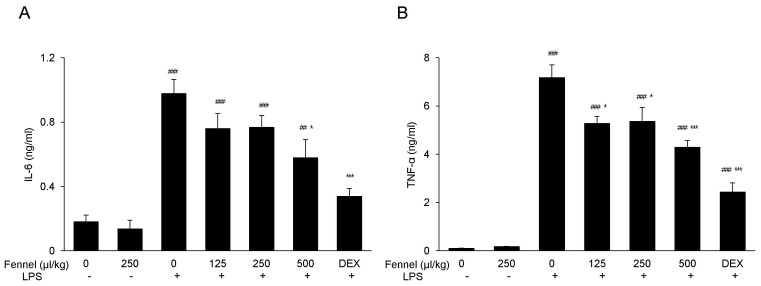
LPS significantly increased the concentrations in BALF of the inflammatory cytokines IL-6 (0.97±0.09 vs. 0.18±0.04 ng/ml, p<0.001; Fig. 4A) and TNF-α (7.18±0.53 vs. 0.10±0.01 ng/ml, 0<0.001; Fig. 4B) compared with vehicle. Pretreatment with fennel 125µl/kg (IL-6, 0.76±0.10 ng/ml, p=0.468; TNF-α, 5.27±0.30 ng/ml, p=0.010), 250µl/kg (IL-6, 0.77±0.07, p=0.517; TNF-α, 5.36±0.58 ng/ml, p=0.016), and 500µl/kg (IL-6, 0.58±0.11, p=0.017; TNF-α, 4.29±0.29 ng/ml, p<0.001), however, significantly and dose-dependently suppressed the production of IL-6 and TNF-α, with 500µl/kg fennel showing maximum reduction.
Fig. 4. Effects of fennel on (A) IL-6 and (B) TNF-α expression in the BALF of LPS-treated mice. IL-6 and TNF-α in BALF were analyzed by ELISA. Data are reported as mean±S.E.M. (n=7~10 per group). ##p<0.01, ###p<0.001 compared with the vehicle group; *p<0.05, ***p<0.001 compared with the vehicle+LPS group.
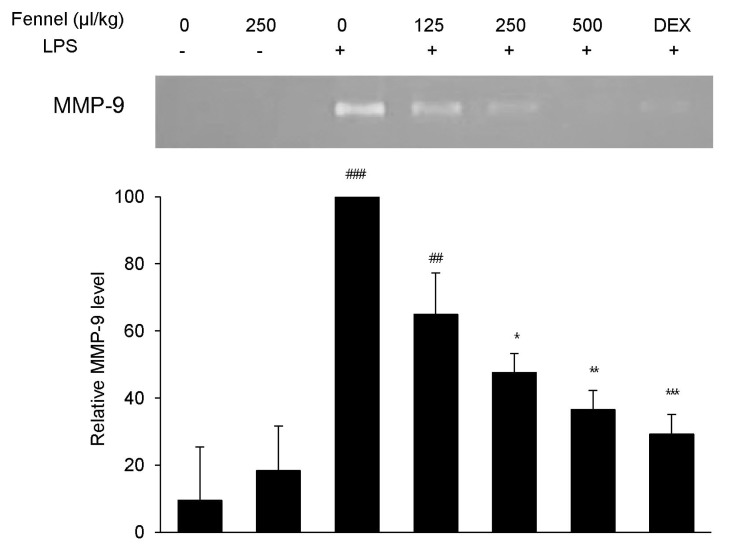
Effect of fennel on MMP-9 activity in LPS-treated mice
MMP-9, a representative proinflammatory mediator that plays an essential role in lung inflammation, was analyzed in BALF by gelatin zymography. BALF from mice treated with vehicle+LPS showed a 10-fold increase in a gelatinolytic band at 92 kDa, the molecular weight of MMP-9 (p<0.001), compared with vehicle-treated mice (Fig. 5). Pretreatment with 250 and 500µl/kg fennel dose-dependently reduced MMP-9 activity, and pretreatment with DEX also reduced MMP-9 activity.
Fig. 5. Effect of fennel on MMP-9 activity in LPS-treated mice. Relative MMP-9 activity in BALF was analyzed by zymography followed by scanning densitometry. Data are reported as mean±S.E.M. (n=7~10 per group). ##p<0.01, ###p<0.001 compared with the vehicle group; *p<0.05, **p<0.01, ***p<0.001 compared with the vehicle+LPS group.
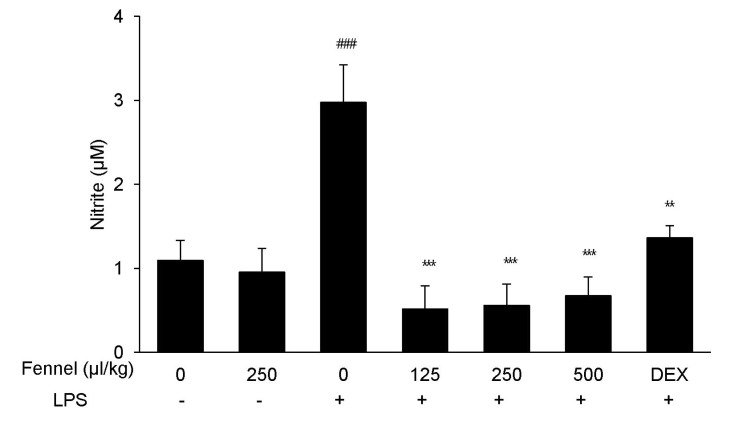
Effect of fennel on nitric oxide (NO) production in BALF
NO is a critical immune modulator in the proinflammatory cytokine response associated with ALI. Treatment with LPS significantly enhanced the production of NO compared with vehicle (2.98±0.45µM vs. 1.09±0.24µM, p=0.001; Fig. 6). However, this increase was significantly and dose-dependently reduced by pretreatment with fennel 125µl/kg (0.52±0.27µM, p<0.001), 250µl/kg (0.56±0.73µM, p<0.001), and 500µl/kg (0.67±0.23µM, p<0.001).
Fig. 6. Effect of fennel on NO production in the BALF of LPS-treated mice. NO concentrations in BALF were measured by nitrite assays. Data are reported as mean±S.E.M. (n=7~10 per group). ###p<0.001 compared with the vehicle group; **p<0.01, ***p<0.001 compared with the vehicle+LPS group.
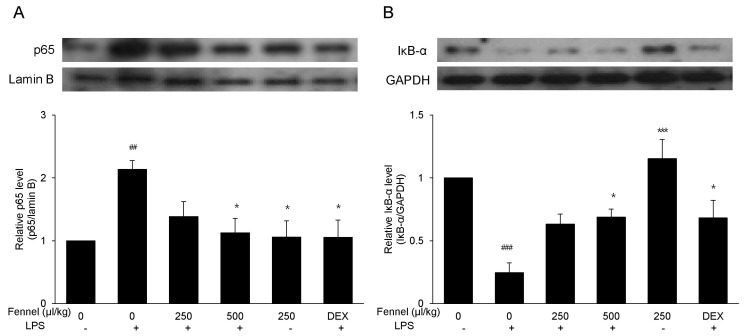
Effect of fennel on activation of NF-κB in LPS-induced ALI mice
NF-κB activation was assessed by western blotting to determine the anti-inflammatory pathways by which fennel reduced LPS-induced ALI in mice. Treatment with vehicle+ LPS increased the level of expression of NF-κB p65 2.13-fold (p=0.007) compared with vehicle alone (Fig. 7A). However, in LPS-treated mice, pretreatment with 500µl/kg fennel reduced the expression of NF-κB p65 1.90-fold compared with pretreatment with vehicle alone (p=0.019). Mice treated with vehicle+LPS showed 4.05-fold lower IκB-α expression compared with those treated with vehicle alone (p<0.001), whereas mice treated with 500µl/kg fennel plus LPS showed 2.79-fold higher IκB-α expression compared with those treated with vehicle+LPS (p=0.023) (Fig. 7B). This finding indicated that fennel suppressed NF-κB activation by blocking IκB-α degradation.
Fig. 7. Effect of fennel on NF-κB activation in LPS-treated mice. Nuclear and cytosolic extracts in lung tissue were fractionated and the expression of NF-κB p65 (A) and IκB-α (B) proteins in nuclear and cytosolic extracts, respectively, were assessed by western blotting. Lamin B and GAPDH were used as internal controls. Data are reported as mean±S.E.M. (n=7~10 per group). ##p<0.01, ###p<0.001 compared with the vehicle group; *p<0.05, ***p<0.001 compared with the vehicle+LPS group.
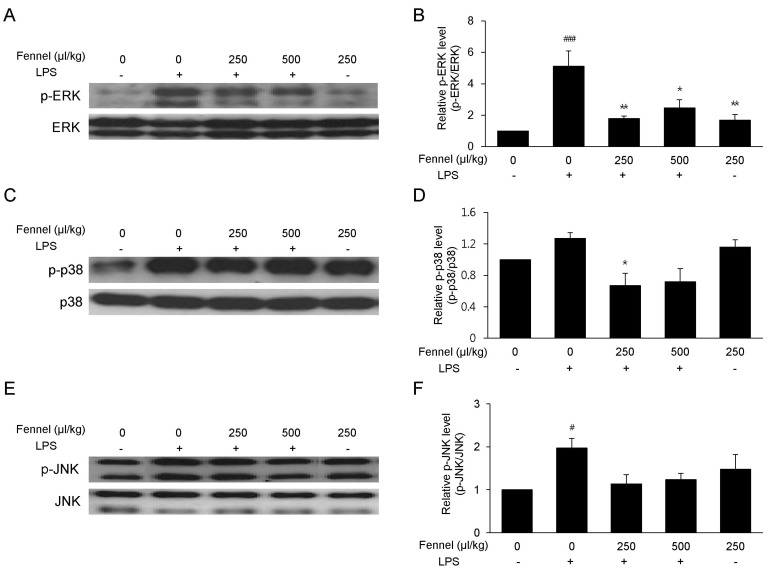
Effect of fennel on the MAPK signaling pathway
The effect of fennel on the MAPK signaling pathway was analyzed to determine its anti-inflammatory mechanism of action. LPS increased the levels of expression levels of phospho-ERK (5.11-fold, p<0.001) (Fig. 8A and 8B), phospho-p38 (1.27-fold, p=0.474) (Fig. 8C and 8D), and phospho-JNK (1.97-fold, p=0.036) (Fig. 8E and 8F). In contrast, 250µl/kg (2.86-fold, p=0.004) and 500µl/kg (2.07-fold, p=0.021) fennel significantly reduced the level of LPS-induced ERK phosphorylation.
Fig. 8. Effect of fennel on the MAPK signaling pathway in LPS-treated mice. Lung tissues were analyzed by western blotting with antibodies to p-ERK (A), p-p38 (C), and p-JNK (E), and quantitative protein expression was normalized to ERK (B), p38 (D), and JNK (F), respectively. Data are reported as mean±S.E.M. (n=7~10 per group). #p<0.05, ###p<0.001 compared with the vehicle group; *p<0.05, **p<0.01 compared with the vehicle+LPS group.
DISCUSSION
Although inflammation is a normal immune reaction, uncontrolled inflammation can lead to organ dysfunction or disease [11]. Clinical ALI involves neutrophilic inflammation and is a common complication of other conditions [12]. Because LPS from Gram-negative bacteria evokes inflammatory responses and endotoxic symptoms [13], LPS is used in experimental models of inflammation. In agreement with previous findings, we found that LPS-treated mice showed inflammatory responses, including elevations in immune system cells and proinflammatory cytokines, as well as alterations in lung histology. Fennel, which has been shown to have anti-inflammatory effects, protected mice against LPS-induced ALI. Fennel reduced lung damage, the numbers of pro-inflammatory cells, and the production of pro-inflammatory mediators induced by LPS.
NF-κB, an important transcription factor in inflammatory responses, has been shown to regulate the production of pro-inflammatory cytokines [14]. Although NF-κB activation is important in normal inflammatory responses, its overproduction is closely associated with inflammatory diseases, such as sepsis [14]. In the absence of stimuli, NF-κB is located in the cytoplasm, where it binds to IκB-α and remains inactive. Thus, regulating IκB-α may control the NF-κB signaling pathway. Trans-anethole, the main constituent of fennel, has been reported to reduce NF-κB concentrations in mice with hepatic ischemia/reperfusion injury [15], as well as to reduce NF-κB levels, while slightly increasing IκB-α levels, in LPS-treated BALB/C mice [9]. Similarly, we found that treatment with fennel not only reduced p65 expression, but increased IκB-α level. Thus, fennel may directly suppress NF-κB activation, perhaps by enhancing the expression of its inhibitor, IκB-α.
Calcium signaling plays an important role in inflammatory conditions [16]. Administration of LPS has been found to transiently elevate intracellular calcium level, leading to ERK phosphorylation and the expression of TNF-α [17]. Fennel was reported to significantly reduce the expression of TNF-α in response to S. aureus [18], suggesting that it modulates intracellular calcium concentration. Fennel induced the relaxation of guinea pig tracheal chains via hyperpolarization, thus inhibiting calcium influx [19]. In addition, high doses of trans-anethole were reported to modify calcium channels on isolated rat aortas [20], further suggesting that the protective effect of fennel on TNF-α and ERK expression was due, at least in part, to calcium modulation.
The MAP kinase pathway, which includes ERKs, JNKs and p38, is also involved in the endotoxic effects of LPS, leading to inflammation. This pathway and TNF-α expression are both upstream and downstream of each other [21]. NO is another important signaling molecule, which regulates physiological functions, including vascular contraction, neuronal signal and inflammation [22]. NO may derive from inducible NO synthase (iNOS) associated pathophysiological processes related to inflammation. Unlike JNK and p38, ERKs negatively activate iNOS. We found that fennel significantly reduced nitrate levels, suppressing LPS-induced ERK expression but having no effect on JNK or p38 levels.
Fennel contains mainly trans-anethole, limonene, and anisole [18]. Trans-anethole was shown to have anti-inflammatory effects, substantially similar to those of fennel, on pro-inflammatory cytokines, NO, and transcription factors [9]. Moreover, d-limonene has shown anti-inflammatory effects in rat kidney by modulating NF-κB and iNOS [23]. Oral administration of limonene to rats suppressed both NF-κB and IL-6 [24]. Taken together, these findings suggest that limonene and trans-anethole, the main components of fennel, are responsible for the anti-inflammatory effects of fennel.
In conclusion, this study confirmed that fennel effectively blocked LPS-induced inflammation, by regulating pro-inflammatory cytokines, transcription factors, and NO. These findings suggest that fennel may have clinical activity in mitigating inflammatory conditions.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (No.2012R1A2A2A02007145).
ABBREVIATIONS
- fennel
Foeniculum vulgare Mill.
- LPS
lipopolysaccharide
- BALF
bronchoalveolar lavage fluid
- NO
nitric oxide
- ALI
acute lung injury
- ROS
reactive oxygen species
- MDA
malondialdehyde
- DEX
dexamethasone
- LDH
lactate dehydrogenase
- H&E
hematoxylin and eosin
- ECL
enhanced chemiluminescence
- ELISA
enzyme-linked immunosorbent assay
References
- 1.Herold S, Gabrielli NM, Vadász I. Novel concepts of acute lung injury and alveolar-capillary barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2013;305:L665–L681. doi: 10.1152/ajplung.00232.2013. [DOI] [PubMed] [Google Scholar]
- 2.Dushianthan A, Grocott MP, Postle AD, Cusack R. Acute respiratory distress syndrome and acute lung injury. Postgrad Med J. 2011;87:612–622. doi: 10.1136/pgmj.2011.118398. [DOI] [PubMed] [Google Scholar]
- 3.Kabir K, Gelinas JP, Chen M, Chen D, Zhang D, Luo X, Yang JH, Carter D, Rabinovici R. Characterization of a murine model of endotoxin-induced acute lung injury. Shock. 2002;17:300–303. doi: 10.1097/00024382-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Mizumura K, Gon Y, Kumasawa F, Onose A, Maruoka S, Matsumoto K, Hayashi S, Kobayashi T, Hashimoto S. Apoptosis signal-regulating kinase 1-mediated signaling pathway regulates lipopolysaccharide-induced tissue factor expression in pulmonary microvasculature. Int Immunopharmacol. 2010;10:1062–1067. doi: 10.1016/j.intimp.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Goswami N, Chatterjee S. Assessment of free radical scavenging potential and oxidative DNA damage preventive activity of Trachyspermum ammi L. (carom) and Foeniculum vulgare Mill. (fennel) seed extracts. Biomed Res Int. 2014;2014:582767. doi: 10.1155/2014/582767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi EM, Hwang JK. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia. 2004;75:557–565. doi: 10.1016/j.fitote.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Mohamad RH, El-Bastawesy AM, Abdel-Monem MG, Noor AM, Al-Mehdar HA, Sharawy SM, El-Merzabani MM. Antioxidant and anticarcinogenic effects of methanolic extract and volatile oil of fennel seeds (Foeniculum vulgare) J Med Food. 2011;14:986–1001. doi: 10.1089/jmf.2008.0255. [DOI] [PubMed] [Google Scholar]
- 8.Ritter AM, Domiciano TP, Verri WA, Jr, Zarpelon AC, da Silva LG, Barbosa CP, Natali MR, Cuman RK, Bersani-Amado CA. Antihypernociceptive activity of anethole in experimental inflammatory pain. Inflammopharmacology. 2013;21:187–197. doi: 10.1007/s10787-012-0152-6. [DOI] [PubMed] [Google Scholar]
- 9.Kang P, Kim KY, Lee HS, Min SS, Seol GH. Anti-inflammatory effects of anethole in lipopolysaccharide-induced acute lung injury in mice. Life Sci. 2013;93:955–961. [PubMed] [Google Scholar]
- 10.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 11.Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–d26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- 12.Gao MY, Chen L, Yang L, Yu X, Kou JP, Yu BY. Berberine inhibits LPS-induced TF procoagulant activity and expression through NF-κB/p65, Akt and MAPK pathway in THP-1 cells. Pharmacol Rep. 2014;66:480–484. doi: 10.1016/j.pharep.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167:5067–5076. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beinke S, Ley SC. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem J. 2004;382:393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho HI, Kim KM, Kwak JH, Lee SK, Lee SM. Protective mechanism of anethole on hepatic ischemia/reperfusion injury in mice. J Nat Prod. 2013;76:1717–1723. doi: 10.1021/np4004323. [DOI] [PubMed] [Google Scholar]
- 16.Zhang T, Feng Q. Nitric oxide and calcium signaling regulate myocardial tumor necrosis factor-α expression and cardiac function in sepsis. Can J Physiol Pharmacol. 2010;88:92–104. doi: 10.1139/Y09-097. [DOI] [PubMed] [Google Scholar]
- 17.Geoghegan-Morphet N, Burger D, Lu X, Sathish V, Peng T, Sims SM, Feng Q. Role of neuronal nitric oxide synthase in lipopolysaccharide-induced tumor necrosis factor-alpha expression in neonatal mouse cardiomyocytes. Cardiovasc Res. 2007;75:408–416. doi: 10.1016/j.cardiores.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Qiu J, Li H, Su H, Dong J, Luo M, Wang J, Leng B, Deng Y, Liu J, Deng X. Chemical composition of fennel essential oil and its impact on Staphylococcus aureus exotoxin production. World J Microbiol Biotechnol. 2012;28:1399–1405. doi: 10.1007/s11274-011-0939-4. [DOI] [PubMed] [Google Scholar]
- 19.Boskabady MH, Khatami A, Nazari A. Possible mechanism(s) for relaxant effects of Foeniculum vulgare on guinea pig tracheal chains. Pharmazie. 2004;59:561–564. [PubMed] [Google Scholar]
- 20.Soares PM, Lima RF, de Freitas Pires A, Souza EP, Assreuy AM, Criddle DN. Effects of anethole and structural analogues on the contractility of rat isolated aorta: Involvement of voltage-dependent Ca2+-channels. Life Sci. 2007;81:1085–1093. doi: 10.1016/j.lfs.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Sabio G, Davis RJ. TNF and MAP kinase signalling pathways. Semin Immunol. 2014;26:237–245. doi: 10.1016/j.smim.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moncada S, Higgs EA. Molecular mechanisms and therapeutic strategies related to nitric oxide. FASEB J. 1995;9:1319–1330. [PubMed] [Google Scholar]
- 23.Rehman MU, Tahir M, Khan AQ, Khan R, Oday-O-Hamiza, Lateef A, Hassan SK, Rashid S, Ali N, Zeeshan M, Sultana S. D-limonene suppresses doxorubicin-induced oxidative stress and inflammation via repression of COX-2, iNOS, and NFκB in kidneys of Wistar rats. Exp Biol Med (Maywood) 2014;239:465–476. doi: 10.1177/1535370213520112. [DOI] [PubMed] [Google Scholar]
- 24.d'Alessio PA, Ostan R, Bisson JF, Schulzke JD, Ursini MV, Béné MC. Oral administration of d-limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life Sci. 2013;92:1151–1156. doi: 10.1016/j.lfs.2013.04.013. [DOI] [PubMed] [Google Scholar]