Abstract
The breast is particularly vulnerable to carcinogenic influences during adolescence due to rapid proliferation of mammary cells and lack of terminal differentiation. We investigated consumption of adolescent red meat and other protein sources in relation to breast cancer risk in the Nurses' Health Study II cohort.
We followed prospectively 44,231 women aged 33-52 years who, in 1998, completed a detailed questionnaire about diet during adolescence. Relative risks (RR) and 95% confidence intervals (95%CI) were estimated using Cox proportional hazard regression.
We documented 1132 breast cancer cases during 13-year follow-up. In multivariable Cox regression models with major breast cancer risk factors adjustment, greater consumption of adolescent total red meat was significantly associated with higher premenopausal breast cancer risk (highest vs lowest quintiles, RR, 1.42; 95%CI, 1.05-1.94; Ptrend=0.007), but not postmenopausal breast cancer. Adolescent poultry intake was associated with lower risk of breast cancer overall (RR, 0.75; 95%CI, 0.59-0.96; for each serving/day). Adolescent intakes of iron, heme iron, fish, eggs, legumes and nuts were not associated with breast cancer. Replacement of one serving/day of total red meat with one serving of combination of poultry, fish, legumes, and nuts was associated with a 16% lower risk of breast cancer overall (RR, 0.84; 95%CI, 0.74-0.96) and a 24% lower risk of premenopausal breast cancer (RR, 0.76; 95%CI, 0.64-0.92).
Higher consumption of red meat during adolescence was associated with premenopausal breast cancer. Substituting other dietary protein sources for red meat in adolescent diet may decrease premenopausal breast cancer risk.
Introduction
The relation between intake of red meat and breast cancer has been examined in many studies; the overall evidence from a pooled analysis of cohort studies (1) and a recent meta-analysis (2) has not supported an important association. However, most of this evidence has been based on diet during midlife and later. In contrast, among women in Nurses' Health Study II (NHSII) cohort, high intake of red meat in early adulthood was associated with increased breast cancer incidence (3). Furthermore, vegetable protein consumption at age 14 was associated with lower risk of benign breast disease in early adulthood (4). Therefore, one potential explanation for the overall lack of association in previous studies is the timing of dietary assessment. Mammary glands may be particularly vulnerable to breast carcinogenesis during childhood due to rapid proliferation of cells and lack of terminal differentiation. The evidence for this window of vulnerability was demonstrated in the atomic bombings of Hiroshima and Nagasaki and was also seen with radiation treatment for Hodgkin's lymphoma; in both cases exposure to radiation in childhood and early adult life was associated with subsequent risk of breast cancer, whereas exposure after age 30 had little effect (5-7).
In an early analysis of the NHSII with 7 years follow-up and with 455 incident invasive breast cancer cases (8), intake of total red meat (processed plus unprocessed) during adolescence was associated with an increased risk of premenopausal breast cancer. This updated analysis provided an examination of animal and vegetable sources of protein during adolescence including intakes of total red meat (unprocessed plus processed red meat), poultry, fish, eggs, legumes and nuts in relation to breast cancer incidence with almost triple the number of cases and twice the length of follow-up. In addition, we were able to examine breast cancers separately by menopausal status and estrogen receptor (ER) and progesterone receptor (PR) status.
Subjects and Methods
Study population
In 1989, the NHSII cohort was established with a total 116,430 female registered nurses aged 24-43 years who completed biennial questionnaires including items on lifestyle and medical events. In 1997, participants were asked whether they would fill out a supplemental food frequency questionnaire about diet during high school (HS-FFQ). 64,380 women (55% of the entire cohort) indicated willingness to complete, and 47,355 of them returned the HS-FFQ in 1998. Participants were excluded if they had any cancer except non-melanoma skin cancer before 1998, had missing information on age or red meat intake or reported implausible daily energy intake (<600 or ≥5000 Kcal). After exclusions, data from 44,231 women were available for the present analysis. The follow-up completed was greater than 98% of potential person-years through 2011.
The study protocol was approved by the institutional review boards of Brigham and Women's Hospital and Harvard School of Public Health (Boston, MA, United States).
Assessment of adolescent and adult food intake
Adolescent food intake was measured using the 124-item food frequency questionnaire (FFQ) which was specifically designed to include foods that were usually consumed between 1960 and 1980 when the participants would have been in high school. Categories on the HS-FFQ included main dishes, bread and cereals, fruits, vegetables, condiments, snack foods, dairy products and beverages. Total red meat intake included unprocessed red meat (hamburger, beef, lamb, pork and meatloaf) and processed red meat items (hot dog, bacon and other processed meat such as sausage, salami and bologna); poultry included chicken and turkey; fish included tuna, dark meat fish (for example, mackerel, salmon, sardines, bluefish, swordfish), and other fish; legumes included beans, peas, lentils, and lima beans; and nuts included peanuts, peanut butter, other nuts and seeds. For each food, a commonly used portion size was indicated, and participants were asked to their dietary habits when they were in high school. Nine categories of frequency were possible ranging from “never or less than once per month” to “6 or more per day”. The validity and reproducibility of the HS-FFQ was described in detail elsewhere (9, 10). Briefly, the reproducibility of the HS-FFQ was examined among 80 young adults by comparing their responses on the HS-FFQ with information they provided using a similar questionnaire 10 years earlier about their current diet while they were in high school. The mean of correlation between the two measures administered 10 years apart was 0.58 (range, 0.40-0.88) (9). In the same group, the mean correlation for nutrients assessed by the HS-FFQ and by three 24-hour recalls while in high school was 0.45. Furthermore, the reproducibility of HS-FFQ was administered twice at a 4-year interval to a random sample of 333 NHSII participants; the correlation for adolescent nutrient intakes reported 4 years earlier was 0.65 (range, 0.50-0.77) and red meat consumed within main dishes had a mean correlation of 0.52, whereas the correlation between adult and adolescent diet was weak (mean nutrient correlation, 0.20, red meat correlation, 0.34) (10), indicated that this is largely independent information. Validity was also assessed by comparing dietary intake reported by 272 mothers of the NHSII participants with adolescent diets using the HS-FFQ; the correlation was 0.40 (range, 0.13–0.59) for nutrients (10).
To assess dietary intake during adulthood, women were asked to complete a semi-quantitative FFQ with approximately 130 items about usual diet and alcohol consumptions during the past year in 1991 and every four years afterwards. Food items included in this FFQ and response categories were similar to those on the HS-FFQ (publicly available at http://www.channing.harvard.edu/nhs/?page_id=246).
Nutrient intakes for both the HS-FFQ and adult FFQ were calculated by multiplying the frequency of consumption by the nutrient content of the specified portions of each unit of food or beverage and summing across all items. The US Department of Agriculture, food manufacturers, and independent academic sources were used to calculate the nutrient values in foods (11-13), using values specific to 1960 to 1980 for the HS-FFQ. To calculate the percentage of energy contributed by fat and other macronutrients, we divided energy intake from that nutrient by total energy intake. Intakes of iron and heme iron were energy-adjusted by using the residuals from the regression of nutrient intake on total energy intake (14, 15).
Documentation of Breast Cancer
Incident cases of breast cancer were ascertained on biennial follow-up questionnaires mailed between 1997 and 2011 and by a search of the National Death Index. We requested permission to obtain medical records and pathology reports, which were used to confirm diagnose and to extract information on estrogen and progesterone receptor status. Deaths in this cohort were reported through family members and the postal service in response to the follow-up questionnaires or identified through annual review of the National Death Index. Medical records were obtained for 88% of NHSII cases included in this analysis. More than 99% of self-report breast cancers were confirmed through pathology report review. Therefore, we included self-reported cases with missing pathology report in the analysis. Cases of carcinoma in situ were not included in the analyses.
Assessment of other variables
Data on age, height, weight, family history of breast cancer, history of benign breast disease, smoking, race, menopausal status, age at menarche, postmenopausal hormone use, and oral contraceptive use were obtained from the biennial NHSII questionnaires and all variables except race, height and age at menarche were updated to the most recent information before date of diagnosis, whenever available. Menopausal status is described in detail elsewhere (16). Briefly, Women were defined as premenopausal if they still had menstrual periods or had hysterectomy with at least one ovary remaining and were younger than 46 years for smokers or younger than 48 years for nonsmokers. Women were defined as postmenopausal if they reported permanent cessation of menstrual periods or had bilateral oophorectomy surgery. Women who had unknown menopausal status or had hysterectomy without bilateral oophorectomy were considered postmenopausal if they were 54 years or older for smokers or 56 years or older for nonsmokers.
Body mass index (BMI) at age 18 was obtained from the 1989 questionnaire and used as a proxy for BMI during high school. Weight change from age 18 was calculated by taking the difference between current weight and recalled weight at age 18. Data on smoking, alcohol consumption and oral contraceptive use during adolescence were obtained from the 1989 NHSII questionnaire.
Statistical analysis
We conducted the analyses in three groups: among all women, premenopausal women and postmenopausal women. Follow-up time began with return of the baseline questionnaire in 1998 until either June 2011, the date of breast cancer diagnosis, or death, whichever came first. In premenopausal women, we stopped follow-up when they reported postmenopausal or uncertain menopausal status. For postmenopausal group, women started contributing person-time from the first 2-year cycle in which they reported postmenopausal status. Cox proportional hazards models, stratified by age in months and 2-year follow-up cycle, were used to estimate relative risks (RR) and 95% confidence intervals (95%CI) (17). Multivariable models adjusted for race, family history of breast cancer in mother or sisters, history of benign breast disease, smoking, height, body mass index (BMI) at age 18, weight gain since age 18, age at menarche, parity and age at first birth, oral contraceptive use, adolescent alcohol intake, adult alcohol intake and adolescent energy intake, and, for postmenopausal women, age at menopause and hormone use. Among all women, we additionally adjusted for hormone use and menopausal status and, age at menopause. In this group, menopausal status and postmenopausal hormone use were categorized as premenopausal, postmenopausal never users, postmenopausal past users, postmenopausal current users and unknown menopausal status. Adjustment for energy provides a measure of dietary composition rather than absolute intake, and also provides an adjustment for over and under reporting (18). Tests for linear trend were conducted by modeling the median value for each quintile and treating this as a continuous variable in the regression model. The time-varying covariates included in the multivariate analyses were updated every 2 or 4 years. We replaced missing covariate data with carried-forward data for continuous variables and missing indicator method for categorical variables (19). We estimated the effect of substituting 1 serving/day of poultry, fish, legumes, eggs or nuts during adolescence for one serving/day of total red meat during adolescence by including simultaneously these food items as well as low fat dairy product and high fat dairy product as continuous variables in the multivariable model. The RR and the 95%CIs for the substitution effect were derived from difference between the regression coefficients, variances and covariance (20). In a sensitivity analysis, to determine whether the additional follow-up independently supported the earlier report (8), the association between total red meat and premenopausal breast cancer was separately evaluated from 1998 to 2005 (similar to prior study (8)) and from 2005 to 2011. To examine differential associations of total red meat intake with breast cancer risk by hormone receptor status, we used Cox proportional cause-specific hazards regression model with a duplication method for competing risk data. This method permits estimation of separate associations of red meat for tumors that are both estrogen and progesterone receptors positive (ER+/PR+) and both receptors negative (ER-/PR-) (21). We examined effect modification of the association between red meat and breast cancer risk by other measures of breast cancer risk factors. A cross product interaction term between each fact and intake of red meat expressed as a continuous variable was included in the multivariable model. P values for tests for interactions were derived by using a likelihood ratio test with one degree of freedom. All P values and 95%CI were 2-sided and performed by using SAS version 9.3 (SAS Institute Inc, Cary NC).
Results
During 602,444 person-years follow-up of 44,231 women, 1132 women were diagnosed with invasive breast cancer (546 premenopausal, 483 postmenopausal and 103 uncertain menopausal status). Table 1 shows the baseline characteristics of participants according to quintiles of total red meat intake in adolescence. Compared to women in the lowest quintile of adolescent dietary red meat intake, those in the highest quintile were more likely to drink more alcohol in adolescence and adulthood, to be smokers and to have age at menarche less than 12 years. They were also more likely to have larger BMI at age 18 and in adulthood.
Table 1. Age-standardized distribution of potential risk factors for breast cancer in 1997 according to adolescent total red meat intake.
| Quintile of total red meat intake (n=44,231) | |||||
|---|---|---|---|---|---|
| Q1 (n=8,820) |
Q2 (n=8,864) |
Q3 (n=8,851) |
Q4 (n=8,845) |
Q5 (n=8,851) |
|
| Adolescence | |||||
| Total red meat, serving/day | 0.7±0.2 | 1.1±0.1 | 1.4±0.1 | 1.8±0.1 | 2.6±0.6 |
| Body mass index at age 18, kg/m2 | 20.9±3.0 | 21.0±3.0 | 21.1±3.2 | 21.3±3.3 | 21.6±3.6 |
| Total energy intake, kcal/day | 2159±669 | 2481±640 | 2707±660 | 2968±677 | 3409±709 |
| Alcohol intake at age 18, g/day | 4.3±7.2 | 4.9±7.5 | 5.1±7.5 | 5.2±8.1 | 5.6±9.0 |
| Poultry, serving/day | 0.4±0.3 | 0.4±0.3 | 0.4±0.3 | 0.4±0.3 | 0.4±0.3 |
| Fish, serving/day | 0.2±0.3 | 0.2±0.2 | 0.2±0.2 | 0.3±0.2 | 0.3±0.2 |
| Legumes, serving/day | 0.5±0.4 | 0.5±0.3 | 0.5±0.3 | 0.6±0.4 | 0.6±0.4 |
| Eggs, serving/day | 0.3±0.3 | 0.3±0.3 | 0.3±0.3 | 0.4±0.3 | 0.5±0.4 |
| Nuts, serving/day | 0.5±0.4 | 0.5±0.4 | 0.5±0.4 | 0.5±0.5 | 0.6±0.5 |
| Fruits and vegetables, serving/day | 4.6±2.9 | 4.5±2.4 | 4.6±2.4 | 4.8±2.4 | 5.1±2.6 |
| Adulthood | |||||
| Age, year | 42.1±4.8 | 42.5±4.7 | 42.6±4.7 | 42.9±4.6 | 43.2±4.5 |
| Body mass index, kg/m2 | 24.9±5.4 | 25.3±5.5 | 25.7±5.7 | 26.2±6.2 | 26.9±6.5 |
| Total energy intake, kcal/day | 1644±520 | 1736±517 | 1805±536 | 1887±548 | 1991±584 |
| Alcohol intake, g/day | 3.1±5.7 | 3.3±5.7 | 3.5±6.0 | 3.5±6.1 | 3.4±6.1 |
| Total red meat, serving/day | 0.5±0.4 | 0.7±0.4 | 0.8±0.4 | 0.8±0.5 | 1.0±0.6 |
| Age at first birth, year | 26.9±4.7 | 26.7±4.6 | 26.4±4.5 | 26.3±4.6 | 26.0±4.6 |
| Current smokers, % | 8 | 9 | 9 | 10 | 10 |
| Current oral contraceptive use, % | 8 | 9 | 8 | 8 | 9 |
| History of benign breast disease, % | 15 | 16 | 16 | 16 | 16 |
| Family history of breast cancer in mother or sisters, % | 16 | 16 | 16 | 16 | 16 |
| Parity≥3, % | 25 | 27 | 27 | 27 | 25 |
| Age at menarche <12, % | 24 | 23 | 24 | 25 | 26 |
Mean ± SD (all such values).
Among all women, intake of total red meat was weakly but significantly associated with breast cancer risk (for highest vs lowest quintiles, multivariate RR, 1.17; 95%CI, 0.94-1.45, Ptrend=0.046). Among premenopausal women, higher adolescent intake of total red meat was associated with greater breast cancer risk (for highest vs lowest quintiles, multivariate RR, 1.42; 95%CI, 1.05-1.94; Ptrend=0.007) (Table 2). The association between total red meat and premenopausal breast cancer remained similar after adjustment for fruits and vegetables intake (RR, 1.38; 95%CI, 1.01-1.88; Ptrend=0.01) or heme iron (RR, 1.49; 95%CI, 1.04-2.14; Ptrend=0.01). Adjustment for adult red meat also did not change the association (for highest vs lowest quintiles, RR, 1.42; 95%CI, 1.04-1.95; Ptrend=0.009). The association between total red meat and premenopausal breast cancer was apparent after adjustment for animal fat (for highest vs lowest quintiles, RR, 1.41; 95%CI, 1.00-2.00; Ptrend=0.02). Among the covariates, total energy intake accounted for most of the difference in RR's for red meat between the age-adjusted and the multivariate models. Total energy intake itself was not significantly associate with either risk of breast cancer overall (for highest vs lowest quintiles RR, 0.94, 95%CI, 0.78-1.14, Ptrend=0.59) or risk of premenopausal breast cancer (for highest vs lowest quintiles RR, 0.79, 95%CI, 0.60-1.03, Ptrend=0.16). We also conducted an analysis for red meat intake per 1000 kcal energy intake; similar results were observed (supplemental Table S1). In both the age-adjusted and multivariable analysis, intakes of total red meat in adolescence were not significantly associated with postmenopausal breast cancer risk (Table 2). When analyses were conducted for unprocessed and processed red meat separately, the estimates were similar (supplemental Table S2).
Table 2. Relative risk (RR) and 95% confidence intervals (95%CI) for breast cancer according to quintile of adolescent intake of meat and other protein sources in premenopausal women, postmenopausal women and all women.
| Quintile of intake | Per 1 serving increase/day | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Ptrend* | ||
| Total red meat (unprocessed and processed red meat) | |||||||
| All women | |||||||
| Median intake, serving/day | 0.70 | 1.13 | 1.42 | 1.78 | 2.43 | ||
| No. of cases/person-years | 223/120,337 | 200/120,929 | 229/120,227 | 236/120,790 | 244/120,161 | ||
| Age-adjusted RR (95%CI) | 1 | 0.88 (0.72-1.06) | 1.01 (0.84-1.22) | 1.01 (0.84-1.21) | 1.05 (0.87-1.26) | 0.29 | 1.03 (0.95-1.12) |
| Multivariable RR (95%CI) | 1 | 0.88 (0.73-1.07) | 1.04 (0.85-1.26) | 1.07 (0.87-1.31) | 1.17 (0.94-1.45) | 0.046 | 1.09 (0.99-1.20) |
| Premenopausal women | |||||||
| Median intake, serving/day | 0.70 | 1.06 | 1.42 | 1.78 | 2.36 | ||
| No. of cases/person-years | 104/67,481 | 98/66,983 | 104/69,164 | 115/66,996 | 125/67,864 | ||
| Age-adjusted RR (95%CI) | 1 | 0.94 (0.71-1.24) | 0.92 (0.70-1.22) | 1.07 (0.82-1.40) | 1.13 (0.87-1.47) | 0.18 | 1.08 (0.96-1.22) |
| Multivariable RR (95%CI) | 1 | 1.01 (0.76-1.34) | 1.04 (0.78-1.39) | 1.27 (0.95-1.70) | 1.42 (1.05-1.94) | 0.007 | 1.21 (1.05-1.39) |
| Postmenopausal women | |||||||
| Median intake, serving/day | 0.77 | 1.13 | 1.49 | 1.85 | 2.50 | ||
| No. of cases/person-years | 103/43,029 | 88/43,449 | 95/43,404 | 93/43,011 | 104/42,986 | ||
| Age-adjusted RR (95%CI) | 1 | 0.83 (0.62-1.10) | 0.90 (0.68-1.20) | 0.88 (0.66-1.16) | 0.97 (0.73-1.27) | 0.94 | 0.98 (0.86-1.11) |
| Multivariable RR (95%CI) | 1 | 0.79 (0.59-1.06) | 0.85 (0.63-1.15) | 0.84 (0.61-1.14) | 0.98 (0.71-1.36) | 0.75 | 0.99 (0.85-1.15) |
| Poultry (chicken and turkey) | |||||||
| All women | |||||||
| Median intake, serving/day | 0.14 | 0.21 | 0.28 | 0.57 | 0.86 | ||
| No. of cases/person-years | 255/121,032 | 176/93,350 | 281/144,564 | 305/161,071 | 115/80,959 | ||
| Age-adjusted RR (95%CI) | 1 | 0.91 (0.75-1.11) | 0.94 (0.79-1.12) | 0.96 (0.81-1.14) | 0.74 (0.59-0.92) | 0.047 | 0.78 (0.62-0.99) |
| Multivariable RR (95%CI) | 1 | 0.90 (0.74-1.09) | 0.92 (0.77-1.09) | 0.93 (0.78-1.11) | 0.72 (0.57-0.90) | 0.03 | 0.75 (0.59-0.96) |
| Premenopausal women | |||||||
| Median intake, serving/day | 0.14 | 0.21 | 0.28 | 0.57 | 0.86 | ||
| No. of cases/person-years | 114/63,133 | 94/53,599 | 130/77,997 | 155/94,322 | 53/48,579 | ||
| Age-adjusted RR (95%CI) | 1 | 0.96 (0.73-1.26) | 0.96 (0.74-1.24) | 0.97 (0.76-1.24) | 0.67 (0.48-0.93) | 0.046 | 0.75 (0.53-1.04) |
| Multivariable RR (95%CI) | 1 | 0.97 (0.73-1.28) | 0.96 (0.74-1.24) | 0.97 (0.75-1.25) | 0.66 (0.47-0.93) | 0.046 | 0.74 (0.52-1.05) |
| Postmenopausal women | |||||||
| Median intake, serving/day | 0.14 | 0.21 | 0.28 | 0.50 | 0.86 | ||
| No. of cases/person-years | 112/47,388 | 72/34,865 | 110/48,463 | 81/33,927 | 108/50,728 | ||
| Age-adjusted RR (95%CI) | 1 | 0.87 (0.65-1.17) | 0.92 (0.71-1.21) | 1.04 (0.78-1.39) | 0.95 (0.72-1.24) | 0.91 | 0.89 (0.63-1.26) |
| Multivariable RR (95%CI) | 1 | 0.85 (0.63-1.14) | 0.90 (0.68-1.18) | 1.01 (0.75-1.36) | 0.91 (0.69-1.21) | 0.92 | 0.85 (0.58-1.24) |
| Fish | |||||||
| All women | |||||||
| Median intake, serving/day | 0.07 | 0.14 | 0.21 | 0.28 | 0.57 | ||
| No. of cases/person-years | 215/119,227 | 244/131,093 | 239/117,381 | 229/122,833 | 205/111,717 | ||
| Age-adjusted RR (95%CI) | 1 | 1.02 (0.85-1.23) | 1.07 (0.89-1.29) | 0.97 (0.80-1.17) | 0.97 (0.80-1.17) | 0.53 | 0.91 (0.69-1.20) |
| Multivariable RR (95%CI) | 1 | 1.00 (0.83-1.21) | 1.07 (0.89-1.29) | 0.97 (0.80-1.17) | 0.96 (0.79-1.18) | 0.57 | 0.92 (0.69-1.23) |
| Premenopausal women | |||||||
| Median intake, serving/day | 0.07 | 0.14 | 0.21 | 0.28 | 0.57 | ||
| No. of cases/person-years | 104/69,339 | 132/75,538 | 114/65,496 | 101/66,359 | 95/61,656 | ||
| Age-adjusted RR (95%CI) | 1 | 1.14 (0.88-1.48) | 1.11 (0.85-1.45) | 0.93 (0.70-1.23) | 0.96 (0.73-1.27) | 0.36 | 0.94 (0.63-1.40) |
| Multivariable RR (95%CI) | 1 | 1.13 (0.87-1.47) | 1.13 (0.86-1.48) | 0.96 (0.72-1.28) | 0.99 (0.74-1.33) | 0.56 | 1.01 (0.67-1.53) |
| Postmenopausal women | |||||||
| Median intake, serving/day | 0.07 | 0.14 | 0.21 | 0.28 | 0.57 | ||
| No. of cases/person-years | 91/40,204 | 93/45,374 | 102/42,534 | 107/46,498 | 90/41,185 | ||
| Age-adjusted RR (95%CI) | 1 | 0.89 (0.66-1.19) | 0.97 (0.73-1.29) | 0.98 (0.74-1.30) | 0.92 (0.69-1.24) | 0.81 | 0.86 (0.56-1.32) |
| Multivariable RR (95%CI) | 1 | 0.84 (0.63-1.13) | 0.94 (0.70-1.26) | 0.94 (0.70-1.25) | 0.89 (0.65-1.21) | 0.72 | 0.84 (0.53-1.32) |
| Egg** | |||||||
| All women | |||||||
| Median intake, serving/day | 0.07 | 0.14 | 0.43 | 0.79 | |||
| No. of cases/person-years | 184/113,018 | 266/145,146 | 538/267,825 | 143/74,591 | |||
| Age-adjusted RR (95%CI) | 1 | 1.11 (0.92-1.34) | 1.15 (0.97-1.36) | 1.05 (0.84-1.31) | 0.67 | 0.99 (0.83-1.18) | |
| Multivariable RR (95%CI) | 1 | 1.11 (0.92-1.34) | 1.15 (0.96-1.36) | 1.07 (0.85-1.34) | 0.62 | 1.00 (0.83-1.19) | |
| Premenopausal women | |||||||
| Median intake, serving/day | 0.07 | 0.14 | 0.43 | 0.79 | |||
| No. of cases/person-years | 95/67,950 | 135/86,017 | 256/146,528 | 60/36,902 | |||
| Age-adjusted RR (95%CI) | 1 | 1.10 (0.84-1.43) | 1.16 (0.91-1.47) | 1.06 (0.77-1.48) | 0.58 | 1.07 (0.84-1.38) | |
| Multivariable RR (95%CI) | 1 | 1.12 (0.86-1.46) | 1.22 (0.95-1.56) | 1.14 (0.81-1.60) | 0.34 | 1.12 (0.87-1.43) | |
| Postmenopausal women | |||||||
| Median intake, serving/day | 0.07 | 0.14 | 0.43 | 0.79 | |||
| No. of cases/person-years | 73/35,851 | 106/48,136 | 231/99,671 | 72/31,587 | |||
| Age-adjusted RR (95%CI) | 1 | 1.08 (0.80-1.47) | 1.10 (0.85-1.44) | 1.06 (0.76-1.48) | 0.79 | 0.97 (0.74-1.26) | |
| Multivariable RR (95%CI) | 1 | 1.05 (0.78-1.42) | 1.06 (0.81-1.39) | 1.04 (0.74-1.47) | 0.85 | 0.96 (0.73-1.27) | |
| Legumes | |||||||
| All women | |||||||
| Median intake, serving/day | 0.14 | 0.28 | 0.42 | 0.64 | 1.00 | ||
| No. of cases/person-years | 215/118,675 | 284/143,289 | 165/83,219 | 256/135,361 | 212/121,637 | ||
| Age-adjusted RR (95%CI) | 1 | 1.06 (0.88-1.26) | 1.07 (0.87-1.31) | 1.01 (0.84-1.21) | 0.91 (0.75-1.10) | 0.16 | 0.87 (0.74-1.02) |
| Multivariable RR (95%CI) | 1 | 1.03 (0.86-1.23) | 1.06 (0.86-1.30) | 1.01 (0.84-1.22) | 0.92 (0.75-1.12) | 0.27 | 0.88 (0.75-1.05) |
| Premenopausal women | |||||||
| Median intake, serving/day | 0.14 | 0.28 | 0.42 | 0.64 | 1.00 | ||
| No. of cases/person-years | 120/71,380 | 110/68,000 | 120/60,761 | 111/73,822 | 85/64,345 | ||
| Age-adjusted RR (95%CI) | 1 | 0.96 (0.74-1.25) | 1.08 (0.84-1.40) | 0.86 (0.66-1.12) | 0.76 (0.57-1.00) | 0.02 | 0.77 (0.60-0.99) |
| Multivariable RR (95%CI) | 1 | 0.98 (0.75-1.27) | 1.10 (0.84-1.42) | 0.91 (0.69-1.18) | 0.80 (0.60-1.07) | 0.08 | 0.81 (0.63-1.05) |
| Postmenopausal women | |||||||
| Median intake, serving/day | 0.14 | 0.28 | 0.43 | 0.71 | 1.00 | ||
| No. of cases/person-years | 81/38,363 | 114/49,926 | 109/44,966 | 91/39,618 | 88/42,943 | ||
| Age-adjusted RR (95%CI) | 1 | 1.06 (0.80-1.42) | 1.18 (0.88-1.58) | 1.09 (0.81-1.48) | 0.94 (0.69-1.28) | 0.56 | 0.87 (0.69-1.10) |
| Multivariable RR (95%CI) | 1 | 1.03 (0.77-1.37) | 1.18 (0.88-1.59) | 1.08 (0.79-1.47) | 0.94 (0.68-1.29) | 0.62 | 0.88 (0.68-1.13) |
| Nuts | |||||||
| All women | |||||||
| Median intake, serving/day | 0.07 | 0.28 | 0.43 | 0.64 | 1.00 | ||
| No. of cases/person-years | 272/149,705 | 175/90,408 | 252/135,697 | 226/111,720 | 207/114,871 | ||
| Age-adjusted RR (95%CI) | 1 | 1.08 (0.89-1.30) | 1.02 (0.86-1.21) | 1.13 (0.94-1.35) | 1.01 (0.84-1.21) | 0.79 | 0.95 (0.84-1.09) |
| Multivariable RR (95%CI) | 1 | 1.07 (0.88-1.30) | 1.01 (0.84-1.20) | 1.11 (0.92-1.33) | 1.02 (0.84-1.24) | 0.75 | 0.96 (0.83-1.10) |
| Premenopausal women | |||||||
| Median intake, serving/day | 0.07 | 0.28 | 0.43 | 0.64 | 1.00 | ||
| No. of cases/person-years | 135/84,058 | 92/51,106 | 111/76,182 | 104/62,542 | 104/64,586 | ||
| Age-adjusted RR (95%CI) | 1 | 1.08 (0.82-1.41) | 0.88 (0.68-1.13) | 1.06 (0.82-1.38) | 1.00 (0.77-1.29) | 0.96 | 0.95 (0.78-1.14) |
| Multivariable RR (95%CI) | 1 | 1.10 (0.84-1.44) | 0.91 (0.70-1.17) | 1.12 (0.85-1.46) | 1.08 (0.82-1.43) | 0.54 | 0.99 (0.81-1.20) |
| Postmenopausal women | |||||||
| Median intake, serving/day | 0.14 | 0.28 | 0.43 | 0.64 | 1.00 | ||
| No. of cases/person-years | 121/53,941 | 70/32,117 | 110/48,705 | 97/40,181 | 85/40,901 | ||
| Age-adjusted RR (95%CI) | 1 | 1.00 (0.74-1.34) | 1.01 (0.78-1.31) | 1.09 (0.83-1.43) | 0.96 (0.72-1.27) | 0.92 | 0.98 (0.80-1.19) |
| Multivariable RR (95%CI) | 1 | 0.97 (0.72-1.31) | 0.98 (0.76-1.28) | 1.05 (0.79-1.39) | 0.95 (0.70-1.28) | 0.88 | 0.97 (0.78-1.21) |
Ptrend calculated with median intake of each variable in each quintile as a continuous variable.
Multivariable model was stratified by age in months at start of follow-up and calendar year of the current questionnaire cycle and was simultaneously adjusted for race (white, non-white), family history of breast cancer in mother or sisters (yes, no), history of benign breast disease (yes, no), smoking (never, past, current 1-14/day, current 15-24/day, current ≥25/day), height (<62, 62 to <65, 65 to <68, ≥68 inches), weight gain since age 18 (≤-5, >-5-5, >5-10, >10-20, >20 kg), BMI at age 18 years (<18.5, 18.5–<22.5, 22.5-<25, 25.0-<30, ≥30.0 kg/m2), age at menarche (<12, 12, 13, ≥14 yr), parity and age at first birth (nulliparous, parity ≤2 and age at first birth <25 yr, parity≤2 and age at first birth 25 to <30 yr, parity ≤2 and age at first birth ≥30 yr, parity 3-4 and age at first birth <25 yr, parity 3-4 and age at first birth 25 to <30 yr, parity 3-4 and age at first birth ≥30 yr, parity ≥5 and age at first birth <25 yr, parity ≥5 and age at first birth ≥25 yr), oral contraceptive use (never, past, current), adolescent alcohol intake (nondrinker, <1.5, 1.5–<5, 5-<10, ≥10 g/day), adult alcohol intake (nondrinker, <5, 5–<15,≥15 g/day), and adolescent energy intake (quintile). In postmenopausal women, we additionally adjusted for hormone use (postmenopausal never users, postmenopausal past users, postmenopausal current users), age at menopause (<45 yr, 45-46 yr, 47-48, 49-50 yr, 51-52 yr, ≥53 yr). Among all women, we additionally adjusted for hormone use and menopausal status (premenopausal, postmenopausal never users, postmenopausal past users, postmenopausal current users, unknown menopausal status) and, age at menopause (premenopausal, unknown menopause, <45 yr, 45-46 yr, 47-48, 49-50 yr, 51-52 yr, ≥53 yr).
Quartile.
Higher intake of poultry during adolescence was associated with lower breast cancer risk (RR, 0.75; 95%CI, 0.59-0.96; for each additional serving/day of intake) among all women. This inverse association was not significant in either premenopausal women (RR for each additional serving/day of intake, 0.74; 95%CI, 0.52-1.05) or postmenopausal women (RR for each additional serving/day of intake, 0.85; 95%CI, 0.58-1.24). The results were similar after adjustment for adolescent animal fat, fruits and vegetables or total red meat intakes (Table 2). There was no significant association between adolescent intake of fish, eggs legumes, or nuts and either premenopausal or postmenopausal breast cancer.
No clear relationship was noted across quintiles of total iron and heme iron intakes in adolescence and breast cancer incidence in either premenopausal or postmenopausal women (Supplemental Table S3).
Replacement of one serving of total red meat with other sources of protein during adolescence is presented in Figure 1. Among all women, substituting one serving of poultry/day for one serving of total red meat was associated with a lower breast cancer risk (RR, 0.70; 95%CI, 0.54-0.92). Replacement of one serving/day of total red meat with one serving of combination of poultry, fish, legumes, and nuts was associated with an 16% lower risk of breast cancer (RR, 0.84; 95%CI, 0.74-0.96). Replacement of red meat with legumes, nuts, fish, or eggs was not associated with breast cancer risk overall. Similar associations were seen in premenopausal women for replacement of one serving/day of total red meat with poultry (RR, 0.62; 95%CI, 0.43-0.91), with legumes (RR, 0.68; 95%CI, 0.51-0.91), and with combination of poultry, fish, legumes, and nuts (RR, 0.76; 95%CI, 0.64-0.92). However, substitution of total red meat with other major sources of protein was not associated with significantly lower postmenopausal breast cancer risk.
Figure 1. Multivariable* relative risk (RR) and 95% confidence intervals (95%CI) for breast cancer associated with replacement of adolescent intake of total red meat with other sources of dietary protein (1 serving/day) in premenopausal women, postmenopausal women and all women.
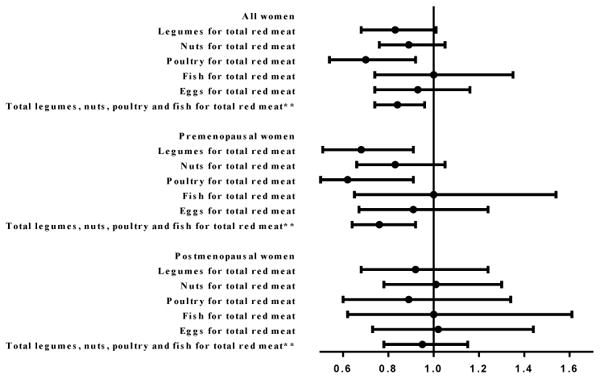
*Multivariable model was stratified by age in months at start of follow-up and calendar year of the current questionnaire cycle and was simultaneously adjusted for race (white, non-white), family history of breast cancer in mother or sisters (yes, no), history of benign breast disease (yes, no), smoking (never, past, current 1-14/day, current 15-24/day, current ≥25/day), height (<62, 62 to <65, 65 to <68, ≥68 inches), weight gain since age 18 (≤-5, >-5-5, >5-10, >10-20, >20 kg), BMI at age 18 years (<18.5, 18.5–<22.5, 22.5-<25, 25.0-<30, ≥30.0 kg/m2), age at menarche (<12, 12, 13, ≥14 yr), parity and age at first birth (nulliparous, parity ≤2 and age at first birth <25 yr, parity≤2 and age at first birth 25 to <30 yr, parity ≤2 and age at first birth ≥30 yr, parity 3-4 and age at first birth <25 yr, parity 3-4 and age at first birth 25 to <30 yr, parity 3-4 and age at first birth ≥30 yr, parity ≥5 and age at first birth <25 yr, parity ≥5 and age at first birth ≥25 yr), oral contraceptive use (never, past, current), adolescent alcohol intake (nondrinker, <1.5, 1.5–<5, 5-<10, ≥10 g/day), adult alcohol intake (nondrinker, <5, 5–<15,≥15 g/day), adolescent energy intake (quintile) and major protein sources (intakes of total red meat (continuous), poultry (continuous), fish (continuous), eggs (continuous), legumes (continuous), nuts (continuous), low fat dairy (continuous) and high fat dairy (continuous)). In postmenopausal women, we additionally adjusted for hormone use (postmenopausal never users, postmenopausal past users, postmenopausal current users), age at menopause (<45 yr, 45-46 yr, 47-48, 49-50 yr, 51-52 yr, ≥53 yr). Among all women, we additionally adjusted for hormone use and menopausal status (premenopausal, postmenopausal never users, postmenopausal past users, postmenopausal current users, unknown menopausal status) and, age at menopause (premenopausal, unknown menopause, <45 yr, 45-46 yr, 47-48, 49-50 yr, 51-52 yr, ≥53 yr). ** In the model for substituting combination of legumes, nuts, poultry and fish for total red meat, the major sources of protein were total red meat (continuous), sum of legumes, nuts, poultry and fish intake (continuous), eggs (continuous), low fat dairy (continuous) and high fat dairy (continuous).
Information on estrogen receptor status was available for 87% (n=982) and progesterone receptor status for 86% (n=974) of the breast cancer cases. We examined the associations with breast cancers that were positive for both (ER+/PR+) and negative for both receptors (ER-/PR-). We did not observe significant heterogeneity between total red meat intake and ER/PR status in either premenopausal or postmenopausal breast cancer (Table 3).
Table 3. Multivariable* relative risk (RR) and 95% confidence intervals (95%CI) of subtypes of breast cancer according to adolescent intake of total red meat in premenopausal women, postmenopausal women and all women.
| Total red meat, per 1 serving/day | ||||||
|---|---|---|---|---|---|---|
| Breast cancer subtype | All women | Premenopausal women | Postmenopausal women | |||
| No. of cases | RR (95%CI) | No. of cases | RR (95%CI) | No. of cases | RR (95%CI) | |
| Estrogen and progesterone receptor positive | 694 | 1.06 (0.94-1.19) | 350 | 1.24 (1.06-1.45) | 283 | 0.91 (0.75-1.10) |
| Estrogen and progesterone receptor negative | 160 | 1.06 (0.84-1.33) | 83 | 1.18 (0.88-1.60) | 65 | 0.90 (0.62-1.30) |
| P heterogeneity | 1.00 | 0.78 | 0.96 | |||
Multivariable model was stratified by age in months at start of follow-up and calendar year of the current questionnaire cycle and was simultaneously adjusted for race (white, non-white), family history of breast cancer in mother or sisters (yes, no), history of benign breast disease (yes, no), smoking (never, past, current 1-14/day, current 15-24/day, current ≥25/day), height (<62, 62 to <65, 65 to <68, ≥68 inches), weight gain since age 18 (≤-5, >-5-5, >5-10, >10-20, >20 kg), BMI at age 18 years (<18.5, 18.5–<22.5, 22.5-<25, 25.0-<30, ≥30.0 kg/m2), age at menarche (<12, 12, 13, ≥14 yr), parity and age at first birth (nulliparous, parity ≤2 and age at first birth <25 yr, parity≤2 and age at first birth 25 to <30 yr, parity ≤2 and age at first birth ≥30 yr, parity 3-4 and age at first birth <25 yr, parity 3-4 and age at first birth 25 to <30 yr, parity 3-4 and age at first birth ≥30 yr, parity ≥5 and age at first birth <25 yr, parity ≥5 and age at first birth ≥25 yr), oral contraceptive use (never, past, current), adolescent alcohol intake (nondrinker, <1.5, 1.5–<5, 5-<10, ≥10 g/day), adult alcohol intake (nondrinker, <5, 5–<15,≥15 g/day), and adolescent energy intake (quintile). In postmenopausal women, we additionally adjusted for hormone use (postmenopausal never users, postmenopausal past users, postmenopausal current users), age at menopause (<45 yr, 45-46 yr, 47-48, 49-50 yr, 51-52 yr, ≥53 yr). Among all women, we additionally adjusted for hormone use and menopausal status (premenopausal, postmenopausal never users, postmenopausal past users, postmenopausal current users, unknown menopausal status) and, age at menopause (premenopausal, unknown menopause, <45 yr, 45-46 yr, 47-48, 49-50 yr, 51-52 yr, ≥53 yr).
When the association between total red meat and premenopausal breast cancer was evaluated in the two follow-up time periods (1998-2005 or 2005-2011), higher intake of total red meat during adolescence was associated with higher breast cancer risk in both follow-up periods (RR, 1.19; 95%CI, 1.00-1.41; for each additional serving/day of intake from 1998 to 2005 and RR, 1.20; 95%CI, 0.94-1.53 for each additional serving/day of intake follow-up from 2005 to 2011).
We also examined whether the association between adolescent intakes of total red meat differed by breast cancer risk factors including family history of breast cancer, BMI, oral contraceptive use, history of benign breast disease, adolescent alcohol intake, adult alcohol intake, age at first birth and parity and smoking. The association between total red meat intake and breast cancer risk was modified by adolescent alcohol intake (P for interaction =0.0004 for total breast cancer and P for interaction =0.01 for premenopausal breast cancer). In women drinking 1.5 g/day or more alcohol at age 18, higher intake of total red meat was significantly associated with higher incidence of breast cancer (among all women: RR, 1.26; 95%CI, 1.09-1.44; among premenopausal women: RR, 1.31; 95%CI, 1.09-1.56 for each serving/day increment). But among women with alcohol intake at age 18 less than 1.5 g/day, there was no significant association (among all women: RR, 0.97; 95%CI, 0.85-1.11; among premenopausal women: RR, 1.09; 95%CI, 0.89-1.33).
Discussion
This prospective study provides further evidence that greater consumption of red meat during adolescence is significantly associated with higher premenopausal breast cancer risk, consistent with our earlier findings with shorter follow-up (8). Adjusting for animal fat, fruits and vegetables or adult red meat intake did not appreciably change the association. In addition, poultry intake was associated with a lower risk of breast cancer overall. Moreover, replacement of red meat with a combination of poultry, fish, legumes and nuts was also associated with lower breast cancer risk.
Evidence from prospective studies of red meat intake and breast cancer risk is inconsistent (22-28). In a recent review and meta-analysis of prospective studies on unprocessed and processed red meat consumption and breast cancer incidence, red meat intake was not independently associated with breast cancer incidence (2), but these studies focused on adult dietary intake. In contrast, a few studies have provided some evidence that diet during adolescent may affect the risk of adult breast cancer. In a population-base case-control study, higher consumption of high fat red meat during adolescence was associated with higher breast cancer risk before age 45 (29). Our finding is consistence with the case-control study (29) and the previous analyses of adolescent (8) and early adult (3) red meat intake and risk of breast cancer in NHSII.
Several biological mechanisms may explain the association between high intake of red meat and breast cancer. Carcinogenic by-products, such as heterocyclic amines and polycyclic aromatic hydrocarbons, created during high-temperature cooking of meat may contribute to breast cancer (30, 31). Heterocyclic amines have estrogenic activity and breast tissue-specific carcinogenic activity has been reported (32). Although a positive association between animal fat consumption in early adulthood and breast cancer was seen in a previous analysis of this cohort (33), the association with adolescent red meat intake that we observed appeared to be independent of animal fat intake during adolescence. Heme iron from meat has also been implicated in the etiology of breast cancer (34). However, heme iron intake was not associated with breast cancer risk in this analysis, and the associations for red meat and premenopausal and total breast cancer remained significant after adjustment for heme iron. Residues of the exogenous hormones used for growth stimulation in beef cattle (35) and dietary nitrosamines mainly found in processed meat may play a role in the etiology of breast cancer (36, 37). The significant interaction that we noted between adolescent red meat intake and alcohol intake at age 18 may be due to chance and this needs to be confirmed in additional studies.
Most previous studies found no association of poultry intake with breast cancer risk (27, 38-41). However, these studies focused on dietary intake in midlife and later. In agreement with our finding, a case-control study has reported an inverse association with poultry intake (42). We also found an inverse association between early adulthood poultry intake and breast cancer risk in NHSII (3). Poultry contains higher amounts of polyunsaturated fat than red meat, and processing and cooking methods for poultry likely differ from red meat. It is also possible that poultry consumers had a healthy diet and the inverse association between poultry and breast cancer may be explained by residual confounding. However, there was only a weak association between red meat and poultry intake during adolescence (r=0.11) and adjustment additionally for fruit and vegetable intakes or red meat did not attenuate the associations.
Anti-carcinogenic effects of beans have been shown in animal models (43) and might contribute to the lower breast cancer risk that we observed with replacement of red meat with legumes. Reductions in insulin, insulin-like growth factor and C-reactive protein, or alterations in lipid metabolism could also contribute to this lower risk (44). Soy isoflavones consumption has been inversely associated with breast cancer incidence (45). However, intake of soy products in our cohort was too low for us to investigate.
One limitation of this study is the measurement of adolescent diet. In our study, the adolescent dietary intake was reported when women were 33–52 years of age. However, the observed association was independent of adult diet, and comparison with maternal reports provides some evidence of validity (9, 10). We examined incident cases and only included breast cancer diagnosed after 1998; our study only addresses the association for breast cancer onset after age 33. Residual confounders are always of concern in any observational studies. Although we adjusted for a wide range of potential confounders for breast cancer, we still could not rule out the possibility that other unmeasured or inadequately measured factors have influenced our findings. Furthermore, the NHSII cohort is predominantly white, limiting the ability to study the meat consumption and breast cancer associations in other races; however it is unlikely that the biology underlying this association differs by race.
Major strengths of this study include the large number of cases, length of follow-up and the ability to examine subtypes of breast cancers. The detailed prospective and updated assessments of diet and lifestyle factors afforded the most comprehensive adjustment of many potential confounders, including adult dietary intake.
In conclusion, we found that higher consumption of red meat during adolescence was associated with elevated rates of premenopausal breast cancer, but not postmenopausal breast cancer. In addition, poultry intake was associated with a lower risk of breast cancer overall. Replacement of red meat with combination of poultry, fish, legumes and nuts in adolescence may help to reduce the breast cancer risk, especially during the premenopausal years. As the data on diet and breast cancer during childhood remain limited, further investigation is needed to better understand the impact of timing of dietary exposures and cancer risk.
Supplementary Material
The Novelty and Impact statementss.
Few studies have evaluated prospectively the relationship between comprehensive dietary protein sources in adolescence and breast cancer risk.
Higher intake of red meat during adolescence was associated with higher premenopausal breast cancer risk. Substituting other dietary protein sources for red meat in adolescent diet may decrease the premenopausal breast cancer risk.
Acknowledgments
The study was supported by the National Institutes of Health Grant (R01CA050385). The study sponsors were not involved in the study design and collection, analysis and interpretation of data, or the writing of the article or the decision to submit it for publication. The authors were independent from study sponsors. We would like to thank the participants and staff of the Nurses' Health Study II, for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. In addition, this study was approved by the Connecticut Department of Public Health (DPH) Human Investigations Committee. Certain data used in this publication were obtained from the DPH.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
The authors' responsibility were as follows: MSF, EC, WYC, HE and WCW: designed the research; MSF: analysis and wrote the manuscript; and MSF and WCW: had primary responsibility for the final content of the manuscript; and all authors: provided critical input in the writing of the manuscript and read and approved the final manuscript. The authors assume full responsibility for analyses and interpretation of these data.
References
- 1.Missmer SA, Smith-Warner SA, Spiegelman D, Yaun SS, Adami HO, Beeson WL, van den Brandt PA, Fraser GE, Freudenheim JL, Goldbohm RA, Graham S, Kushi LH, et al. Meat and dairy food consumption and breast cancer: a pooled analysis of cohort studies. Int J Epidemiol. 2002;31:78–85. doi: 10.1093/ije/31.1.78. [DOI] [PubMed] [Google Scholar]
- 2.Alexander DD, Morimoto LM, Mink PJ, Cushing CA. A review and meta-analysis of red and processed meat consumption and breast cancer. Nutr Res Rev. 2010;23:349–65. doi: 10.1017/S0954422410000235. [DOI] [PubMed] [Google Scholar]
- 3.Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC. Dietary protein sources in early adulthood and breast cancer incidence: prospective cohort study. BMJ. 2014;348:g3437. doi: 10.1136/bmj.g3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkey CS, Willett WC, ScD, Tamimi RM, Rosner B, Frazier AL, Colditz GA. Vegetable Protein and Vegetable Fat Intakes in Pre-Adolescent and Adolescent Girls, and Risk for Benign Breast Disease in Young Women. Breast Cancer Res Treat. 2013;141:299–306. doi: 10.1007/s10549-013-2686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Land CE, Tokunaga M, Koyama K, Soda M, Preston DL, Nishimori I, Tokuoka S. Incidence of female breast cancer among atomic bomb survivors, Hiroshima and Nagasaki, 1950-1990. Radiat Res. 2003;160:707–17. doi: 10.1667/rr3082. [DOI] [PubMed] [Google Scholar]
- 6.Swerdlow AJ, Barber JA, Hudson GV, Cunningham D, Gupta RK, Hancock BW, Horwich A, Lister TA, Linch DC. Risk of second malignancy after Hodgkin's disease in a collaborative British cohort: the relation to age at treatment. J Clin Oncol. 2000;18:498–509. doi: 10.1200/JCO.2000.18.3.498. [DOI] [PubMed] [Google Scholar]
- 7.Wahner-Roedler DL, Nelson DF, Croghan IT, Achenbach SJ, Crowson CS, Hartmann LC, O'Fallon WM. Risk of breast cancer and breast cancer characteristics in women treated with supradiaphragmatic radiation for Hodgkin lymphoma: Mayo Clinic experience. Mayo Clin Proc. 2003;78:708–15. doi: 10.4065/78.6.708. [DOI] [PubMed] [Google Scholar]
- 8.Linos E, Willett WC, Cho E, Colditz G, Frazier LA. Red meat consumption during adolescence among premenopausal women and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:2146–51. doi: 10.1158/1055-9965.EPI-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maruti SS, Feskanich D, Rockett HR, Colditz GA, Sampson LA, Willett WC. Validation of adolescent diet recalled by adults. Epidemiology. 2006;17:226–9. doi: 10.1097/01.ede.0000198181.86685.49. [DOI] [PubMed] [Google Scholar]
- 10.Maruti SS, Feskanich D, Colditz GA, Frazier AL, Sampson LA, Michels KB, Hunter DJ, Spiegelman D, Willett WC. Adult recall of adolescent diet: reproducibility and comparison with maternal reporting. Am J Epidemiol. 2005;161:89–97. doi: 10.1093/aje/kwi019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nutrient Database for Standard Reference, Release 14 Department of Agriculture. ARS; 2001. [Google Scholar]
- 12.Holland GWA, Unwin ID, Buss DH, Paul AA, Dat S. The Composition of Foods. Cambridge UK: The Royal Society of Chemistry and Ministry of Agriculture, Fisheries and Food; 1991. [Google Scholar]
- 13.Dial S. Tocopherols and tocotrienols in key foods in the US diet. AOCS Press; 1995. pp. 327–42. [Google Scholar]
- 14.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 15.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1984;65:1220–8S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 16.Colditz GA, Stampfer MJ, Willett WC, Stason WB, Rosner B, Hennekens CH, Speizer FE. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol. 1987;126:319–25. doi: 10.1093/aje/126.2.319. [DOI] [PubMed] [Google Scholar]
- 17.Cox DR, O D. Analysis of Survival Data. London, England: Chapman & Hall; 1984. [Google Scholar]
- 18.Rhee JJ, Cho E, Willett WC. Energy adjustment of nutrient intakes is preferable to adjustment using body weight and physical activity in epidemiological analyses. Public Health Nutr. 2014;17:1054–60. doi: 10.1017/S1368980013001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huberman M, Langholz B. Application of the missing-indicator method in matched case-control studies with incomplete data. Am J Epidemiol. 1999;150:1340–5. doi: 10.1093/oxfordjournals.aje.a009966. [DOI] [PubMed] [Google Scholar]
- 20.Halton TL, Willett WC, Liu S, Manson JE, Stampfer MJ, Hu FB. Potato and French fry consumption and risk of type 2 diabetes in women. Am J Clin Nutr. 2006;83:284–90. doi: 10.1093/ajcn/83.2.284. [DOI] [PubMed] [Google Scholar]
- 21.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–32. [PubMed] [Google Scholar]
- 22.Holmes MD, Colditz GA, Hunter DJ, Hankinson SE, Rosner B, Speizer FE, Willett WC. Meat, fish and egg intake and risk of breast cancer. Int J Cancer. 2003;104:221–7. doi: 10.1002/ijc.10910. [DOI] [PubMed] [Google Scholar]
- 23.Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, Sinha R. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med. 2007;4:e325. doi: 10.1371/journal.pmed.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pala V, Krogh V, Berrino F, Sieri S, Grioni S, Tjønneland A, Olsen A, Jakobsen MU, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, Romieu I, et al. Meat, eggs, dairy products, and risk of breast cancer in the European Protective Investigation into Cancer and Nutrition (EPIC) cohort. Am J Clin Nutr. 2009;90:602–12. doi: 10.3945/ajcn.2008.27173. [DOI] [PubMed] [Google Scholar]
- 25.Genkinger JM, Makambi KH, Palmer JR, Rosenberg L, Adams-Campbell LL. Consumption of dairy and meat in relation to breast cancer risk in the Black Women's Health Study. Cancer Causes Control. 2013;24:675–84. doi: 10.1007/s10552-013-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsson SC, Bergkvist L, Wolk A. Long-term meat intake and risk of breast cancer by oestrogen and progesterone receptor status in a cohort of Swedish women. Eur J Cancer. 2009;45:3042–6. doi: 10.1016/j.ejca.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 27.Egeberg R, Olsen A, Autrup H, Christensen J, Stripp C, Tetens I, Overvad K, Tjønneland A. Meat consumption, N-acetyl transferase 1 and 2 polymorphism and risk of breast cancer in Danish postmenopausal women. Eur J Cancer Prev. 2008;17:39–47. doi: 10.1097/CEJ.0b013e32809b4cdd. [DOI] [PubMed] [Google Scholar]
- 28.Taylor EF, Burley VJ, Greenwood DC, Cade JE. Meat consumption and risk of breast cancer in the UK Women's Cohort Study. Br J Cancer. 2007;96:1139–46. doi: 10.1038/sj.bjc.6603689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potischman N, Weiss HA, Swanson CA, Coates RJ, Gammon MD, Malone KE, Brogan D, Stanford JL, Hoover RN, Brinton LA. Diet during adolescence and risk of breast cancer among young women. J Natl Cancer Inst. 1998;90:226–33. doi: 10.1093/jnci/90.3.226. [DOI] [PubMed] [Google Scholar]
- 30.De Stefani E, Ronco A, Mendilaharsu M, Guidobono M, Deneo-Pellegrini H. Meat intake, heterocyclic amines, and risk of breast cancer: a case-control study in Uruguay. Cancer Epidemiol Biomarkers Prev. 1997;6:573–81. [PubMed] [Google Scholar]
- 31.Steck SE, Gaudet MM, Eng SM, Britton JA, Teitelbaum SL, Neugut AI, Santella RM, Gammon MD. Cooked meat and risk of breast cancer lifetime versus recent dietary intake. Epidemiology. 2007;18:373–82. doi: 10.1097/01.ede.0000259968.11151.06. [DOI] [PubMed] [Google Scholar]
- 32.Lauber SN, Ali S, Gooderham NJ. The cooked food derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine is a potent oestrogen: a mechanistic basis for its tissue-specific carcinogenicity. Carcinogenesis. 2004;25:2509–17. doi: 10.1093/carcin/bgh268. [DOI] [PubMed] [Google Scholar]
- 33.Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC. Premenopausal dietary fat in relation to pre and post-menopausal breast cancer. Breast Cancer Res Treat. 2014;145:255–65. doi: 10.1007/s10549-014-2895-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kallianpur AR, Lee SA, Gao YT, Lu W, Zheng Y, Ruan ZX, Dai Q, Gu K, Shu XO, Zheng W. Dietary animal-derived iron and fat intake and breast cancer risk in the Shanghai Breast Cancer Study. Breast Cancer Res Treat. 2008;107:123–32. doi: 10.1007/s10549-007-9538-3. [DOI] [PubMed] [Google Scholar]
- 35.Andersson AM, Skakkebaek NE. Exposure to exogenous estrogens in food: possible impact on human development and health. Eur J Endocrinol. 1999;140:477–85. doi: 10.1530/eje.0.1400477. [DOI] [PubMed] [Google Scholar]
- 36.Bartsch H, Montesano R. Relevance of nitrosamines to human cancer. Carcinogenesis. 1984;5:1381–1393. doi: 10.1093/carcin/5.11.1381. [DOI] [PubMed] [Google Scholar]
- 37.Mirvish SS. Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett. 1995;93:17–48. doi: 10.1016/0304-3835(95)03786-V. [DOI] [PubMed] [Google Scholar]
- 38.Zhang CX, Ho SC, Chen YM, Lin FY, Fu JH, Cheng SZ. Meat and egg consumption and risk of breast cancer among Chinese women. Cancer Causes Control. 2009;20:1845–53. doi: 10.1007/s10552-009-9377-0. [DOI] [PubMed] [Google Scholar]
- 39.Shannon J, Cook LS, Stanford JL. Dietary intake and risk of postmenopausal breast cancer (United States) Cancer Causes Control. 2003;14:19–27. doi: 10.1023/a:1022506507984. [DOI] [PubMed] [Google Scholar]
- 40.Dai Q, Shu XO, Jin F, Gao YT, Ruan ZX, Zheng W. Consumption of animal foods, cooking methods, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:801–8. [PubMed] [Google Scholar]
- 41.Hu J, La Vecchia C, DesMeules M, Negri E, Mery L Canadian Cancer Registries Epidemiology Research Group. Meat and fish consumption and cancer in Canada. Nutr Cancer. 2008;60:313–24. doi: 10.1080/01635580701759724. [DOI] [PubMed] [Google Scholar]
- 42.Franceschi S, Favero A, La Vecchia C, Negri E, Dal Maso L, Salvini S, Decarli A, Giacosa A. Influence of food groups and food diversity on breast cancer risk in Italy. Int J Cancer. 1995;63:785–9. doi: 10.1002/ijc.2910630606. [DOI] [PubMed] [Google Scholar]
- 43.Thompson MD, Mensack MM, Jiang W, Zhu Z, Lewis MR, McGinley JN, Brick MA, Thompson HJ. Cell signaling pathways associated with a reduction in mammary cancer burden by dietary common bean (Phaseolus vulgaris L) Carcinogenesis. 2012;33:226–32. doi: 10.1093/carcin/bgr247. [DOI] [PubMed] [Google Scholar]
- 44.Thompson MD, Thompson HJ. Physiological effects of bean (Phaseolus vulgaris L.) consumption on cellular signaling in cancer. Cell Cycl. 2012;11:835–6. doi: 10.4161/cc.11.5.19676. [DOI] [PubMed] [Google Scholar]
- 45.Dong JY, Qin LQ. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2011;125:315–23. doi: 10.1007/s10549-010-1270-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


