Abstract
Prostate cancer is one of the leading causes of cancer death among men in the United States. Although, recent advancement in diagnostic tools has significantly increased early detection and decreased cases of advanced diseases, high numbers of patients who underwent treatment still experience recurrent disease at distant sites, after long periods of remission. Therefore, it is of paramount importance to elucidate the pathological mechanism of the tumor recurrence in order to identify better therapeutic targets and also to distinguish patients with indolent disease from patients with recurrent disease at the time of surgery for optimization of therapeutic intervention. Recurrent tumor cells disseminate at a very early stage even before diagnosis of localized disease and remain dormant as “residual disease” in patients for a long period of time. Colonization and recurrent growth at distant site requires acquisition of genetic and epigenetic alterations and remodeling of signaling pathways. This review focuses on recent advances on identification of biomarkers associated with early and late recurrent disease as well as mechanisms involved during recurrence of prostate cancer.
Keywords: Prostate cancer, Recurrence, Biomarkers, Mechanisms, Review
2. INTRODUCTION
Prostate cancer is one of the common causes of cancer in men. The incidence of prostate cancer is expected to increase up to 1.7 million cases by 2030 due to aging of global population (1, 2). More than 90 % of death associated with prostate cancer can be attributed to metastatic disease (3). Nevertheless, with the recent advancement in diagnostic technologies, prostate cancer has been detected in patients at very early stages, leading to improved therapeutic and treatment outcome (4). Surgical resection of primary tumor at localized state has been established practice and is also associated with increased overall survival (5). Despite this significant achievement in early detection and treatment, approximately 35% of patients who had surgically resected primary tumor experience recurrent disease after years (6, 7, 8). On the other hand, there are evidences in which prostatic foci remain indolent without further progression for a long period of time. The lifetime mortality risk associated with these patients was ~3% (9). So, it is important to distinguish patients who will suffer recurrence from patients who will retain indolent disease for proper management of this disease. This can be accomplished by understanding the biology behind dormancy and recurrence.
Increasing line of evidences support the role of epithelial to mesenchymal transition (EMT) in early dissemination of cells from primary tumor (10, 11). EMT is also known to induce stem-like phenotype in cancer cells, which is associated with tumor growth, proliferation and drug resistance (12, 13). Thus, EMT induced cells with stem-like phenotype remain in patients either as Circulating tumor cells (CTCs) in blood or disseminated tumor cells (DTCs) at distant site and are potential seed for recurrence. Mesenchymal to epithelial transition (MET), the phenomenon which is opposite of EMT is known to be essential for colonization at distant site (10) suggesting one potential mechanism behind recurrence. Similarly, prostate specific androgen mediated signaling can also influence disseminated cells for achieving recurrent phenotype. Additionally, loss of tumor metastasis suppressor genes (MSG), dormancy inducer genes, and organ-specific homing genes can play major roles in recurrence. The mechanisms involved in recurrence are essential for therapeutic targeting of this disease. Alternatively, primary prostate tumor can also be examined for the presence of biomarkers that can successfully predict recurrence at distant sites. This approach will enable aggressive therapeutic intervention to patients with aggressive disease. Thus patients can be distinguished for recurrence either by identification of molecular components involved in recurrence of CTCs and DTCs or by prognosis based on primary biopsy sample.
3. DISSEMINATION OF PROSTATE TUMOR CELLS AND RESIDUAL DISEASE
The pathological process of tumor progression is complex and involves multiple steps. This process is known as metastatic cascade and involves breaching of extracellular matrix by primary tumor followed by invasion to nearby stroma, extravasation of tumor cells into blood stream, circulation, intravasation into distant organ and finally formation of secondary metastasis (14). The properties of the disseminated and circulating cells within the bloodstream or distant organs can be explored to gain significant insight in the mechanisms associated with recurrence.
The classical understanding of the metastatic cascade has been changed recently after successful isolation and enumeration of circulating tumor cells (CTCs) in the blood of patients at a very early stage. Patients with localized prostate tumor were found to be positive for CTCs suggesting early dissemination of tumor cells to the bloodstream (15). Early dissemination can be explained by phenomenon of EMT which involves acquisition of molecular and genetic changes in the cells leading to increased motility (10). In fact, it has been recently shown that patients with localized prostate tumor were found to be positive for EMT markers such as Twist and Vimentin further corroborating the role of EMT in dissemination of tumor cells into blood (11). During tumor progression, cells are continuously shed to bloodstream via EMT. The number of disseminated CTCs decreases significantly after resection of localized primary tumor (15). Although, primary tumor resection decreases the cell number in the blood, there are no evidences that CTCs are completely eliminated from circulation and even after the successful treatment with chemotherapy or radiotherapy, the remnant cells survive and potentially lead to growth of secondary tumor (16). It is possible that CTCs home to distant organs as DTCs and seed for recurrence before diagnosis and treatment of primary tumor. Hence, resection of primary tumor even at a very early stage might have minimal effect in diminishing incidence of recurrence. On the other hand, in order to regrow at a distant site, these cells must survive the barriers imposed by the immediate environment or by therapeutic treatments. The potential of tumor cells to survive can be explained by recent cancer stem cell theory which suggests that metastatic characteristic are inherent properties of small fraction of the primary tumor cells that can overcome restrictive barriers (12, 13). It is possible that recurrent cells possess cancer stem-like cells (CSCs) properties. In support of this notion, EMT has been found to be associated with acquisition of stem cells phenotype which further corroborates that recurrent cell population possesses CSCs properties (13). Therefore, CTCs in blood with CSCs properties survives the systemic circulation and seed to multiple distant organs for recurrence. Homing of tumor cells at target organs is practically evident as DTCs in bone marrow of the patients. DTCs reside at target organ for long periods of time, and can be detected in patients either as solitary tumor cell or as micrometastasis before and after prostatectomy (6, 17, 18). However, before recurrence as a secondary tumor, these cells must establish colonization in target organs (10). Therefore, the time for recurrence might be regulated at two steps (i) CTCs to extravasation and homing and (ii) DTCs to recurrence. However correlation between detection of DTCs and recurrence was found to be inconclusive. Both negative and positive correlation in patients with no previous evidence of disease was found by two different studies thereby necessitating further verification of the role of DTCs in larger cohorts (6, 17).
It is well recognized that a small population of tumor cells evading chemotherapy is frequently correlated with the properties of CTCs and DTCs in clinical settings. Interestingly, this small population can regrow back into the primary tumor. Toivanen et al have recently shown that when tumor cells were isolated from patients with early staged androgen dependent disease and inoculated into mice followed by androgen ablation therapy, small population of tumor cells survived and was able to awake from quiescence phase in the absence of therapy. Additionally, these quiescent cells were positive for stem-like markers such as CD44, ALDH and Nanog suggesting that these cells are potential residual CSCs (19). This finding also provides insight in the role of “disease remains” or “residual disease” with stem-like characteristic, which might exist in patients as CTCs or DTCs. Moreover, the fact that “disease remains” are non-proliferating, can overcome therapies and exist in patients for years after successful resection of primary tumor suggests that these cells are comprised with both the characteristics of CSCs and dormant cell (6, 20, 21). On the other hand, these cells can survive at distant organs (as DTCs) focuses toward the role of microenvironment in modulating phenotype of tumor cells. Recent evidences also suggest that dormant cell can survive in niche provided by distant organ as a solitary tumor cell (22, 23). All these evidences indicate that recurrent cells possess CSCs properties, disseminate from primary tumor before resection, reside at distant organ as a dormant disease and finally are evident as recurred tumor.
Switching of dormant tumor cells to aggressive phenotype requires remodeling of inherent signaling pathways within specific niche that can be acquired by genetic, epigenetic and metabolic changes inherited by the tumor cells. Further investigation on the identification of evolutionary process involved during circulation and arrest at distant sites may provide definitive clue to therapeutically target these cells to prevent relapse. Hence, it is important to decipher intrinsic properties of disseminated cells to distinguish tumor cells with high recurrent potential from cells with high dormancy potential.
4. MECHANISMS INVOLVED IN PROSTATE CANCER RECURRENCE
The disseminated cells from primary prostate have to face multiple challenges before colonizing to the target organ. Most of the tumor cells disseminated to blood are fated to die by anoikis, by shear stress in circulation and by Natural killer (NK) cell mediated lysis (24, 25). However, some tumor cells survive and escape these barriers by forming aggregate with platelets (26, 27). Tumor cells also have to face challenges when patients are treated with radiation or chemotherapy drugs. Only cells capable of overcoming these stresses can survive in circulation. Furthermore, after successful homing to the target organ, tumor cells are exerted with inhibitory signaling from immediate microenvironment (28). Therefore, a recurrent tumor cell must acquire mechanisms to resist these barriers by regulating multiple signaling. Various external and internal factors are considered to be involved during the switch from dormancy to recurrence as described below.
4.1. Mesenchymal to epithelial transition
The concept of EMT promoting CSCs phenotype as well as dissemination is well established. It has also been proposed that MET leads to successful seeding as well as distant metastasis (10). Recently, Tsai et al. have shown that MET is indeed a critical process in augmenting metastasis, using squamous cell carcinoma model (29). However, whether MET is required for seeding or homing to distant organ is yet to be verified. The fact that EMT has been shown to be a marker of therapeutic resistance in breast cancer suggests that, in patients treated with drugs, dormant cells must possess mesenchymal phenotype (30). Additionally, solitary survival of these cells in target organs also indicates that MET is not required for homing which raises possibility that recurrent switch is partly regulated by EMT/MET axis.
Notch and TGF-β associated signalings are known to induce EMT in several cancers including prostate cancer (31, 32, 33, 34, 35). A study has shown the role of Notch signaling in aggressive prostate disease. Expression of JAGGED1, a ligand in Notch signaling was found to be high on metastatic cells compared to non-metastatic cells. This study has also shown that patients with localized disease can be predicted for recurrence based on JAGGED1 expression in primary tumors (36). On the other hand, JAGGED induced Notch signaling is associated with CSCs properties via EMT and is known to promote self-renewal of CSCs as well as HSC (31, 37, 38, 39). These findings provide insight into the role of Notch signaling in dissemination of prostate CSCs and their survival in circulation. Similarly, TGF-β signaling also increases metastasis through induction of invasive ability of cells (40, 41, 42). However, TGF-β is also known to suppress secondary tumor growth by activating p38 mitogen activated protein kinases (MAPK) (43, 44, 45). Therefore, it is plausible that tumor cell programs dissemination through EMT by activating Notch and TGF-β signalings, and these signaling increases the number of cells at the target site. However, these cells remain dormant due to EMT and are modulated by factors at target site for recurrence via MET. Nevertheless, precise mechanisms behind the switch from mesenchymal-state to epithelial state are yet to be elucidated.
Other studies suggest that disseminated prostate tumor cells occupy bone marrow niche by expression of Annexin II receptor and replace bone marrow hematopoietic stem cells (HSC). Annexin II receptor facilitates homing of prostate tumor cells to osteoblasts and bone marrow endothelial cells which are major components of HSC niche (46). Normally, HSC niche involves regulated signaling for differentiation and retention of normal HSC (47). HSC maintains mesenchymal phenotype and possesses multilineage potential (48). HSC can exert same signaling that might possibly induce dormancy of tumor cells by enhancing mesenchymal state for extended period of time. Recurrence can then be driven by perturbing the normal homeostasis by secreting bone remodeling cytokines leading to MET followed by expansion of osteoblast which favors tumor progression in bone (49). Bone microenvironment would otherwise inhibit secretion of these cytokine restraining tumor growth. Additionally, HSC niche is also known to play a prominent role in maintaining stemness of tumor cells which is crucial for survival of these cells as well as metastasizing in new environment (50).
Similarly, metastasis of prostate cancer cells to bone is mediated by stromal derived factor 1 (SDF-1) cytokine and CXCR4 receptor axis (51, 52). Prostate cancer cells express CXCR4 receptor to localize itself to bone, while SDF-1 is secreted by bone marrow cells (53). The binding of SDF-1 to CXCR4 induces expression of Annexin II from osteoblast. In response to Annexin II signaling, prostate cancer cells express growth arrest specific 6 (GAS6) receptors. On the other hand, osteoblast expresses GAS6 proteins. This interaction of GAS6 and the receptor in the HSC niche has been shown to induce dormancy. Moreover binding between GAS6 and its receptor, specifically AXL, makes tumor cells become chemo resistant (54). Therefore, this signaling must be disrupted to recur in bone environment. One possibility for induction of dormancy by this signaling can be related to phenomenon of EMT. AXL receptor tyrosine kinase expression is known to be stimulated by EMT in breast cancer (55). Although this stimulation has not been shown in prostate cancer, it can be speculated that dormancy induced by Annexin II- is potentially due to EMT of prostate cancer cells as evident by AXL expression. Considering this scenario, MET can be a switch for recurrence which might be mediated by disruption of Annexin II based signaling.
4.2. Reactivation of androgen signaling
The phenomenon of re-activation of androgen-mediated signaling can be observed in local recurrence, in which tumor grows back at the same site after hormone deprivation therapy or tumor resection. At physiological level of adrenal androgen, local recurrence has been observed in patients treated hormone deprivation therapy due to re-activation of androgen signaling (19, 56, 57). Several studies have shown relation of increased levels of androgen receptor (AR) and its co-activators in patients with recurrent disease (56, 58, 59). Due to AR gene amplifications and mutations in these patients, both mRNA and protein level were found to be increased (60, 61). In contrast, similar studies that have confirmed that androgen dependency in recurrence has also shown that there was no difference in expression of AR level between benign prostate and primary tumor, rather, recurrence was mediated by stabilization of receptors in tumors by both ligand dependent and independent manner (62, 63, 64). These results were further supported by the finding that prostate specific antigen (PSA) levels showed no significant difference between benign and recurrent prostate tissue (65). Moreover, level of dihydrotestosterone (DHT), one of the preferred ligand of AR, was found to be sufficient in prostate tissues for recurrence to occur, which corroborates the notion of role of androgen signaling in local recurrence of androgen dependent prostate disease (66).
On the other hand, androgen independent prostate cancer or castration resistant prostate cancer (CRPC) develops via adoption and activation of several other pathways and is more metastatic and lethal (62). Activation of several androgen receptor splice variants is associated with progression toward androgen independent disease and distant recurrence after radical prostatectomy (67, 68, 69). Distant recurrence is known to be seeded by disseminated cells (CTCs and DTCs) residing at distant organ (17, 70). However, involvement of androgen signaling in awakening of quiescent DTCs at distant site is rarely investigated. One of the clues in relation to the role of androgen signaling in distant recurrence was established when CRPC patients were found to express high levels of PSA (71). AR as well as PSA was also found to be expressed in bone, epidural space and periosteum metastases of these patients, suggesting that driver factor for progression at secondary site involves activation of androgen signaling (72). Similarly, CTCs isolated from CRPC patients were driven in hormone dependent manner which was evident as CTCs were positive for ERG oncogene and ER (71). Additionally, it has also been found that glucocorticoid and its metabolites are able to stimulate growth of certain androgen independent prostate cancers with double mutations in their AR. Cortisol, which is the main circulating glucocorticoid in humans, is present in the physiological concentration greater than required to bind and activate mutated receptor (73). These mechanisms may explain incidence of the recurrent disease that grows back in androgen independent manner. However, the precise mechanism behind recurrence of wild type AR is yet to be determined. One possible explanation involves activation of anti-apoptotic signaling via expression of Bcl2, which renders cells with proliferative capacity. Bcl2 expression was found to be increased in cells that were recovered from castrated mice; however, no association has been observed between CTCs and Bcl2 till date (74). Alternatively, androgen deprivation may lead to selection of population with androgen responsiveness, that remains quiescent in the absence of androgen and lead to recurrent growth in the suitable environment (19). These findings emphasize that mechanisms for recurrence at distant organs involve both androgen-dependent and –independent signaling and the time period it takes for recurrence represents time required to acquire genetic, epigenetic and metabolic changes that enables signaling associated with recurrent growth.
4.3. Loss of metastasis suppressor genes
The difference between metastasis and recurrence should be considered very carefully in designing effective therapeutic strategy for recurrence. Emerging role of metastasis suppressor genes may not exemplify the exact setting of recurrence; however, elucidating further mechanistic insight into the role of these genes may provide a clue in recurrence. Many genes are known to significantly suppress metastasis without inhibiting primary tumor, and these suppressors act as a secondary barrier at different steps of metastasis at different target organs (75). Several lines of evidences have also supported the role of many metastasis suppressor genes including KAI1, RKIP, NDRG1 in prostate cancer dormancy (76, 77, 78).
KAI1 is capable of suppressing metastasis of prostate cancer. This gene was shown to interact with Duffy antigen receptor of chemokines (DARC) present on the endothelial cells to suppress metastasis by inducing senescence and preventing extravasation. Indeed DARC-knockout mice were shown to significantly promote metastasis (77). It can be inferred that disseminated CTCs with high level of KAI1 expression avoid anoikis mediated cell death and remain dormant by binding to endothelial cells. Therefore, the recurrent switch may involve switching-off of this pathway followed by extravasation of cancer cells. One approach to distinguish dormant population of cells from recurrent population would be evaluation of expression level of KAI1 in isolated CTCs. Additionally, the phenomenon of senescence to induce dormancy was also evident at the target site such as bone. Bone microenvironment has been shown to restrict the proliferating phenotype of prostate cancer stem like cells by inducing senescence. Moreover, the microenvironment-induced senescence was found to upregulate NDRG1, another well studied metastasis suppressor gene. However, senescence was found to be reversible and this reversibility was correlated to recurrence and formation of secondary tumor (28). Taken together, reversal of cell state from senescent to active proliferating type together with loss of metastasis suppressor gene may be one of the key recurrence mechanisms.
Another metastasis inhibitor gene Raf kinase Inhibitory Protein (RKIP) was found to suppress prostate cancer metastasis by regulating cell cycle and angiogenesis (76, 79). On the other hand, RKIP is known to function by negatively regulating Extracellular regulated protein kinases 1/2 (ERK1/2) (80, 81). Recently it has been shown that the balance of dormancy and recurrence is regulated by the ratio between p38 MAPK and ERK1/2 MAPK level in several tumors including prostate cancer. High ratio of p38:ERK1/2 favored proliferation whereas low ratio was associated to dormancy (82, 83). The finding that p38 can negatively regulate Raf mediated growth, suggests that p38 potentially inhibits ERK (83, 84). Moreover, p38 activation is also associated with G0-G1 arrest as well as senescence (85). In this context, RKIP is also considered to be associated with regulation of p38 and ERK levels and induction of cell cycle arrest or senescence. Therefore, it would be interesting to examine RKIP levels in solitary DTCs together with p38 and ERK1/2 levels to decipher potential role of RKIP in dormancy. On the other hand, RKIP expression is also associated with spindle arrest which suggests that loss of this gene will increase number of chromosomal aberrations leading to acquirement of potential to recur. However, chromosomal aberration linked to a loss of RKIP has not been validated yet (79).
Tumor-inherent factors are responsible for retention of dormant state. These factors involve changes associated with morphology, reduced level of intra-cellular signaling and alteration of cellular state (Figure 1). Tumor cells must overcome resistive effect of one or more of these factors for gaining proliferative phenotype. It is also possible that Androgen mediated signaling, metastasis suppressing factors and MET activating factors cross-talk with each other and together drive recurrent cell proliferation. These factors can be exploited for their ability to retain tumor cells in dormant state which provides an alternative in treatment of recurrent disease. Moreover, molecular characterization of CTCs/DTCs in patients can also be used to predict the risk of recurrence (Table 1). Alternatively, patients can be segregated based on the prognostic markers expressed either on the biopsy or resected tumor sample to selectively intervene patients who require aggressive intervention (Table 2).
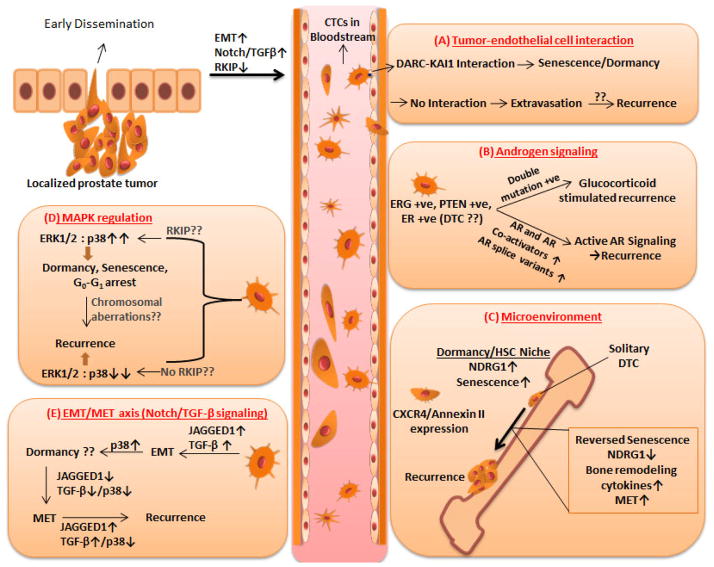
Figure 1.
Mechanisms involved in recurrence: Early dissemination of localized prostate tumor leads to increased number of CTC in the bloodstream. CTC can obtain various shapes during circulation. (A) Senescence is induced when CTC expressing KAI-1 binds to DARC expressed by endothelial cells leading to inhibition of extravasation. Lack of this interaction leads to extravasation and recurrence. (B) Disseminated cells can acquire double mutation on their androgen receptor evolving itself to grow and proliferate based on glucocorticoid. Double mutation negative cells can reactivate androgen signaling by increased expression of androgen receptor, its co-activators and splice variants. (C) Bone-specific homing of prostate cancer is mediated by expression of CXCR4 receptor. Bone niche can regulate residing cancer cells to induce dormancy via augmentation of NDRG1 and induction of senescence. Activation from dormancy involves reversal of senescence, expression of bone remodeling cytokines, decreased expression of NDRG1 and MET. (D) Ratio between expression levels of ERK1/2 and p38 decides fate of disseminated cells (high ratio of ERK1/2:p38 leads to dormancy, senescence, and G0-G1 arrest. It is possible that RKIP expression and state of chromosomal aberrations can regulate the switch. (E) Notch and TGF-β signaling based EMT via decreased expression of JAGGED1 and p38 can reverse the mesenchymal phenotype of prostate cancer cells to epithelial phenotype that re-activates Notch and TGF-β signaling leading to recurrence.
Table 1.
Predicting risk of recurrence by expression levels of major genes in CTC/DTC
Table 2.
Segregating recurrent patients based on primary biopsy or tumor sample or CTC count
| Affected Pathway/signaling | Predicting Genes/Proteins/factors | References |
|---|---|---|
| PSA-based recurrence | ||
| Androgen signaling | AR, LSD1, FLH2, TIF2 | 89, 90, 91 |
| - | DNA Copy number Alterations | 92 |
| Cell growth and Apoptosis | p53, Bcl2 | 74, 93 |
| MET | E-cadherin | 94 |
| Stromal associated factors | WFDC1/ps20, HS, Clusterin | 95, 96, 97, 98 |
| Volume of reactive stroma | ||
| CTC-based recurrence | ||
| - | CTC count > 5 | 21, 102, 103, 105 |
| Clinical recurrence | ||
| Angiogenesis | CD34/Micro-vascularity | 106 |
| - | Chromogranin A, Sialyltransferase I, PDGFR-β, HOX6C, ITPR3 | 108, 109 |
| Epigenetic regulation | H3/H4 Acetylation and dimethylation | 110 |
| PCR2 target genes | 112 | |
5. BIOMARKERS FOR PROSTATE CANCER RECURRENCE
5.1. Markers based on level of PSA
The effective treatment of prostate cancer recurrence can be enhanced by examining the established biomarkers which can precisely predict the chances of recurrences. Present biomarkers include PSA testing and biopsy Gleason grading. Gleason grading for biopsy involves examination of differentiation rate and has already proven to be a poor predictor of recurrence (86, 87). PSA testing, a measure of tumor burden, which is determined by quantitating the levels of PSA in blood serum, is widely used to predict incidence among asymptomatic men as well as disease relapse in patients after prostatectomy. Quantifying PSA levels for predicting recurrence is also known as biochemical recurrence. Examining PSA level with time as well as time for PSA recurrence after radical prostatectomy can independently predict recurrence based on survival of the patients (88). It should be noted that PSA recurrence is correlated with high expression of Androgen Receptor (AR) in primary tumor (89). Similarly, expression of different co-factors or co-activators associated with AR such as LSD1, FLH2, and TIF2 has also been shown to predict biochemical relapse of prostate tumor (90, 91). On the other hand, a recent elegant study by Taylor et. al has demonstrated that DNA aberrations correlate with the biochemical relapse, by analyzing relatively large number of primary tumors, metastases, prostate cell lines and xenografts. In this study, DNA copy number alteration (CNA) in primary prostate was also shown to significantly relate to biochemical recurrence. Tumors with high CNA showed less time to recur compared to tumors with low CNA (92). Various molecules associated with the progression of tumors have been shown to possess relevance in predicting biochemical recurrence. Tumor suppressive master regulator p53 and anti-apoptotic factor Bcl2 are actively involved in regulation of cell growth and apoptosis. PSA-based recurrence was associated with augmented expression of both p53 and Bcl2 in patients who had undergone radical prostatectomy (93). Similarly, aberrant expression of E-Cadherin, protein responsible for maintenance of epithelial state of cells, was also demonstrated to independently predict biological relapse (94). This finding suggests the potential role of MET in activation of androgen mediated signaling pathway as evident by PSA expression. Alternatively, factors in microenvironment of primary prostate tumor are also known to render prognostic value in evaluating patients for biochemical recurrence. Decreased expression of stromal WAP-type four disulfide core (WFDC1)/ps20 and hyaluronan (HA) in primary prostate was found to shorten recurrence-free survival time of patients independently (95, 96). Similarly, increased volume of reactive stroma and augmented expression of Clusterin have been also shown to increase the incidence of PSA recurrence in shorter period of time (97, 98).
Ironically, prediction of recurrence based on PSA has been controversial as two large screening trails showed that PSA level has no correlation to overall survival of patients and has limitations such as poor specificity and sensitivity (99, 100, 101). Although, PSA levels do not provide insight into clinical recurrence, it can segregate patients with high risk or low risk for recurrence based on primary tumor characteristics. The predictive value of chromosomal abnormalities, MET associated factor and anti-apoptotic factor suggests that these factors play significant role in recurrence. New scientific approaches should be focused more toward finding alternative biomarkers to pinpoint patients for either clinical relapse or tumor specific biochemical relapse.
5.2. Circulating tumor cells
Recent evidences direct toward the role of CTCs in prediction of recurrence. CTCs are emerging in itself as a biomarker to predict recurrence in multiple cancers including prostate cancer (102, 103). The number of shed CTCs was initially found to be correlated with progression of prostate tumor regardless of the androgen status. Increased tumor burden was shown to be associated with high number of isolation of CTCs (70). In case of castration resistant prostate cancer (CRPC), CTCs count (>5 CTCs per 7.5ml blood) was found to be the most accurate predictor, better than PSA-based prediction, for overall survival in post-treatment patients (21). Similarly, in an independent study, CTCs count in CRPC patients was found to be correlated to bone scan index, the percentage of tumor associated with bony skeleton which indicates that CTCs may feed a growing mass of tumor in bone (104). Furthermore isolated CTCs were also found to be positive for tumor specific markers such as EGFR, ERG and AR which may play significant role in recurrence (71, 105). The emerging prognostic value of CTCs for overall survival as well as therapeutic efficiency extends the idea that these cells possess CSCs features and with its malignant properties are able to form secondary metastases.
5.3. Other genetic markers for recurrence
Similar to PSA recurrence, tumor cell specific properties have also been shown to offer predictive values of clinical recurrence in patients. The level of angiogenesis, process of formation of new blood vessels, in both invasive and localized primary prostate tumor was found to serve as predictor of recurrence. Microvascularity as assessed by CD34 expression was found to be significantly correlated with clinical recurrence (106). With the advancement of technology for high throughput gene expression analysis, the trend in identification of biomarkers has been extended to gene expression profiling of the primary tumor followed by the prognosis prediction based on clinical and pathological information. The combination of genes obtained based on this approach predicted clinical recurrence in greater than 95% of patients (107). Another study proposed 5-genes model to distinguish recurrent and non-recurrent cases using small dataset of patients. These five genes, Chromogranin A, Sialyltransferase I, PDGFR-β, HOX6C and ITPR3, if expressed in high levels in primary tumor were found to be significantly co-related to recurrence (108, 109). In a different approach of predicting clinical outcome, Seligson et al have shown the correlation between global histone acetylation and prediction for recurrence. This observation was confirmed by examining five residues in Histone 3 (H3) and Histone 4 (H4) for acetylation and dimethylation in clinical samples. It was found that high expression of these histone markers in primary prostate predicted biological recurrence independent of tumor stage and preoperative PSA level. However, mechanistically how this bulk histones modification could actually confer recurrence is yet to be determined (110). Another epigenetic based prognosis for recurrence is based on a polycomb repression signature. The polycomb repression group complexes (PRC2), EZH2 (enhancer of zeste homolog 2), SUZ12 (Suppressor of zeste 12) and EED (embryonic ectoderm development) possess histone methyltransferases activity and are involved in transcriptional silencing (111, 112). PCR2 target genes were significantly correlated to clinical outcome of the patients (112).
The identification of accurate biomarkers that hold high prognostic value for prostate cancer recurrence is still an unmet goal. All biomarkers can confer advantages on prediction and rationalizing decision making during treatment (Table 2). Perhaps, capability of these biomarkers should be examined on bigger cohorts. Patients can be stratified based on the subtype of disease and biomarker appropriate for each subtype can be identified. Moreover, identification of organ specific recurrence biomarkers can be ideal treatment of specific disease. On the other hand, focus should be driven toward mechanistic side to understand how these biomarkers are responsible to predict recurrence efficiently. This mechanistic approach should elucidate role of these genes in altering microenvironment and assisting cells for recurrence. It might also be plausible that these genes aid only for augmenting aggressiveness of tumor cells, however, the fact that early stage localized disease can be predicted for recurrence based on these markers necessitates determination of exact mechanism behind it. In a different approach, CTCs positive for these markers can precisely be correlated with recurrence. Although current studies have shown that gene profile in CTCs and DTCs are much more heterogeneous than previously expected, expression profiling of CTCs for site-specific recurrence may establish expression signature associated with dormant and recurrent phenotype (113).
7. DISCUSSIONS AND FUTURE DIRECTIONS
Prostate cancer is becoming a curable disease due to early diagnosis and efficient therapeutic approach. However, the re-emergence of tumor has made this curable disease fatal. The variable period of dormancy among patients necessitates differentiation of patients susceptible for early recurrent disease from patients with indolent disease at molecular level. This segregation restricts therapeutic intervention only to patients with aggressive disease. Tumor can be differentiated as aggressive based on molecular characterization at three levels (i) Primary, (ii) Circulating (CTCs) and (iii) Disseminated and homed (DTCs) (Table 2). Examining the levels of biochemical or clinical recurrence-associated biomarkers can distinguish patients with aggressive disease. Similarly, CTCs count and detection of DTCs on itself can also differentiate patients based on prognosed survival-time. On the other hand, recurrent disease can be targeted based on current knowledge of recurrence-involved mechanisms (Figure 1). Alternatively, recurrent disease can be treated by inducing dormancy, the concept of making cancer a chronic disease. Drug inhibiting Androgen mediated signaling or Specific peptide drugs that share homology to metastasis suppressing genes can also be promising if delivered in an efficient way.
The future approach in treatment of recurrent disease should involve molecular characterization of residual disease to identify definitive clues involved in recurrence. Molecular mechanism associated with CTCs and DTCs, the overt seed for recurrence, should be analyzed at single-cell level for organ-specific recurrence. Recurrent cells known to remain dormant at target site must adopt evolutionary switch to activate itself from dormant state. Retrospective studies involving analysis of DTCs and CTCs in a cohort of patients with organ-specific recurrence can aid in finding of such targetable factors. Association of pathological conditions with genetic or epigenetic events can be validated via this approach. Similarly, experimental tool can be established by isolating tumor cells from clinical samples (CTCs or DTCs) that mimic recurrent or dormant growth on specific organ. This tool can be used to establish organ specific dormancy and recurrence signatures. Alternatively, animal model recapitulating recurrent and dormant growth can facilitate isolation and characterization of CTCs and DTCs. Monitoring these animal models can establish link between pathological conditions and distant recurrence. Similarly, transgenic animal models for prostate cancer can also be used for identification of novel next-generation biomarkers for prognosis and monitoring of therapeutic response.
Acknowledgments
This work was supported by NIH (R01CA124650, R01CA129000 to KW) and the US Department of Defense (PC PC110060. PC101369 to KW).
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007;121(7):1571–8. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peinado H, Rafii S, Lyden D. Inflammation joins the “niche”. Cancer Cell. 2008;14(5):347–9. doi: 10.1016/j.ccr.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marberger M, Barentsz J, Emberton M, Hugosson J, Loeb S, Klotz L, Koch M, Shariat SF, Vickers A. Novel approaches to improve prostate cancer diagnosis and management in early-stage disease. BJU Int. 2012;109(Suppl 2):1–7. doi: 10.1111/j.1464-410X.2011.10870.x. [DOI] [PubMed] [Google Scholar]
- 5.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172(3):910–4. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 6.Weckermann D, Muller P, Wawroschek F, Harzmann R, Riethmuller G, Schlimok G. Disseminated cytokeratin positive tumor cells in the bone marrow of patients with prostate cancer: detection and prognostic value. J Urol. 2001;166(2):699–703. [PubMed] [Google Scholar]
- 7.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 8.Pfitzenmaier J, Ellis WJ, Arfman EW, Hawley S, McLaughlin PO, Lange PH, Vessella RL. Telomerase activity in disseminated prostate cancer cells. BJU Int. 2006;97(6):1309–13. doi: 10.1111/j.1464-410X.2006.06194.x. [DOI] [PubMed] [Google Scholar]
- 9.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 10.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 11.Behnsawy HM, Miyake H, Harada K, Fujisawa M. Expression patterns of epithelial-mesenchymal transition markers in localized prostate cancer: significance in clinicopathological outcomes following radical prostatectomy. BJU Int. 2013;111(1):30–7. doi: 10.1111/j.1464-410X.2012.11551.x. [DOI] [PubMed] [Google Scholar]
- 12.Pantel K, Alix-Panabieres C. The clinical significance of circulating tumor cells. Nat Clin Pract Oncol. 2007;4(2):62–3. doi: 10.1038/ncponc0737. [DOI] [PubMed] [Google Scholar]
- 13.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8(5):341–52. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 15.Stott SL, Lee RJ, Nagrath S, Yu M, Miyamoto DT, Ulkus L, Inserra EJ, Ulman M, Springer S, Nakamura Z, Moore AL, Tsukrov DI, Kempner ME, Dahl DM, Wu CL, Iafrate AJ, Smith MR, Tompkins RG, Sequist LV, Toner M, Haber DA, Maheswaran S. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl Med. 2010;2(25):25ra23. doi: 10.1126/scitranslmed.3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreno JG, Miller MC, Gross S, Allard WJ, Gomella LG, Terstappen LW. Circulating tumor cells predict survival in patients with metastatic prostate cancer. Urology. 2005;65(4):713–8. doi: 10.1016/j.urology.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Morgan TM, Lange PH, Porter MP, Lin DW, Ellis WJ, Gallaher IS, Vessella RL. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res. 2009;15(2):677–83. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verkooijen RB, Smit JW, Romijn JA, Stokkel MP. The incidence of second primary tumors in thyroid cancer patients is increased, but not related to treatment of thyroid cancer. Eur J Endocrinol. 2006;155(6):801–6. doi: 10.1530/eje.1.02300. [DOI] [PubMed] [Google Scholar]
- 19.Toivanen R, Frydenberg M, Murphy D, Pedersen J, Ryan A, Pook D, Berman DM, Taylor RA, Risbridger GP B Australian Prostate Cancer. A preclinical xenograft model identifies castration-tolerant cancer-repopulating cells in localized prostate tumors. Sci Transl Med. 2013;5(187):187ra71. doi: 10.1126/scitranslmed.3005688. [DOI] [PubMed] [Google Scholar]
- 20.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4(6):448–56. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 21.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 22.Naumov GN, MacDonald IC, Chambers AF, Groom AC. Solitary cancer cells as a possible source of tumour dormancy? Semin Cancer Biol. 2001;11(4):271–6. doi: 10.1006/scbi.2001.0382. [DOI] [PubMed] [Google Scholar]
- 23.Naumov GN, MacDonald IC, Weinmeister PM, Kerkvliet N, Nadkarni KV, Wilson SM, Morris VL, Groom AC, Chambers AF. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 2002;62(7):2162–8. [PubMed] [Google Scholar]
- 24.Gassmann P, Haier J. The tumor cell-host organ interface in the early onset of metastatic organ colonisation. Clin Exp Metastasis. 2008;25(2):171–81. doi: 10.1007/s10585-007-9130-6. [DOI] [PubMed] [Google Scholar]
- 25.Hanna N, Burton RC. Definitive evidence that natural killer (NK) cells inhibit experimental tumor metastases in vivo. J Immunol. 1981;127(5):1754–8. [PubMed] [Google Scholar]
- 26.Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295–300. [PubMed] [Google Scholar]
- 27.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirouskova M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105(1):178–85. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C, Wilber A, Watabe K. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med. 2011;208(13):2641–55. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22(6):725–36. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, Sequist LV, Brachtel E, Sgroi D, Baselga J, Ramaswamy S, Toner M, Haber DA, Maheswaran S. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–4. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong D, Banerjee S, Ahmad A, Li Y, Wang Z, Sethi S, Sarkar FH. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS One. 2010;5(8):e12445. doi: 10.1371/journal.pone.0012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 33.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24(37):5764–74. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 34.Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23(5):1155–65. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhau HE, Odero-Marah V, Lue HW, Nomura T, Wang R, Chu G, Liu ZR, Zhou BP, Huang WC, Chung LW. Epithelial to mesenchymal transition (EMT) in human prostate cancer: lessons learned from ARCaP model. Clin Exp Metastasis. 2008;25(6):601–10. doi: 10.1007/s10585-008-9183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santagata S, Demichelis F, Riva A, Varambally S, Hofer MD, Kutok JL, Kim R, Tang J, Montie JE, Chinnaiyan AM, Rubin MA, Aster JC. JAGGED1 expression is associated with prostate cancer metastasis and recurrence. Cancer Res. 2004;64(19):6854–7. doi: 10.1158/0008-5472.CAN-04-2500. [DOI] [PubMed] [Google Scholar]
- 37.Mancini SJ, Mantei N, Dumortier A, Suter U, MacDonald HR, Radtke F. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood. 2005;105(6):2340–2. doi: 10.1182/blood-2004-08-3207. [DOI] [PubMed] [Google Scholar]
- 38.Xing F, Kobayashi A, Okuda H, Watabe M, Pai SK, Pandey PR, Hirota S, Wilber A, Mo YY, Moore BE, Liu W, Fukuda K, Iiizumi M, Sharma S, Liu Y, Wu K, Peralta E, Watabe K. Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain. EMBO Mol Med. 2013;5(3):384–96. doi: 10.1002/emmm.201201623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, de la Pompa JL. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18(1):99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Li Y, Banerjee S, Kong D, Ahmad A, Nogueira V, Hay N, Sarkar FH. Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-kappaB signaling pathways. J Cell Biochem. 2010;109(4):726–36. doi: 10.1002/jcb.22451. [DOI] [PubMed] [Google Scholar]
- 41.Wikstrom P, Stattin P, Franck-Lissbrant I, Damber JE, Bergh A. Transforming growth factor beta1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate. 1998;37(1):19–29. doi: 10.1002/(sici)1097-0045(19980915)37:1<19::aid-pros4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 42.Akhurst RJ, Derynck R. TGF-beta signaling in cancer--a double-edged sword. Trends Cell Biol. 2001;11(11):S44–51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 43.Han J, Sun P. The pathways to tumor suppression via route p38. Trends Biochem Sci. 2007;32(8):364–71. doi: 10.1016/j.tibs.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, Matsumoto K, Nishida E. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J Biol Chem. 1999;274(38):27161–7. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- 45.Hickson JA, Huo D, Vander Griend DJ, Lin A, Rinker-Schaeffer CW, Yamada SD. The p38 kinases MKK4 and MKK6 suppress metastatic colonization in human ovarian carcinoma. Cancer Res. 2006;66(4):2264–70. doi: 10.1158/0008-5472.CAN-05-3676. [DOI] [PubMed] [Google Scholar]
- 46.Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, Pienta MJ, Song J, Wang J, Loberg RD, Krebsbach PH, Pienta KJ, Taichman RS. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121(4):1298–312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung Y, Wang J, Song J, Shiozawa Y, Wang J, Havens A, Wang Z, Sun YX, Emerson SG, Krebsbach PH, Taichman RS. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood. 2007;110(1):82–90. doi: 10.1182/blood-2006-05-021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 49.Sugihara A, Maeda O, Tsuji M, Tsujimura T, Nakata Y, Akedo H, Kotake T, Terada N. Expression of cytokines enhancing the osteoclast activity, and parathyroid hormone-related protein in prostatic cancers before and after endocrine therapy: an immunohistochemical study. Oncol Rep. 1998;5(6):1389–94. doi: 10.3892/or.5.6.1389. [DOI] [PubMed] [Google Scholar]
- 50.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62(6):1832–7. [PubMed] [Google Scholar]
- 52.Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, Pienta KJ, Taichman RS. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003;89(3):462–73. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- 53.Sun YX, Schneider A, Jung Y, Wang J, Dai J, Wang J, Cook K, Osman NI, Koh-Paige AJ, Shim H, Pienta KJ, Keller ET, McCauley LK, Taichman RS. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20(2):318–29. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 54.Shiozawa Y, Pedersen EA, Patel LR, Ziegler AM, Havens AM, Jung Y, Wang J, Zalucha S, Loberg RD, Pienta KJ, Taichman RS. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010;12(2):116–27. doi: 10.1593/neo.91384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gjerdrum C, Tiron C, Hoiby T, Stefansson I, Haugen H, Sandal T, Collett K, Li S, McCormack E, Gjertsen BT, Micklem DR, Akslen LA, Glackin C, Lorens JB. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci U S A. 2010;107(3):1124–9. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Kwast TH, Schalken J, Ruizeveld de Winter JA, van Vroonhoven CC, Mulder E, Boersma W, Trapman J. Androgen receptors in endocrine-therapy-resistant human prostate cancer. Int J Cancer. 1991;48(2):189–93. doi: 10.1002/ijc.2910480206. [DOI] [PubMed] [Google Scholar]
- 57.Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9(4):401–6. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 58.de Vere White R, Meyers F, Chi SG, Chamberlain S, Siders D, Lee F, Stewart S, Gumerlock PH. Human androgen receptor expression in prostate cancer following androgen ablation. Eur Urol. 1997;31(1):1–6. doi: 10.1159/000474409. [DOI] [PubMed] [Google Scholar]
- 59.Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, Wilson EM. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61(11):4315–9. [PubMed] [Google Scholar]
- 60.Buchanan G, Yang M, Harris JM, Nahm HS, Han G, Moore N, Bentel JM, Matusik RJ, Horsfall DJ, Marshall VR, Greenberg NM, Tilley WD. Mutations at the boundary of the hinge and ligand binding domain of the androgen receptor confer increased transactivation function. Mol Endocrinol. 2001;15(1):46–56. doi: 10.1210/mend.15.1.0581. [DOI] [PubMed] [Google Scholar]
- 61.Gottlieb B, Beitel LK, Wu JH, Trifiro M. The androgen receptor gene mutations database (ARDB): 2004 update. Hum Mutat. 2004;23(6):527–33. doi: 10.1002/humu.20044. [DOI] [PubMed] [Google Scholar]
- 62.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1(1):34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 63.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20(13):3001–15. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 64.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93(22):1687–97. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 65.Stege R, Tribukait B, Lundh B, Carlstrom K, Pousette A, Hasenson M. Quantitative estimation of tissue prostate specific antigen, deoxyribonucleic acid ploidy and cytological grade in fine needle aspiration biopsies for prognosis of hormonally treated prostatic carcinoma. J Urol. 1992;148(3):833–7. doi: 10.1016/s0022-5347(17)36736-8. [DOI] [PubMed] [Google Scholar]
- 66.Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10(2):440–8. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 67.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69(6):2305–13. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, Page ST, Coleman IM, Nguyen HM, Sun H, Nelson PS, Plymate SR. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120(8):2715–30. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, Bova GS, Luo J. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69(1):16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghossein RA, Scher HI, Gerald WL, Kelly WK, Curley T, Amsterdam A, Zhang ZF, Rosai J. Detection of circulating tumor cells in patients with localized and metastatic prostatic carcinoma: clinical implications. J Clin Oncol. 1995;13(5):1195–200. doi: 10.1200/JCO.1995.13.5.1195. [DOI] [PubMed] [Google Scholar]
- 71.Attard G, Swennenhuis JF, Olmos D, Reid AH, Vickers E, A’Hern R, Levink R, Coumans F, Moreira J, Riisnaes R, Oommen NB, Hawche G, Jameson C, Thompson E, Sipkema R, Carden CP, Parker C, Dearnaley D, Kaye SB, Cooper CS, Molina A, Cox ME, Terstappen LW, de Bono JS. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69(7):2912–8. doi: 10.1158/0008-5472.CAN-08-3667. [DOI] [PubMed] [Google Scholar]
- 72.Hobisch A, Culig Z, Radmayr C, Bartsch G, Klocker H, Hittmair A. Distant metastases from prostatic carcinoma express androgen receptor protein. Cancer Res. 1995;55(14):3068–72. [PubMed] [Google Scholar]
- 73.Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, Feldman D. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6(6):703–6. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 74.Liu AY, Corey E, Bladou F, Lange PH, Vessella RL. Prostatic cell lineage markers: emergence of BCL2+ cells of human prostate cancer xenograft LuCaP 23 following castration. Int J Cancer. 1996;65(1):85–9. doi: 10.1002/(SICI)1097-0215(19960103)65:1<85::AID-IJC15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 75.Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nat Rev Cancer. 2003;3(1):55–63. doi: 10.1038/nrc967. [DOI] [PubMed] [Google Scholar]
- 76.Fu Z, Kitagawa Y, Shen R, Shah R, Mehra R, Rhodes D, Keller PJ, Mizokami A, Dunn R, Chinnaiyan AM, Yao Z, Keller ET. Metastasis suppressor gene Raf kinase inhibitor protein (RKIP) is a novel prognostic marker in prostate cancer. Prostate. 2006;66(3):248–56. doi: 10.1002/pros.20319. [DOI] [PubMed] [Google Scholar]
- 77.Bandyopadhyay S, Pai SK, Gross SC, Hirota S, Hosobe S, Miura K, Saito K, Commes T, Hayashi S, Watabe M, Watabe K. The Drg-1 gene suppresses tumor metastasis in prostate cancer. Cancer Res. 2003;63(8):1731–6. [PubMed] [Google Scholar]
- 78.Bandyopadhyay S, Zhan R, Chaudhuri A, Watabe M, Pai SK, Hirota S, Hosobe S, Tsukada T, Miura K, Takano Y, Saito K, Pauza ME, Hayashi S, Wang Y, Mohinta S, Mashimo T, Iiizumi M, Furuta E, Watabe K. Interaction of KAI1 on tumor cells with DARC on vascular endothelium leads to metastasis suppression. Nat Med. 2006;12(8):933–8. doi: 10.1038/nm1444. [DOI] [PubMed] [Google Scholar]
- 79.Eves EM, Shapiro P, Naik K, Klein UR, Trakul N, Rosner MR. Raf kinase inhibitory protein regulates aurora B kinase and the spindle checkpoint. Mol Cell. 2006;23(4):561–74. doi: 10.1016/j.molcel.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yeung K, Seitz T, Li S, Janosch P, McFerran B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H, Sedivy JM, Kolch W. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature. 1999;401(6749):173–7. doi: 10.1038/43686. [DOI] [PubMed] [Google Scholar]
- 81.Yeung K, Janosch P, McFerran B, Rose DW, Mischak H, Sedivy JM, Kolch W. Mechanism of suppression of the Raf/MEK/extracellular signal-regulated kinase pathway by the raf kinase inhibitor protein. Mol Cell Biol. 2000;20(9):3079–85. doi: 10.1128/mcb.20.9.3079-3085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK) Cancer Res. 2003;63(7):1684–95. [PubMed] [Google Scholar]
- 83.Aguirre-Ghiso JA, Liu D, Mignatti A, Kovalski K, Ossowski L. Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol Biol Cell. 2001;12(4):863–79. doi: 10.1091/mbc.12.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen G, Hitomi M, Han J, Stacey DW. The p38 pathway provides negative feedback for Ras proliferative signaling. J Biol Chem. 2000;275(50):38973–80. doi: 10.1074/jbc.M002856200. [DOI] [PubMed] [Google Scholar]
- 85.Haq R, Brenton JD, Takahashi M, Finan D, Finkielsztein A, Damaraju S, Rottapel R, Zanke B. Constitutive p38HOG mitogen-activated protein kinase activation induces permanent cell cycle arrest and senescence. Cancer Res. 2002;62(17):5076–82. [PubMed] [Google Scholar]
- 86.Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep. 1966;50(3):125–8. [PubMed] [Google Scholar]
- 87.Bunting PS. Screening for prostate cancer with prostate-specific antigen: beware the biases. Clin Chim Acta. 2002;315(1–2):71–97. doi: 10.1016/s0009-8981(01)00717-3. [DOI] [PubMed] [Google Scholar]
- 88.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, Partin AW. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294(4):433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 89.Cordon-Cardo C, Kotsianti A, Verbel DA, Teverovskiy M, Capodieci P, Hamann S, Jeffers Y, Clayton M, Elkhettabi F, Khan FM, Sapir M, Bayer-Zubek V, Vengrenyuk Y, Fogarsi S, Saidi O, Reuter VE, Scher HI, Kattan MW, Bianco FJ, Wheeler TM, Ayala GE, Scardino PT, Donovan MJ. Improved prediction of prostate cancer recurrence through systems pathology. J Clin Invest. 2007;117(7):1876–83. doi: 10.1172/JCI31399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Agoulnik IU, Vaid A, Nakka M, Alvarado M, Bingman WE, 3rd, Erdem H, Frolov A, Smith CL, Ayala GE, Ittmann MM, Weigel NL. Androgens modulate expression of transcription intermediary factor 2, an androgen receptor coactivator whose expression level correlates with early biochemical recurrence in prostate cancer. Cancer Res. 2006;66(21):10594–602. doi: 10.1158/0008-5472.CAN-06-1023. [DOI] [PubMed] [Google Scholar]
- 91.Kahl P, Gullotti L, Heukamp LC, Wolf S, Friedrichs N, Vorreuther R, Solleder G, Bastian PJ, Ellinger J, Metzger E, Schule R, Buettner R. Androgen receptor coactivators lysine-specific histone demethylase 1 and four and a half LIM domain protein 2 predict risk of prostate cancer recurrence. Cancer Res. 2006;66(23):11341–7. doi: 10.1158/0008-5472.CAN-06-1570. [DOI] [PubMed] [Google Scholar]
- 92.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bauer JJ, Sesterhenn IA, Mostofi FK, McLeod DG, Srivastava S, Moul JW. Elevated levels of apoptosis regulator proteins p53 and bcl-2 are independent prognostic biomarkers in surgically treated clinically localized prostate cancer. J Urol. 1996;156(4):1511–6. [PubMed] [Google Scholar]
- 94.Loric S, Paradis V, Gala JL, Berteau P, Bedossa P, Benoit G, Eschwege P. Abnormal E-cadherin expression and prostate cell blood dissemination as markers of biological recurrence in cancer. Eur J Cancer. 2001;37(12):1475–81. doi: 10.1016/s0959-8049(01)00143-5. [DOI] [PubMed] [Google Scholar]
- 95.Aaltomaa S, Lipponen P, Tammi R, Tammi M, Viitanen J, Kankkunen JP, Kosma VM. Strong Stromal Hyaluronan Expression Is Associated with PSA Recurrence in Local Prostate Cancer. Urol Int. 2002;69(4):266–72. doi: 10.1159/000066123. [DOI] [PubMed] [Google Scholar]
- 96.McAlhany SJ, Ayala GE, Frolov A, Ressler SJ, Wheeler TM, Watson JE, Collins C, Rowley DR. Decreased stromal expression and increased epithelial expression of WFDC1/ps20 in prostate cancer is associated with reduced recurrence-free survival. Prostate. 2004;61(2):182–91. doi: 10.1002/pros.20085. [DOI] [PubMed] [Google Scholar]
- 97.Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, Wheeler M, Spitler J, Rowley DR. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res. 2003;9(13):4792–801. [PubMed] [Google Scholar]
- 98.Pins MR, Fiadjoe JE, Korley F, Wong M, Rademaker AW, Jovanovic B, Yoo TK, Kozlowski JM, Raji A, Yang XJ, Lee C. Clusterin as a possible predictor for biochemical recurrence of prostate cancer following radical prostatectomy with intermediate Gleason scores: a preliminary report. Prostate Cancer Prostatic Dis. 2004;7(3):243–8. doi: 10.1038/sj.pcan.4500722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O’Brien B, Clapp JD, Rathmell JM, Riley TL, Hayes RB, Kramer BS, Izmirlian G, Miller AB, Pinsky PF, Prorok PC, Gohagan JK, Berg CD, Team PP. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Maattanen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A, Investigators E. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 101.Brawer MK. Prostate-specific antigen: current status. CA Cancer J Clin. 1999;49(5):264–81. doi: 10.3322/canjclin.49.5.264. [DOI] [PubMed] [Google Scholar]
- 102.Danila DC, Fleisher M, Scher HI. Circulating tumor cells as biomarkers in prostate cancer. Clin Cancer Res. 2011;17(12):3903–12. doi: 10.1158/1078-0432.CCR-10-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Punnoose EA, Atwal SK, Spoerke JM, Savage H, Pandita A, Yeh RF, Pirzkall A, Fine BM, Amler LC, Chen DS, Lackner MR. Molecular biomarker analyses using circulating tumor cells. PLoS One. 2010;5(9):e12517. doi: 10.1371/journal.pone.0012517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, Lilja H, Schwartz L, Larson S, Fleisher M, Scher HI. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13(23):7053–8. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- 105.Shaffer DR, Leversha MA, Danila DC, Lin O, Gonzalez-Espinoza R, Gu B, Anand A, Smith K, Maslak P, Doyle GV, Terstappen LW, Lilja H, Heller G, Fleisher M, Scher HI. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13(7):2023–9. doi: 10.1158/1078-0432.CCR-06-2701. [DOI] [PubMed] [Google Scholar]
- 106.Bettencourt MC, Bauer JJ, Sesterhenn IA, Connelly RR, Moul JW. CD34 immunohistochemical assessment of angiogenesis as a prognostic marker for prostate cancer recurrence after radical prostatectomy. J Urol. 1998;160(2):459–65. [PubMed] [Google Scholar]
- 107.Bettuzzi S, Scaltriti M, Caporali A, Brausi M, D’Arca D, Astancolle S, Davalli P, Corti A. Successful prediction of prostate cancer recurrence by gene profiling in combination with clinical data: a 5-year follow-up study. Cancer Res. 2003;63(13):3469–72. [PubMed] [Google Scholar]
- 108.Febbo PG, Sellers WR. Use of expression analysis to predict outcome after radical prostatectomy. J Urol. 2003;170(6 Pt 2):S11-9. doi: 10.1097/01.ju.0000095567.03807.a1. discussion S19–20. [DOI] [PubMed] [Google Scholar]
- 109.Singh D, Febbo PG, Ross K, Jackson DG, Manola J, Ladd C, Tamayo P, Renshaw AA, D’Amico AV, Richie JP, Lander ES, Loda M, Kantoff PW, Golub TR, Sellers WR. Gene expression correlates of clinical prostate cancer behavior. Cancer Cell. 2002;1(2):203–9. doi: 10.1016/s1535-6108(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 110.Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, Kurdistani SK. Global histone modification patterns predict risk of prostate cancer recurrence. Nature. 2005;435(7046):1262–6. doi: 10.1038/nature03672. [DOI] [PubMed] [Google Scholar]
- 111.Kirmizis A, Bartley SM, Kuzmichev A, Margueron R, Reinberg D, Green R, Farnham PJ. Silencing of human polycomb target genes is associated with methylation of histone H3 Lys 27. Genes Dev. 2004;18(13):1592–605. doi: 10.1101/gad.1200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yu J, Yu J, Rhodes DR, Tomlins SA, Cao X, Chen G, Mehra R, Wang X, Ghosh D, Shah RB, Varambally S, Pienta KJ, Chinnaiyan AM. A polycomb repression signature in metastatic prostate cancer predicts cancer outcome. Cancer Res. 2007;67(22):10657–63. doi: 10.1158/0008-5472.CAN-07-2498. [DOI] [PubMed] [Google Scholar]
- 113.van de Stolpe A, Pantel K, Sleijfer S, Terstappen LW, den Toonder JM. Circulating tumor cell isolation and diagnostics: toward routine clinical use. Cancer Res. 2011;71(18):5955–60. doi: 10.1158/0008-5472.CAN-11-1254. [DOI] [PubMed] [Google Scholar]