Abstract
Background
Immune hemolytic anemia is a well-known complication after allogeneic hematopoietic stem cell transplantation (HSCT). Posttransplant hemolytic anemia results in increased red blood cell transfusions and medical sequelae including iron overload.
Case Report
We present a case report of immune hemolytic anemia that occurred after allogeneic HSCT from an ABO major–mismatched, HLA-matched unrelated donor. The patient had high anti-donor A type antibodies that were unresponsive to treatment with steroids and rituximab, resulting in persistent transfusion dependence. A detailed time course of anti-A titers, plasma cell content of the marrow, and B-cell content of the blood is presented. Treatment with bortezomib, a protease inhibitor, eliminated residual host-type plasma cells secreting anti-A and restored normal donor-derived erythropoiesis.
Conclusion
This report, and a review of literature for treatment of immune hemolytic anemia after allogeneic HSCT, supports the utility of bortezomib as plasma cell–targeted therapy in this setting.
Immune hemolytic anemia is a well-known complication of allogeneic hematopoietic stem cell transplantation (HSCT).1 Hemolysis typically occurs from 2 to 25 months after HSCT2,3 with an incidence of 3.1% to 6%.2,4 For the purpose of relative comparison to the incidence of hemolytic anemia observed outside of the transplant setting, this is much higher than the incidence of immune hemolytic anemia in the general population (incidence rates per year, 0.0008%; prevalence, 0.17%).5,6
Severe immune hemolytic anemia alone rarely causes death in adults4 but is often associated with other complications and significant morbidity.4,7 Thus, early effective treatment can prevent transfusion-related complications. Conventional options to treat immune hemolytic anemia after allogeneic HSCT include corticosteroids, intravenous immunoglobulin (IVIG), splenectomy, donor lymphocyte infusion, plasma exchange, erythropoietin, rituximab, and other immunosuppressing agents such as cyclosporine.3,8 Bortezomib is an inhibitor of the 26S proteasome and approved for the treatment of multiple myeloma and mantle cell lymphoma in the United States; it has recently been reported as treatment for immune hemolytic anemia,9,10 but the efficacy and safety of bortezomib on secondary immune disorders in allogeneic HSCT recipients has not been extensively described. In this case report, we describe the successful use of bortezomib as treatment of persistent immune hemolytic anemia after allogeneic HSCT and review published articles related to management of immune hemolytic anemia in this setting.
Case Report
A 57-year-old man presented with pancytopenia and a marrow examination that showed International Prognostic Scoring System intermediate-2 myelodysplastic syndrome, with deletion of 3q26 on cytogenetic analysis. He developed secondary acute myeloid leukemia after three cycles of treatment with decitabine and achieved a complete remission (CR) after 7 + 3 induction therapy and two cycles of high-dose cytarabine consolidation.
He received an allogeneic granulocyte–colony-stimulating factor–mobilized peripheral blood stem cell transplantation from a male HLA-matched unrelated donor in first CR. The donor's blood group was A+ D+ and the recipient's was O D+. The allogeneic blood stem cell graft contained 9.8 × 106 CD34+ cells/kg and 114.4 × 106 CD19+ cells/kg. The patient received a reduced-intensity conditioning regimen consisting of 5 days of 25 mg/m2/day fludarabine and 2 days of 70 mg/m2/day melphalan. Prograf and methotrexate (15 mg/m2 on Day 1 and 10 mg/m2 on Days 3, 6, and 11) were given for prophylaxis of graft-versus-host disease (GVHD). He developed Grade I acute GVHD of skin on Day 34 after transplantation that responded well to topical steroids and resolved within a week. The remainder of his immediate posttransplant course was uncomplicated.
He remained red blood cell (RBC) transfusion dependent after achieving neutrophil and platelet engraftment on Day 17. A marrow aspiration on Day 97 showed decreased erythroblasts and excess plasma cells along with reduced reticulocytes in the blood (Figs. 1B and 2). Plasma cells in the marrow were stained in marrow sections with anti-CD138 monoclonal antibody (MoAb; Fig. 2) and estimated CD138 immunohistochemical score blinded to case identical number and date (Fig. 1D). Anti-A IgG and IgM antibody titers were elevated consistent with persistence of host-type plasma cells (Fig. 1C).
Fig 1.
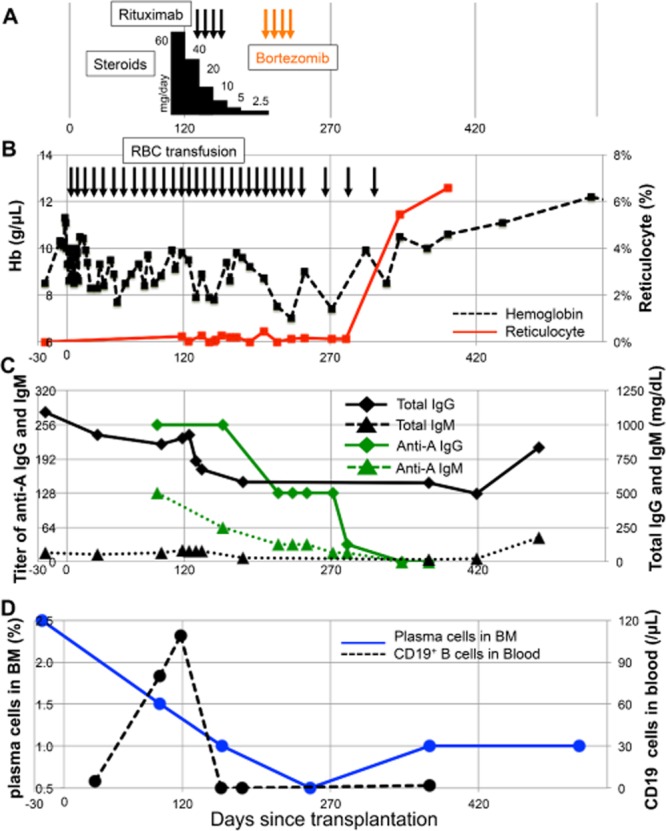
Clinical course of the reported patient with immune hemolytic anemia. (A) Treatment course of steroids (black bars), rituximab (black arrows), and bortezomib (orange arrows). The height of the black bars shows the relative dose of steroids. (B) Graph of Hb (black dashed line) and reticulocyte percentage (red solid line) with timing of RBC transfusions (black arrows). (C) Graph of total IgG (black solid line with diamonds) and IgM (black dashed line with triangles) and anti-A IgG titers (green solid line with diamonds) and anti-A IgM titers (green dashed line with triangles). Semiquantitative titration of anti-A of both the IgM and the IgG subclasses were performed using standard methods. Briefly, 2% suspensions of A1 reagent RBCs were mixed with patient plasma in test tubes. For IgM detection, after 15 minutes of incubation at room temperature the samples were centrifuged and macroscopic agglutination was quantified; for IgG, samples were incubated for 60 minutes at 37°C, washed to remove unbound antibody, incubated with anti-human IgG, and then centrifuged to score agglutination.13 (D) Numbers of CD19+ cells in blood (black dashed line) were assessed by flow cytometry, and plasma cells percentage score for nucleated cells in bone marrow (blue solid line) were estimated blinded to case identical number and date.
Fig 2.
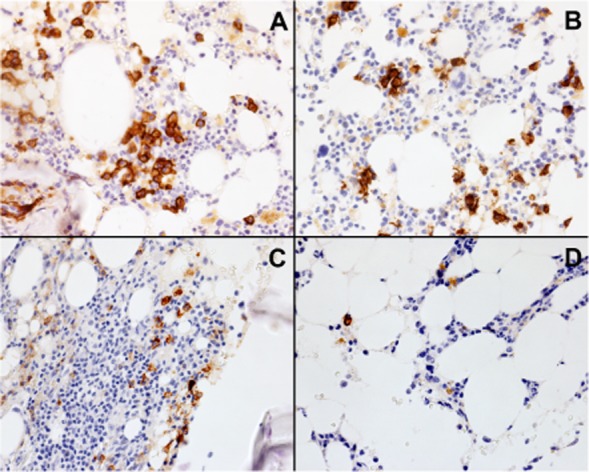
Histologic sections of marrow from the patient with immune hemolytic anemia. Tissue sections were immune stained with anti-CD138 MoAb, and CD138+ cells were visualized after secondary staining with a horseradish peroxidase–labeled secondary antibody. Immunohistochemical analysis for CD138+ plasma cells present in marrow biopsies obtained on Day –22 (A), Day +97 (B), Day +160 (C), and Day +249 (D) with respect to the day of transplant are shown. Overall cellularities of the marrow at the times represented in A, B, C, and D were 40, 60, 50, and 15%.
On Day 118, the patient's hemolytic anemia was treated with 1 mg/kg/day methylprednisolone without a change in the anti-A titer or improved erythropoiesis (Figs. 1B and 1C). He then received 375 mg/m2 rituximab weekly × 4 doses starting on Day 132 posttransplant with residual anti-A IgG and IgM antibodies and continued reliance on RBC transfusions every 10 days (Figs. 1B and 1C). CD19+ cells were eliminated from the blood after rituximab administration (Fig. 1D) although anti-A IgG and IgM antibodies in the blood and plasma cells in the marrow persisted (Figs. 1C and 2). As a third-line therapy, he received weekly subcutaneous injections of 1.3 mg/m2 bortezomib starting on Day 175 (Fig. 1A).
After four doses of bortezomib, serum anti-A levels decreased and then disappeared (Fig. 1C). Likewise, reticulocytes started to increase in the blood from Day 342 (Fig. 1B) and hemoglobin (Hb) increased with resolution of his transfusion-dependent hemolysis. In aggregate, he had received a total of 30 RBC units before erythropoiesis recovered.
Immunohistochemical analysis of CD138+ cells in serial histologic sections of marrow obtained before transplant (Day −22, Fig. 2A) and after transplant on Days +97 (Fig. 2B), +160 (Fig. 2C), and +249 (Fig. 2D) showed an increased frequency of plasma cells (relative to marrow from healthy controls) present before transplantation (Fig. 2A) and persistence of CD138+ plasma cells during the first 3 months after transplantation (Fig. 2B). Treatment with steroids and rituximab decreased the numbers of plasma cells in the marrow (Fig. 2C), and subsequent treatment with bortezomib further reduced the relative frequency of plasma cells in the marrow (Fig. 2D). While rituximab treatment may have contributed to mild impairment of hematopoiesis and reduced overall cellularity of the marrow, bortezomib treatment was associated with a further reduction in the frequency of CD138+ plasma cells in the marrow relative to nucleated cells from approximately 1.8% (Fig. 2C) to 0.6% (Fig. 2D).
Discussion
There are three possible mechanisms for immune hemolytic anemia after allogeneic HSCT.11 The first mechanism is that the antibodies against donor ABO type RBCs derived from recipient lymphocytes persist or recur after allogeneic SCT causing hemolysis. This can be seen in ABO major–mismatched allogeneic HSCT. The second mechanism is that the antibodies derived from donor lymphocytes attack recipient ABO type RBCs. This is called passenger lymphocyte syndrome and can be seen in ABO minor–mismatched allogeneic HSCT. The third is that donor plasma cells produce immune antibodies that cause hemolysis of donor ABO RBCs after engraftment. The hemolysis caused by this mechanism can be seen in any type of allogeneic HSCT even in ABO-matched transplantation. With all three mechanisms, successful treatment requires suppression of the plasma cells secreting antibodies to RBCs or erythroid progenitors.
A review of the literature found 18 reports of treatment of immune hemolytic anemia that developed after allogeneic HSCT in patients with hematologic disease (Table 1). Clinical outcomes on a total of 92 patients were reported, with 39 deaths and 33 survivors. Treatment with oral prednisone or IV methylprednisolone, with an initial dose of 1 mg/kg/day, was administrated as first-line treatment in almost all cases. Hemolytic anemia resolved with steroids alone in 12 of 92 cases, with at least another 60 cases remaining transfusion dependent. Reported second-line therapy of post–allogeneic HSCT immune hemolytic anemia included IVIG and, after Food and Drug Administration (FDA) approval, expansion to bulky non-Hodgkin's lymphoma in 2001,23 rituximab, a MoAb against the CD20 protein.4,9 Although rituximab has been reported as effective treatment for immune hemolytic anemia in the nontransplant setting,24 13 of 32 patients treated with rituximab for hemolytic anemia after allogeneic transplant did not achieve CR from RBC transfusion dependence. For patients with immune hemolytic anemia that persists after administration of steroids, IVIG, and rituximab, less immunosuppressive treatment that is more specifically targeted to plasma cells is needed, as many patients with immune hemolytic anemia die from opportunistic infections during immunosuppressive drug therapy.3,7
TABLE 1.
Published data about immune hemolytic anemia after allogeneic HSCT for hematologic malignancies
| Publication | Number of patients | Primary disease | Stem cell source | First line | Second or more line | Clinical course | Reference |
|---|---|---|---|---|---|---|---|
| 1990 | 1 | CML | BMT | Steroids | 1 alive | 14 | |
| 1994 | 1 | CML | BMT | Steroids | 1 alive | 15 | |
| 1996 | 1 | AA | BMT | Steroids | AZA, VCR, IVIG, SPLX, TLI, 2nd HSCT | 1 dead | 16 |
| 1996 | 7 | TCD-BMT | Steroids | IVIG, PE, EPO | 3 alive, 4 dead | 3 | |
| 1997 | 5 | 3 ALL, 1 CML, 1 MDS | BMT | Steroids | IVIG | 5 alive | 17 |
| 2001 | 9 | CML | TCD-BMT | Steroids | SPLX, DLI, IVIG | 7 alive, 2 dead | 8 |
| 2001 | 1 | ALL | BMT | Steroids | CsA, CY, THAL, IVIG, ALG | 1 alive | 7 |
| 2001 | 1 | Lymphoma | PBSCT | Steroids | 1 dead | 18 | |
| 2005 | 4 | HD, NHL, ALL, AML | PBSCT | Steroids | IVIG, Ritux | 4 alive | 19 |
| 2007 | 9 | CML, AA, MPD | BMT | Steroids | IVIG, AZA, SPLX, VCR, TLI | 5 alive, 4 dead | 2 |
| 2007 | 1 | Hemophagocytic lymphohistiocytosis | CBT | Steroids | IVIG, Ritux | 1 alive | 20 |
| 2007 | 12 | Leukemia, MDS, lymphoma, AA, MM, others | BMT | Steroids | Ritux | 2 alive, 10 dead | 4 |
| 2009 | 1 | CML | CBT | Steroids | IVIG, Ritux, MMF, CY, PE, SPLX | 1 dead | 21 |
| 2011 | 1 | AML | BMT | Steroids | 1 alive | 22 | |
| 2012 | 1 | AML | PBSCT | Ritux | DLI, BTZ | 1 alive | 9 |
| 2013 | 20 | Malignancies and nonmalignancies | CBT | Steroids | Ritux, CsA, IVIG | 33 | |
| 2013 | 2 | AML | CBT | Steroids | IVIG, Ritux, CsA, SPLX, BTZ, Eculiz, PE | 2 dead | 10 |
| 2014 | 15 | Leukemia, AA, FA, MPS, others | 9 BMT, 2 PBSCT, 4 CBT | Steroids | IVIG, Ritux | 1 dead, 14 alive | 34 |
| Summary | 92 patients | 32 alive, 38 dead |
AA = aplastic anemia; ALG = antilymphocyte globulin; ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; AZA = azathioprine; BMT = bone marrow transplantation; BTZ = bortezomib; CBT = cord blood cell transplantation; CML = chronic myeloid leukemia; CsA = cyclosporine; CY = cyclophosphamide; DLI = donor lymphocyte infusion; Eculiz = eculizumab; EPO = erythropoietin; FA = Fanconi anemia; HD = Hodgkin's lymphoma; MDS = myelodysplastic syndrome; MM = multiple myeloma; MMF = mycophenolate mofetil; MPD = myeloproliferative disorder; MPS = mucopolysaccaridosis; NHL = non-Hodgkin's lymphoma; PBSCT = peripheral blood stem cell transplantation; PE = plasma exchange; Ritux = rituximab; SPLX = splenectomy; TCD = T-cell depleted; THAL = thalidomide; TLI = total lymph node irradiation; VCR = vincristine.
Bortezomib is a dipeptide boronate proteasome inhibitor, which reversibly inhibits the 26S proteasome function and leads to the accumulation of polyubiquitinated proteins, inducing the death of both short- and long-lived plasma cells by activation of the terminal unfolded protein response.25–27 The US FDA approved bortezomib to treat multiple myeloma and mantle cell lymphoma patients. In view of its effect on malignant B cells and plasma cells, bortezomib has been used to treat other plasma disorders, with case reports of successful treatment of immune hemolytic anemia related to cryoglobulinemia, systemic lupus erythematosus, and myasthenia gravis.28–30 In addition, bortezomib can deplete alloreactive T cells in allo-HSCT recipients, decrease T-helper Type 1 cells that secrete interferon-γ and interleukin-2,31 and impair activation of monocyte-derived dendritic cells.32 Koreth and colleagues12 have recently demonstrated that bortezomib is efficacious as part of GVHD prophylaxis for patients who received HLA-mismatched allogeneic HSCT after reduced-intensity conditioning regimens. The literature on post–allo-HSCT hemolytic anemia reports the use of bortezomib as third-line therapy in only three patients, with one surviving responder (Table 1).
We report successful treatment with bortezomib of a case of immune hemolytic anemia after allogeneic HSCT that was resistant to steroids and rituximab. For our patient, rituximab depleted CD19+ cells in the blood, but marrow plasma cells were resistant to steroids and rituximab, with persistence of anti-A. After bortezomib administration, anti-A titers decreased and marrow plasma cells were eliminated with a concomitant increase in reticulocytes in the blood and RBC transfusion independence. This case supports the utility of bortezomib treatment to specifically target the residual host-type plasma cells responsible for production of anti-A.
In conclusion, immune hemolytic anemia after allogeneic HSCT for hematologic malignancy is a well-recognized complication that occurs rarely but is responsible for considerable morbidity and reported mortality of more than 50%. Although many cases with hemolytic anemia respond to standard treatment including steroids, IVIG, and rituximab, effective third-line therapy has not been established. We describe successful treatment of immune hemolytic anemia resistant to steroids and rituximab with bortezomib. Disappearance of anti-A was concomitant with elimination of CD138+ plasma cells from the marrow.
Glossary
- CR
complete remission
- HSCT
hematopoietic stem cell transplantation
Conflict of Interest
The authors have disclosed no conflicts of interest.
References
- 1.Petz LD. Hemolysis associated with transplantation. Transfusion. 1998;38:224–228. doi: 10.1046/j.1537-2995.1998.38398222864.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen FE, Owen I, Savage D, et al. Late onset haemolysis and red cell autoimmunisation after allogeneic bone marrow transplant. Bone Marrow Transplant. 1997;19:491–495. doi: 10.1038/sj.bmt.1700677. [DOI] [PubMed] [Google Scholar]
- 3.Drobyski WR, Potluri J, Sauer D, et al. Autoimmune hemolytic anemia following T cell-depleted allogeneic bone marrow transplantation. Bone Marrow Transplant. 1996;17:1093–1099. [PubMed] [Google Scholar]
- 4.Sanz J, Arriaga F, Montesinos P, et al. Autoimmune hemolytic anemia following allogeneic hematopoietic stem cell transplantation in adult patients. Bone Marrow Transplant. 2007;39:555–561. doi: 10.1038/sj.bmt.1705641. [DOI] [PubMed] [Google Scholar]
- 5.Klein NP, Ray P, Carpenter D, et al. Rates of autoimmune diseases in Kaiser Permanente for use in vaccine adverse event safety studies. Vaccine. 2010;28:1062–1068. doi: 10.1016/j.vaccine.2009.10.115. [DOI] [PubMed] [Google Scholar]
- 6.Eaton WW, Rose NR, Kalaydjian A, et al. Epidemiology of autoimmune diseases in Denmark. J Autoimmun. 2007;29:1–9. doi: 10.1016/j.jaut.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pratt G, Kinsey SE. Remission of severe, intractable autoimmune haemolytic anaemia following matched unrelated donor transplantation. Bone Marrow Transplant. 2001;28:791–793. doi: 10.1038/sj.bmt.1703232. [DOI] [PubMed] [Google Scholar]
- 8.Cwynarski K, Goulding R, Pocock C, et al. Immune haemolytic anaemia following T cell-depleted allogeneic bone marrow transplantation for chronic myeloid leukaemia: association with leukaemic relapse and treatment with donor lymphocyte infusions. Bone Marrow Transplant. 2001;28:581–586. doi: 10.1038/sj.bmt.1703206. [DOI] [PubMed] [Google Scholar]
- 9.Poon LM, Koh LP. Successful treatment of isohemagglutinin-mediated pure red cell aplasia after ABO-mismatched allogeneic hematopoietic cell transplant using bortezomib. Bone Marrow Transplant. 2012;47:870–871. doi: 10.1038/bmt.2011.176. [DOI] [PubMed] [Google Scholar]
- 10.Rovira J, Cid J, Gutierrez-Garcia G, et al. Fatal immune hemolytic anemia following allogeneic stem cell transplantation: report of 2 cases and review of literature. Transfus Med Rev. 2013;27:166–170. doi: 10.1016/j.tmrv.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Watz E, Remberger M, Ringden O, et al. Analysis of donor and recipient ABO incompatibility and antibody-associated complications after allogeneic stem cell transplantation with reduced-intensity conditioning. Biol Blood Marrow Transplant. 2014;20:264–271. doi: 10.1016/j.bbmt.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Koreth J, Stevenson KE, Kim HT, et al. Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. J Clin Oncol. 2012;30:3202–3208. doi: 10.1200/JCO.2012.42.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AABB. Technical methods and procedures. 17th ed. Bethesda, MD: American Association of Blood Banks; 2011. [Google Scholar]
- 14.Klumpp TR, Caligiuri MA, Rabinowe SN, et al. Autoimmune pancytopenia following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1990;6:445–447. [PubMed] [Google Scholar]
- 15.Tamura T, Kanamori H, Yamazaki E, et al. Cold agglutinin disease following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1994;13:321–323. [PubMed] [Google Scholar]
- 16.De Lord C, Marsh JC, Smith JG, et al. Fatal autoimmune pancytopenia following bone marrow transplantation for aplastic anaemia. Bone Marrow Transplant. 1996;18:237–239. [PubMed] [Google Scholar]
- 17.Godder K, Pati AR, Abhyankar SH, et al. De novo chronic graft-versus-host disease presenting as hemolytic anemia following partially mismatched related donor bone marrow transplant. Bone Marrow Transplant. 1997;19:813–817. doi: 10.1038/sj.bmt.1700746. [DOI] [PubMed] [Google Scholar]
- 18.Tiplady CW, Fitzgerald JM, Jackson GH, et al. Massive haemolysis in a group A recipient of a group O peripheral blood stem cell allogeneic transplant. Transfus Med. 2001;11:455–458. doi: 10.1046/j.1365-3148.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 19.Raj K, Narayanan S, Augustson B, et al. Rituximab is effective in the management of refractory autoimmune cytopenias occurring after allogeneic stem cell transplantation. Bone Marrow Transplant. 2005;35:299–301. doi: 10.1038/sj.bmt.1704705. [DOI] [PubMed] [Google Scholar]
- 20.Radhi M, Rumelhart S, Tatman D, et al. Severe autoimmune hemolytic anemia after unrelated umbilical cord blood transplant for familial hemophagocytic lymphohistiocytosis: significant improvement after treatment with rituximab. J Pediatr Hematol Oncol. 2007;29:125–127. doi: 10.1097/MPH.0b013e3180320b23. [DOI] [PubMed] [Google Scholar]
- 21.Rokicka M, Styczynski J, Michalewska B, et al. Fatal combined immune hemolytic anemia after double cord blood transplantation in imatinib-resistant CML. Bone Marrow Transplant. 2009;44:383–385. doi: 10.1038/bmt.2009.25. [DOI] [PubMed] [Google Scholar]
- 22.Nakata J, Tamaki H, Ikegame K, et al. Direct antiglobulin test-negative autoimmune hemolytic anemia associated with HLA-haploidentical stem cell transplantation. Int J Hematol. 2011;93:558–560. doi: 10.1007/s12185-011-0833-8. [DOI] [PubMed] [Google Scholar]
- 23.Rastetter W, Molina A, White CA. Rituximab: expanding role in therapy for lymphomas and autoimmune diseases. Annu Rev Med. 2004;55:477–503. doi: 10.1146/annurev.med.55.091902.104249. [DOI] [PubMed] [Google Scholar]
- 24.Rigal D, Meyer F. [Autoimmune haemolytic anemia: diagnosis strategy and new treatments] Transfus Clin Biol. 2011;18:277–285. doi: 10.1016/j.tracli.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Dasanu CA. Bortezomib: friend or foe of hemolytic anemia? J Oncol Pharm Pract. 2011;17:233–235. doi: 10.1177/1078155210374240. [DOI] [PubMed] [Google Scholar]
- 26.Meister S, Schubert U, Neubert K, et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007;67:1783–1792. doi: 10.1158/0008-5472.CAN-06-2258. [DOI] [PubMed] [Google Scholar]
- 27.Neubert K, Meister S, Moser K, et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14:748–755. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- 28.Frohlich K, Holle JU, Aries PM, et al. Successful use of bortezomib in a patient with systemic lupus erythematosus and multiple myeloma. Ann Rheum Dis. 2011;70:1344–1345. doi: 10.1136/ard.2010.133256. [DOI] [PubMed] [Google Scholar]
- 29.Carson KR, Beckwith LG, Mehta J. Successful treatment of IgM-mediated autoimmune hemolytic anemia with bortezomib. Blood. 2010;115:915. doi: 10.1182/blood-2009-09-242917. [DOI] [PubMed] [Google Scholar]
- 30.Gomez AM, Vrolix K, Martinez-Martinez P, et al. Proteasome inhibition with bortezomib depletes plasma cells and autoantibodies in experimental autoimmune myasthenia gravis. J Immunol. 2011;186:2503–2513. doi: 10.4049/jimmunol.1002539. [DOI] [PubMed] [Google Scholar]
- 31.Blanco B, Perez-Simon JA, Sanchez-Abarca LI, et al. Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood. 2006;107:3575–3583. doi: 10.1182/blood-2005-05-2118. [DOI] [PubMed] [Google Scholar]
- 32.Nencioni A, Schwarzenberg K, Brauer KM, et al. Proteasome inhibitor bortezomib modulates TLR4-induced dendritic cell activation. Blood. 2006;108:551–558. doi: 10.1182/blood-2005-08-3494. [DOI] [PubMed] [Google Scholar]
- 33.Daikeler T, Labopin M, Ruggeri A, et al. New autoimmune diseases after cord blood transplantation: a retrospective study of EUROCORD and the Autoimmune Disease Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2013;121:1059–1064. doi: 10.1182/blood-2012-07-445965. [DOI] [PubMed] [Google Scholar]
- 34.Faraci M, Zecca M, Pillon M, et al. Autoimmune hematological diseases after allogeneic hematopoietic stem cell transplantation in children: an Italian multicenter experience. Biol Blood Marrow Transplant. 2014;20:272–278. doi: 10.1016/j.bbmt.2013.11.014. [DOI] [PubMed] [Google Scholar]


