Abstract
This editorial highlights a study by Mellone et al. (2014) entitled “Zinc transporter-1 (ZNT-1): a novel NMDA receptor binding protein at the postsynaptic density” in this issue of Journal of Neurochemistry, which reports for the first time that the zinc-transporter ZnT1 interacts directly and in an activity-dependent manner with the cytoplasmic tail of the zinc-sensitive N-methyl-D-aspartate (NMDA) receptors. The highlighted article suggests a novel pathway towards ion homeostasis through balancing zinc and calcium ions (Zn2+ and Ca2+) and involving receptor modulation at synapses.
Keywords: NMDA receptors, zinc transport, synaptic transmission, spine development
Glutamatergic synapses represent the major mode of intercellular communication in the central nervous system (CNS). The number and morphology of these excitatory synapses correlate in fundamental ways with both normal neurophysiology and behaviors and with pernicious disorders and pathologies. NMDA receptors are molecular hallmarks of glutamatergic synapses; in the post-synaptic density (PSD), they form the core of a massive and dynamic protein signaling hub; and their activation initiates the remodeling of the PSD but can also trigger calcium-dependent cellular death. In this issue of the Journal of Neurochemistry, Mellone and her colleagues report for the first time that the zinc-transporter ZnT1 associates directly and in an activity-dependent manner with the cytoplasmic tail of the zinc-sensitive NMDA receptors, and its protein levels can influence the morphology of post-synaptic spines (Mellone et al. 2014). This is the latest in a series of recent reports that demonstrate new functions for ZnT1 and by placing it at postsynaptic sites they open new hypotheses about specific roles of post-synaptic zinc in the physiology and pathology of excitatory synapses (Sindreu et al. 2014, Shusterman et al. 2014, Grabrucker et al. 2011).
ZnT1 was discovered in the mid-nineties as a protein that confers cellular resistance to zinc toxicity (Palmiter & Findley 1995). It belongs to a family of 10 transmembrane proteins (ZnT1 – ZnT10) that serve to efflux zinc from cytosol into intracellular organelles or into extracellular space, depending on their subcellular distribution. ZnT1 is expressed at the plasma membrane; assembles as a functional dimer; and extrudes zinc in a sodium-independent, proton-driven, and calcium-sensitive manner (Figure 1) (Shusterman et al. 2014, Palmiter & Findley 1995). Because ZnT1 expression is induced by zinc (McMahon & Cousins 1998) and protects cells against zinc toxicity in several experimental paradigms (Palmiter 2004, Nolte et al. 2004, Palmiter & Findley 1995, Tsuda et al. 1997) it is currently believed that ZnT1 is a major player in cellular zinc homeostasis and functions to protect a variety of cells from otherwise noxious actions of physiologic zinc transients.
Figure 1.
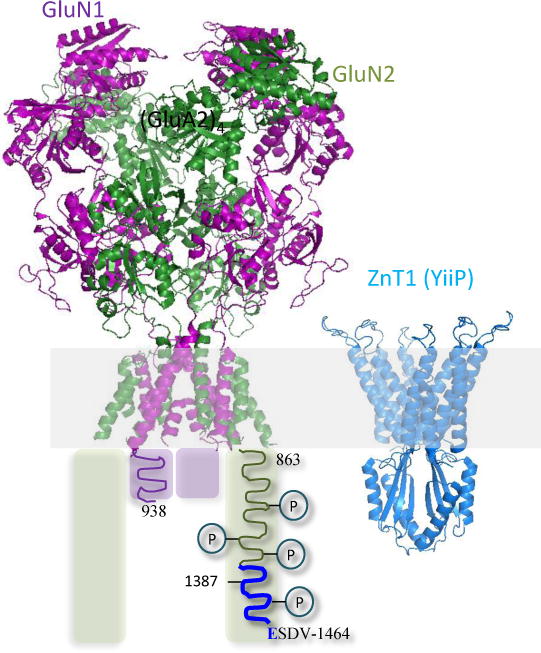
Structural models for an NMDA receptor and a zinc transporter. Left, Model of a tetrameric NMDA receptor combines the atomic arrangement for the ecto- and transmembrane domains of GluN1/GluN2B receptor (PDB 4PE5, Karakas and Furukawa, 2014) with a hypothetical cartoon of the cytoplasmic domain, which is presumed to be intrinsically disordered. Each GluN1 (purple) and GluN2A (green) subunit extends short (839 to 938 in GluN1) and long (863 to 1482, in GluN2A) intracellular tails; both include numerous phosphorylation sites; the sequence (1387–1461) required for interaction with ZnT1 is highlighted in blue. Right, Atomic model of a homo dimeric bacterial zinc transporter (YiiP, PDB: 3H90, Lu and Fu, 2007), which shares sequence homology with the mammalian zinc transporter ZnT1.
Zinc is an essential micro nutrient and among other systemic dysfunctions, nutritional zinc deficiency brings about neurologic problems (Frederickson et al. 2005). How zinc contributes to brain physiology and pathology is poorly understood. Relative to other transition metals, zinc is relatively non-poisonous to humans, most likely due to its low redox reactivity. Notably, extracellular zinc at low micromolar concentrations is lethal to neurons and glial cells (Yokoyama et al. 1986). Little is known about how zinc enters the brain or how its levels are controlled but is clear that zinc accumulates in brain in distinct pools of labile ions that can be visualized histochemically (Maske 1955, Timm 1958). This accumulation makes for often spectacular staining of specific populations of glutamatergic neurons in the neocortex, amygdala, hippocampus, and olfactory bulb (Jo et al. 2000, Frederickson et al. 1982, Frederickson et al. 2000). This zinc is considered a neurotransmitter because it is concentrated into glutamate-containing presynaptic vesicles; it is co-released with glutamate (and protons) in an activity-dependent manner; it interacts with post-synaptic receptors and influences excitability; and it can produce intracellular zinc transient in the post-synaptic neuron (Baranano et al. 2001, Assaf & Chung 1984, Howell et al. 1984). Thus in addition to its prominent role as structural and catalytic component of many proteins, diffusible zinc serves as a signaling factor in the extracellular space and within the intracellular cytosolic compartment. A well-documented function for extracellular zinc is its fast and potent inhibition of NMDA receptor currents.
NMDA receptors are glutamate-gated calcium-permeable ion-channels of high interest to modern neurochemistry. They are large transmembrane proteins that assemble as obligate heterotetramers of GluN subunits (Figure 1A). Two GluN1 protomers are present in all functional NMDA receptors and are expressed ubiquitously in the CNS; they pair with GluN2 and/or GluN3 subunits to form receptors with distinct functional and pharmacologic properties. Of the four GluN2 proteins encoded in mammalian genomes, GluN2A and GluN2B are the most widely distributed and have characteristic developmental profiles. GluN2B is the first to be expressed in early development and is abundant at immature synapses, whereas GluN2A appears later in development and dominates at mature synapses. Among the 17 members of the ionotropic glutamate receptor family, GluN2A and GluN2B are the subunits with the largest cytoplasmic tails. Their intracellular domains are as massive (~400 residues) as the receptors’ ectodomains (Figure 1A); are intrinsically disordered; are subject to enzymatic post-translational modification at multiple site; and they associate, often in a dynamic manner, with a remarkable array of postsynaptic proteins. In the PSD, NMDA receptors form dynamic complexes of densely packed proteins, of which more than 70 can bind directly to the cytoplasmic tails of NMDA receptors (Husi et al. 2000, Sheng & Lee 2000). In the article highlighted here, a yeast-two hybrid screen identified ZnT1 as the newest component of the NMDA receptor signaling complex (Mellone et al. 2014). Together with recent reports that ZnT1 localizes at post-synaptic sites that are not necessarily correlated with sites of vesicular zinc release, these findings engender new hypotheses regarding the role of labile zinc ions at central synapses and more generally in the physiology and pathology of the central nervous system (Sindreu et al. 2014, Grabrucker et al. 2014).
Mellone et al. screened a brain library for proteins likely to bind to the GluN2A C-terminal domain. This approach identified five novel gene products, of which one was ZnT1. Consistent with a physiologic interaction, the authors confirm that ZnT1 is present in synaptosomes isolated from rat hippocampi, striatum, cortex, and cerebellum and also show that ZnT1 expression is developmentally regulated in a manner that correlates with the delayed expression of GluN2A subunits. Notably, when ZnT1 was co-expressed with NMDA receptor subunits in a heterologous system, it induced clustering at the plasma membrane of GluN2A- but not GluN2B- containing receptors, indicating a sub-type specific interaction. Is the interaction direct? Both GluN2A and ZnT1 have on their most distal C-terminal sequence a PDZ binding motif (ESDV and ESSL, respectively), which raises the possibility that they each may be anchored individually to a common structural protein within the PSD network. Biochemical evidence presented by Mellone et al. demonstrates that the interaction between GluN2A and ZnT1 is independent of the GluN2A ESDV motif and is mediated by a GluN2A segment that immediately precedes it, specifically the 1387–1461 sequence. First, the C-terminal tail used as bait in the initial yeast two-hybrid screen (1046–1461) lacked the last three SDV residues; second, the shorter GluN2A(1387–1464) peptide was able to co-precipitate ZntT1 in a pull-down assay.
What may be the role of such direct and subunit-specific interaction between the zinc-sensitive calcium-injecting NMDA receptor and the calcium-sensitive, zinc-ejecting ZnT1? Does it affect NMDA receptor mediated calcium influx and ZnT1 mediated zinc efflux? And if so, does the interaction control function through direct allosteric effects or rather serves to keep the two proteins in close proximity such that their ionic outputs may influence each other’s activities? In heart cells, ZnT1 reduces ionic flux through L-Type Cav channels (Beharier et al. 2007) through functional interaction with the beta subunit of Cav channels (Levy et al. 2009); it also increases expression of and currents through T-type Cav channels, by stimulating the ERK pathway (Mor et al. 2012). It will be important to establish whether ZnT1 can also modulate NMDA receptor activity. In addition, the juxtaposition of the two proteins may influence each other’s local ionic environment. High NMDA receptor activity may lower extracellular calcium levels, which would inhibit ZnT1 activity; conversely, acidification brought about by synaptic activity may increase zinc efflux by ZnT1 and inhibit proximal NMDA receptors, of which the GluN2A subtype is highly sensitive to zinc. These questions however will have to wait quantitative and mechanistic studies to delineate whether and how the interaction or the close proximity between the two electrogenic proteins affects one-another’s functions.
Importantly, cellular treatments that induce NMDA receptor-dependent synaptic plasticity reduced the ability of ZnT1 to co-precipitate GluN2A and reduced the ZnT1 levels in synaptosomes, suggesting that the interaction is dynamic and depends on synaptic state (Mellone et al. 2014). How does synaptic plasticity intersect with ZnT1 affinity for NMDA receptors and its synaptic levels? Post-synaptic kinase/phosphatase systems may play a role given that the GluN2A(1387–1461) segment contains 17 potential phosphorylation sites (serine, threonine and tyrosine residues). Of note is that this same sequence identified here as required for binding ZnT1, is also required for NMDA receptor interaction with CamKII (Gardoni et al. 1999). Specifically PKC phosphorylation of serine 1416 promotes dissociation of αCamKII from GluN2A (Gardoni et al. 2001). Therefore it will be important to determine whether serine 1416 and more generally PKC activity are also responsible for NMDA receptor interaction with ZnT1. Regardless of mechanism the observation still stands that simply manipulating the levels of ZnT1 in cultured hippocampal neurons produced observable changes in synaptic morphology: reducing ZnT1 translation produced spine shrinkage and ZnT1 overexpression produced spine enlargement (Mellone 2014). These observations deserve more in depth investigation because they suggest a role for ZnT1 in determining the shape and size of spines that correlates well with recent reports that as a component of scaffolding proteins, post-synaptic zinc is essential for the normal morphology and maturation of the PSD and may play a role in the etiology of autism spectrum disorders (Grabrucker et al. 2011, Grabrucker et al. 2014).
These new findings for a physiologic role of zinc in post-synaptic physiology may correlate with the initially surprising observation the zinc toxicity in ischemia- or seizure-induced neuronal damage is independent of vesicular zinc, and together argue for more general roles of zinc in neuronal physiology and pathology (Cole et al. 2000, Choi & Koh 1998). Although ZnT1 was initially cloned as a factor conferring zinc tolerance to non-neuronal cells, it also showed up as one of the genes induced by transient ischemia and its protein levels increased specifically in the ischemia-vulnerable pyramidal neurons of the CA1 region, possibly by ischemia-related increase in cytoplasmic zinc (Tsuda et al. 1997). Earlier this year, Sindreu and his colleagues reported for the first time a post-synaptic localization for ZnT1 in normal brain tissue (Sindreu et al. 2014). Importantly, they find no association between sites of vesicular zinc accumulation and sites of ZnT1 expression. Given that in some cells ZnT1 activity may lower intracellular zinc to sub nanomolar levels (Shusterman et al. 2014), the report highlighted here that documents its delayed developmental expression (Mellone et al. 2014) may correlate rather than coincide with the expression of zinc-independent scaffolding proteins as reported by Grabrucker and colleagues (Grabrucker et al. 2011, Grabrucker et al. 2014).
Given the accumulating evidence that zinc is essential for normal synaptic development and its deficiency may contribute to developmental disorders it will be necessary to undertake a systematic evaluation of ZnT1 expression across brain regions, developmental stages, and in pathologic states and to delineate the mechanisms by which it impacts brain physiology and pathology. More generally, these studies call attention to the still nascent understanding of zinc uptake, efflux and homeostasis in CNS, natural processes that may represent valuable targets in the prevention and therapy of mental and neurologic disorders and pathologies.
Abbreviations
- Ca2+
calcium ions
- CNS
central nervous system
- GluN1 & GluN2 (Glun2A–D)
ionotropic glutamate receptors
- NMDA
N-methyl-D-aspartate receptors
- PDB 4PE5
Protein Data Bank ID: 4PE5
- PSD
post-synaptic density
- ZNT-1
zinc transporter-1
- Zn2+
zinc ions
Footnotes
Conflict of interest disclosure
The author has no conflicts of interest to declare. Dr. Popescu is a handling editor with the Journal of Neurochemistry
References
- Assaf SY, Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Baranano DE, Ferris CD, Snyder SH. Atypical neural messengers. Trends Neurosci. 2001;24:99–106. doi: 10.1016/s0166-2236(00)01716-1. [DOI] [PubMed] [Google Scholar]
- Beharier O, Etzion Y, Katz A, Friedman H, Tenbosh N, Zacharish S, Bereza S, Goshen U, Moran A. Crosstalk between L-type calcium channels and ZnT-1, a new player in rate-dependent cardiac electrical remodeling. Cell Calcium. 2007;42:71–82. doi: 10.1016/j.ceca.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Choi DW, Koh JY. Zinc and brain injury. Annu Rev Neurosci. 1998;21:347–375. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- Cole TB, Robbins CA, Wenzel HJ, Schwartzkroin PA, Palmiter RD. Seizures and neuronal damage in mice lacking vesicular zinc. Epilepsy Res. 2000;39:153–169. doi: 10.1016/s0920-1211(99)00121-7. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Manton WI, Frederickson MH, Howell GA, Mallory MA. Stable-isotope dilution measurement of zinc and lead in rat hippocampus and spinal cord. Brain Res. 1982;246:338–341. doi: 10.1016/0006-8993(82)91188-x. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Suh SW, Silva D, Frederickson CJ, Thompson RB. Importance of Zinc in the Central Nervous System: The Zinc-Containing Neuron. J Nutr. 2000;130:1471S–1483. doi: 10.1093/jn/130.5.1471S. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Bellone C, Cattabeni F, Di Luca M. Protein kinase C activation modulates alpha-calmodulin kinase II binding to NR2A subunit of N-methyl-D-aspartate receptor complex. J Biol Chem. 2001;276:7609–7613. doi: 10.1074/jbc.M009922200. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Schrama LH, van Dalen JJ, Gispen WH, Cattabeni F, Di Luca M. AlphaCaMKII binding to the C-terminal tail of NMDA receptor subunit NR2A and its modulation by autophosphorylation. FEBS Lett. 1999;456:394–398. doi: 10.1016/s0014-5793(99)00985-0. [DOI] [PubMed] [Google Scholar]
- Grabrucker AM, Knight MJ, Proepper C, et al. Concerted action of zinc and ProSAP/Shank in synaptogenesis and synapse maturation. 2011;3 doi: 10.1038/emboj.2010.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabrucker S, Jannetti L, Eckert M, et al. Zinc deficiency dysregulates the synaptic ProSAP/Shank scaffold and might contribute to autism spectrum disorders. 2014;1 doi: 10.1093/brain/awt303. [DOI] [PubMed] [Google Scholar]
- Howell GA, Welch MG, Frederickson CJ. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984;308:736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- Jo SM, Won MH, Cole TB, Jensen MS, Palmiter RD, Danscher G. Zinc-enriched (ZEN) terminals in mouse olfactory bulb. Brain Res. 2000;865:227–236. doi: 10.1016/s0006-8993(00)02227-7. [DOI] [PubMed] [Google Scholar]
- Levy S, Beharier O, Etzion Y, et al. Molecular basis for zinc transporter 1 action as an endogenous inhibitor of L-type calcium channels. J Biol Chem. 2009;284:32434–32443. doi: 10.1074/jbc.M109.058842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maske H. A new method for demonstrating A and B cells in the islands of Langerhans. Klin Wochenschr. 1955;33:1058. doi: 10.1007/BF01467958. [DOI] [PubMed] [Google Scholar]
- McMahon RJ, Cousins RJ. Regulation of the zinc transporter ZnT-1 by dietary zinc. Proc Natl Acad Sci U S A. 1998;95:4841–4846. doi: 10.1073/pnas.95.9.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellone M, Pelucchi S, Alberti L, Genazzani A, Di Luca M, Gardoni F. Zinc transporter-1 (ZNT-1): a novel NMDA receptor-binding protein at postsynaptic density. J Neurochem. 2014 doi: 10.1111/jnc.12968. [DOI] [PubMed] [Google Scholar]
- Mor M, Beharier O, Levy S, et al. ZnT-1 enhances the activity and surface expression of T-type calcium channels through activation of Ras-ERK signaling. Am J Physiol Cell Physiol. 2012;303:C192–203. doi: 10.1152/ajpcell.00427.2011. [DOI] [PubMed] [Google Scholar]
- Nolte C, Gore A, Sekler I, Kresse W, Hershfinkel M, Hoffmann A, Kettenmann H, Moran A. ZnT-1 expression in astroglial cells protects against zinc toxicity and slows the accumulation of intracellular zinc. Glia. 2004;48:145–155. doi: 10.1002/glia.20065. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Protection against zinc toxicity by metallothionein and zinc transporter 1. Proc Natl Acad Sci U S A. 2004;101:4918–4923. doi: 10.1073/pnas.0401022101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Findley SD. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995;14:639–649. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Lee SH. Growth of the NMDA receptor industrial complex. Nat Neurosci. 2000;3:633–635. doi: 10.1038/76576. [DOI] [PubMed] [Google Scholar]
- Shusterman E, Beharier O, Shiri L, et al. ZnT-1 extrudes zinc from mammalian cells functioning as a Zn(2+)/H(+) exchanger. Metallomics. 2014;6:1656–1663. doi: 10.1039/c4mt00108g. [DOI] [PubMed] [Google Scholar]
- Sindreu C, Bayes A, Altafaj X, Perez-Clausell J. Zinc transporter-1 concentrates at the postsynaptic density of hippocampal synapses. Molecular brain. 2014;7:16. doi: 10.1186/1756-6606-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm F. Histochemistry of the region of Ammon’s horn. Z Zellforsch Mikrosk Anat. 1958;48:548–555. [PubMed] [Google Scholar]
- Tsuda M, Imaizumi K, Katayama T, Kitagawa K, Wanaka A, Tohyama M, Takagi T. Expression of zinc transporter gene, ZnT-1, is induced after transient forebrain ischemia in the gerbil. J Neurosci. 1997;17:6678–6684. doi: 10.1523/JNEUROSCI.17-17-06678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama M, Koh J, Choi DW. Brief exposure to zinc is toxic to cortical neurons. Neuroscience letters. 1986;71:351–355. doi: 10.1016/0304-3940(86)90646-4. [DOI] [PubMed] [Google Scholar]


