Abstract
Over recent years, modelling approaches from nutritional ecology (known as Nutritional Geometry) have been increasingly used to describe how animals and some other organisms select foods and eat them in appropriate amounts in order to maintain a balanced nutritional state maximising fitness. These nutritional strategies profoundly affect the physiology, behaviour and performance of individuals, which in turn impact their social interactions within groups and societies. Here, we present a conceptual framework to study the role of nutrition as a major ecological factor influencing the development and maintenance of social life. We first illustrate some of the mechanisms by which nutritional differences among individuals mediate social interactions in a broad range of species and ecological contexts. We then explain how studying individual‐ and collective‐level nutrition in a common conceptual framework derived from Nutritional Geometry can bring new fundamental insights into the mechanisms and evolution of social interactions, using a combination of simulation models and manipulative experiments.
Keywords: Collective animal behaviour, individual‐based models, nutritional ecology, nutritional geometry, social biology, social interactions
Introduction
Explaining social behaviour – any interaction between members of the same species that changes their subsequent behaviour (Sokolowski 2010) – is a major challenge in biology. For decades, behavioural and evolutionary biologists have focused on the role of genetic factors in driving cooperation in the kin‐structured societies of insects, birds and primates (Wilson 1975). Over recent years, integrative approaches to the study of social behaviour have considerably broadened the scope of social biology to a wider diversity of organisms, social systems and ecological contexts (Székely et al. 2010). At a mechanistic level, research on collective animal behaviour (Box 1) has made fundamental progress in understanding how complex social phenomena, such as insect swarms or human crowds, emerge from relatively simple interactions among individuals (Camazine et al. 2001; Couzin 2009; Sumpter 2010). From a functional point of view, modern inclusive fitness theory provides a general framework for investigating the evolution of social traits across levels of biological organisation, from genomes to societies (Maynard‐Smith & Szathmary 1995; Bourke 2011). Importantly, these conceptual advances also shed new light on the role of ecological factors in the mechanisms and evolution of social interactions (Gordon 2014; Hofmann et al. 2014).
Box 1. Key principles of collective animal behaviour.
The mechanistic approach to collective animal behaviour uses concepts from statistical physics to study how complex collective phenomena in animal groups (such as collective motion or swarm intelligence) emerge from relatively simple interactions between individuals (Camazine et al. 2001; Couzin 2009; Sumpter 2010). By combining behavioural observations of individuals and groups with mathematical modelling (analytical models or individual‐based models – a class of computational models for simulating the actions and interactions of autonomous agents and assessing their effects on the global system), many collective behaviours can be described in terms of three key principles: (1) quorum responses, in which the probability of an individual taking a particular action varies non‐linearly with the number of individuals already performing it (e.g. Ward et al. 2008); (2) positive feedbacks, when repeated interactions between individuals amplify this probability (e.g. Amé et al. 2006); and (3) negative feedbacks, when repeated interactions between individuals reduce this probability (e.g. Seeley et al. 2012). Collective animal behaviours typically arise from self‐organisation, through a cascade of local interactions among individuals, without the necessity that any of them possess global information or acts as a leader. These generic principles have been identified in a wide range of animal species, group types and ecological contexts, including insect swarms, fish schools, bird flocks, mammal herds and human crowds (Camazine et al. 2001; Couzin 2009; Sumpter 2010). They are also relevant to the study of collective behaviours at lower organisational levels, for example in populations of neurons or cancer cells (Deisboeck & Couzin 2009). Although research on collective behaviour has often focused on how groups solve food‐related problems, such as finding the shortest path to a food source (e.g. Goss et al. 1989) or selecting the most energetic foods among many other alternatives (e.g. Seeley et al. 1991; Sumpter & Beekman 2003), very few studies have investigated the influence of individual nutrition on group behaviour or its evolution.
Nutrition, which impinges on virtually all aspects of an animal's life, is central to many of these interactions (Simpson & Raubenheimer 2012). Animals often provision their young, or search, consume and defend food resources collectively (Krause & Ruxton 2002). In some cases, individuals even cooperate to cultivate and protect their crops in well‐organised gardens (Aanen et al. 2009). It has long been argued that nutritional constraints, such as limited access to water or food (sources of energy or specific nutrients), could have promoted the evolution of cooperation and division of labour in several taxa (Wheeler 1928; Rubenstein & Wrangham 1986; Hunt & Nalepa 1994; West‐Eberrhard 2003; Amdam et al. 2006; Hunt et al. 2007; Amdam & Page 2010). However, the lack of a conceptual framework to study the influence of nutrition on social interactions has hampered the testing of such hypotheses and their incorporation into theories of social evolution (Székely et al. 2010; Bourke 2011).
The rapidly growing field of nutritional ecology – the study of the nutritional interactions between organisms and their environment – now provides theoretical and methodological foundations for a framework to examine the links between nutrition and social interactions (Raubenheimer et al. 2009). Specifically, the state‐space modelling approach of Nutritional Geometry (NG; Box 2) has increasingly been used to describe how individuals regulate their intake of multiple nutrients simultaneously and how this varies, inter alia, across taxonomic groups, developmental stages and feeding guilds, in organisms spanning from slime moulds to humans (Raubenheimer et al. 2012; Simpson & Raubenheimer 2012). In the NG framework, the challenge for an individual is to select foods and eat them in appropriate amounts in order to achieve a state of nutritional balance (referred to as intake target) that maximises its fitness, and when constrained from doing so to arrive at an optimal compromise between the over‐ and under‐ingestion of imbalanced nutrients (see theoretical examples in Fig. 1). A major advantage of NG is that it enables the derivation and testing of specific predictions about physiology (e.g. nutritional homoeostasis), behaviour (e.g. foraging, eating) and evolution (e.g. fitness consequences) based on the nutritional state of the individual in relation to its foraging opportunities in the environment. Although NG was originally developed within the context of nutritional strategies of individual animals (Raubenheimer & Simpson 1993), recent studies have gone a step further, showing how individual‐level nutrition directly influences social interactions (e.g. Simpson et al. 2006; Grover et al. 2007), collective behaviour (e.g. Dussutour & Simpson 2009; Bazazi et al. 2011) and social organisation (e.g. Eggert et al. 2008; Salomon et al. 2008). Investigating nutritional processes and their interactions at different organisational levels thus holds considerable promise to unravel the nutritional underpinnings of complex social phenomena.
Box 2. The geometric framework for nutrition.
Nutritional Geometry (NG) is a state‐based modelling approach to investigate the nutritional strategies of animals (Raubenheimer & Simpson 1993; Simpson & Raubenheimer 2012). In NG models, individual animals, foods and their interactions are represented graphically in a geometric space (a nutrient space) defined by two or more food components (typically, but not necessarily, the macronutrients protein, carbohydrates and fat). Foods are represented as radials through the nutrient space at angles determined by the balance of the component nutrients they contain (nutritional rails), and the animal's nutritional state as a point or region that changes over time (see theoretical examples in Fig. 1). As the animal eats, its nutritional state changes along the rail for the chosen food. The functional aim for animals is to select foods and eat them in appropriate amounts and ratios to direct them to their optimal nutritional state (intake target). Knowing the position in the nutrient space of an individual's nutritional state and intake target provides a basis for making predictions about its physiological, behavioural and fitness responses to the nutrient supply in the environment. An animal can reach its intake target by eating a single nutritionally balanced food (Fig. 1a) or by mixing its intake from two or more nutritionally complementary foods (Fig. 1b). If the animal is restricted to a nutritionally imbalanced food, it must reach a compromise between over‐ingesting some food components and under‐ingesting others (Fig. 1c), for instance by minimising the Euclidean distance between its nutritional state and its intake target (known as the ‘closest distance rule of compromise’; see option 3 in Fig. 1c). NG models have been increasingly used to describe how animals and some other organisms regulate their intake of multiple nutrients when experimentally challenged with perturbations to their nutritional environment or nutritional state (Behmer 2009; Simpson & Raubenheimer 2012). Recently, this framework has also proved useful to address problems in applied nutrition, for instance to improve diets for domestic animals (Hewson‐Hughes et al. 2011), characterise the nutritional ecology of endangered species (Rothman et al. 2011; Nie et al. 2014) or explore ways to improve health and prevent human obesity (Solon‐Biet et al. 2014).
Figure 1.
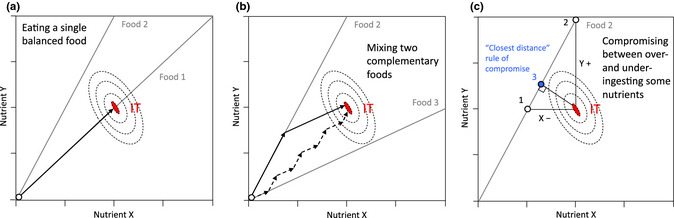
Nutritional Geometry models for a hypothetical individual. Nutritional rails (grey lines) represent the ratio of nutrients X and Y in foods. The white dot is the individual's nutritional state. Fitness levels (dashed lines) decrease with increasing distance from the intake target (IT, red surface). (a) Food 1 is nutritionally balanced (contains the same nutrient ratio as the IT). Food 2 is imbalanced. The individual can reach its IT by exclusively eating Food 1 (arrow). (b) Foods 2 and 3 are individually imbalanced but complementary (fall on opposite sides of the IT). The individual can reach its IT by combining its intake from the two foods (arrows). (c) The individual is restricted to a single imbalanced food and can: (1) satisfy its needs for Y but suffer a shortfall of X; (2) satisfy its needs for X but over‐ingest Y; (3) suffer a moderate shortage of X and excess of Y.
Here, we examine the role of nutrition as a major ecological organiser of social life. First, we discuss some mechanisms by which nutrition mediates social interactions in animals and some other organisms. Next, we explain how concepts of nutritional ecology and social biology can be integrated in a single framework for studying the mechanisms and evolution of social interactions.
Nutrition Influences Social Interactions
Nutrition, broadly defined as all processes by which an organism acquires and uses nutrients for growth and reproduction, is involved in a wide range of social phenomena, from group formation to division of labour. In this section, we present examples of how conceptual advances in NG have helped to understand the nutritional mechanisms mediating some of these phenomena by disentangling the influences of different nutrients, and quantifying both their individual and interactive effects on physiology and behaviour.
Mass migrations
At the most conspicuous level, the spatiotemporal distribution of nutrients defines the foraging areas of individuals and the frequency at which they interact (Simpson & Raubenheimer 2012). When nutrients are scarce or patchily distributed on the landscape, individuals are more likely to encounter one another between and on feeding sites, thus favouring group formation (Collett et al. 1998). If resources are depleted, nutrient deprivation can coordinate the migratory movements of individuals towards new favourable habitats (Simpson et al. 2006; Gregor et al. 2010). For example, in the cellular slime mould Dictyostelium discoideum, local variation in nutrient abundance triggers the transition from solitary‐living single‐celled individuals to swarming multicellular aggregations (Li & Purugganan 2011). When prey bacteria are scarce, solitary‐living slime moulds secrete cyclic adenosine monophosphate, which attracts neighbouring cells, ultimately leading to the aggregation and migration of tens of thousands of cells as a single slug‐like mass (Gregor et al. 2010).
NG‐based analyses in swarming insects, such as Mormon crickets (Anabrus simplex) and desert locusts (Schistocerca gregaria), revealed how nutrient imbalance (not just starvation levels) can trigger the subtle chain of physiological and behavioural changes leading to migratory mass movements (Simpson et al. 2006; Bazazi et al. 2011). During population outbreaks, crickets and locusts congregate on patchy resources such as roosting sites or receding vegetation patches. In locusts, the resulting contact among individuals triggers a phenotypic shift from an isolated and cryptic ‘solitarious’ phase to an actively aggregating ‘gregarious’ phase (Simpson & Sword 2008). This gregarising effect of patchy food distributions (Collett et al. 1998) is further enhanced if foods are nutritionally imbalanced or low in nutrient density, as a result of increased movement by individuals between patches to attain nutrient balance (Despland & Simpson 2000). High‐density aggregations provide anti‐predatory benefits to locusts and crickets (Reynolds et al. 2009), but also increase the competition and the risk of cannibalism. Measures of the nutritional states of crickets (Simpson et al. 2006) and locusts (Bazazi et al. 2011) in diet choice experiments revealed that individuals specifically seek protein (and mineral salts in the case of crickets) at concentrations matching those found in the tissues of conspecifics. By eating each other, insects can supplement their intake of nutrients that are limiting in the environment. These cannibalistic interactions result in a ‘push‐pull’ mechanism in which individuals move in order to reduce their own risk of being bitten, while chasing ahead for potential victims. At critical population densities, cannibalism triggers an autocatalytic marching activity whereby millions of insects align with their neighbours and move in cohesive bands extending over several kilometres (Buhl et al. 2006). Supplementation of protein to crickets (Simpson et al. 2006) and locusts (Bazazi et al. 2011) reduces their locomotion and cannibalistic tendency, which ultimately slows mass movements. In these insects, the synchronisation of the nutritional states of individuals (through the deprivation of specific nutrients) thus modulates the onset and decay of the collective movements. Individual‐based models of collective movement (Box 1) have further shown that cannibalism can also act as the selective force underlying the initial evolution of behavioural phase change expressed by locusts (Guttal et al. 2012), thereby implicating nutrition as a powerful factor in both the proximate and ultimate causes of mass migration.
Social foraging
Social interactions provide individuals with the opportunity to share critical information about nutritional resources in their environment (Danchin et al. 2004). When food is patchily distributed or only temporarily available, animals can use this information to increase their individual rate of finding the required nutrients (Giraldeau & Caraco 2000; Grüter & Leadbeater 2014). If animals forage collectively, social information transfer can have dramatic consequences on group foraging dynamics and efficiency, often leading to ‘consensus’ decisions whereby most (if not all) individuals feed from a single food source despite several alternatives being present (Conradt & Roper 2005).
Collective foraging decisions are common in group‐living insects, such as cockroaches (Lihoreau et al. 2010), tent caterpillars (Dussutour et al. 2008), honeybees (Seeley 1995) and trail‐laying ants (Detrain & Deneubourg 2008), where groups given an opportunity to choose between two identical food sources tend to exploit one randomly chosen source rather than both simultaneously. Despite marked differences in the mechanisms mediating information transfer in these insects, the collective dynamics follow similar rules of positive feedback and quorum responses (Box 1), such that the probability of joining and leaving a particular food source varies non‐linearly with the number of individuals already exploiting that source (Sumpter 2010; Jeanson et al. 2012). By adjusting the intensity of signals about the estimated profitability of sources, insect groups can exploit the best available foods in their environment. Through their ‘waggle dance’ (von Frisch 1967), honeybees (Apis mellifera) communicate locational information about remote food sources enabling them to robustly select nectar sources with the highest concentration of sugar from a dozen or more possibilities and rapidly switch to an alternative source if its relative profitability becomes the highest (Seeley et al. 1991). Cooperative foraging in these insects is not simply limited to choosing the largest or the richest available food source, but also enables bees to adjust their collection of nectar, pollen and water to match the multiple nutrient needs of their colony, both for immediate consumption and reserves (Seeley 1995).
Since social groups are often composed of individuals with distinct nutritional requirements, for instance males and females or individuals at various age classes, many collective foraging decisions must also integrate the multiple and changing needs of all group members (Simpson & Raubenheimer 2012). NG‐based studies taking into account the different nutritional needs of group members have brought new insights into the mechanisms of social foraging in complex societies. In eusocial insects, where food collection tasks are performed by a minority of individuals, foragers need to satisfy their own nutrient requirements, those of the non‐foraging workers, as well as the larvae and queen(s), which have significantly higher protein needs (Hölldobler & Wilson 2009). The challenge is to find appropriate food items and recruit other foragers towards them to collect carbohydrate and protein at a collective‐level target that optimises colony growth and survival (Dussutour & Simpson 2009, 2012; Cook et al. 2010; Paoli et al. 2014). In the green‐headed ant (Rhytidoponera metallica), foragers are informed about the colony's nutritional state by a feedback emanating from the larvae, allowing them to adjust nutrient collection (Dussutour & Simpson 2009). If constrained to an imbalanced diet that is higher than optimal in protein relative to carbohydrate, the excess protein not consumed by the colony is deposited outside the nest as pelleted discard signalling to foragers the need for carbohydrate (Dussutour & Simpson 2009). In these highly integrated societies, or ‘superorganisms’ (Hölldobler & Wilson 2009), nutritional homoeostasis is thus achieved collectively through information transfer that coordinates the actions of individuals for food assessment, collection, processing, storage and waste disposal. Variation in the nutritional needs among classes of individuals (castes) not only modulates the behaviour of foragers but also the complex network of social interactions among colony members once food is brought back into the nest.
Division of labour
Even if all members of a group have similar nutritional needs, there can be some degree of variance in the extent to which each individual will satisfy them, depending on the availability of nutrients in the environment, the foraging performance of the individual and its social interactions (Simpson & Raubenheimer 2012). Variation in the amount and balance of nutrient acquisition among group members can generate some degree of division of labour, by which individuals specialise in different tasks (Salomon et al. 2008; McClure et al. 2011).
Temporary behavioural roles
Many group‐living animals, such as domesticated sheep, chickens and pigs, alternate between resting and feeding phases in a coordinated manner (Sumpter 2010). In these behaviourally synchronised groups, an individual's probability of feeding increases sharply with the number of conspecifics already feeding, and decreases with the number of conspecifics resting (Collins & Sumpter 2007; Gautrais et al. 2007). Models of synchronised behaviour predict that the collective decisions to switch activities can arise from individuals taking transient roles primarily based on their nutritional state (Rands et al. 2003; Conradt et al. 2009). In a simple scenario with only two individuals, the animal with the lower energetic reserves (the ‘leader’) initiates foraging, whilst the one with the higher reserves (the ‘follower’) follows and decides when to stop (Rands et al. 2003). Diet manipulation experiments confirm that such physiological regulation of leadership, where individuals with the highest nutrient requirements are more prone to lead groups may naturally occur in various species (Krause et al. 1992; Fischhoff et al. 2007; McClure et al. 2011). In schools of roaches (Rutilus rutilus), for instance, unfed individuals tend to take front positions (Krause et al. 1992), which provide them with the highest food intake and a dominant influence on directional decisions of the group (Bumann & Krause 1993). In the nomadic forest tent, caterpillar (Malacosoma disstria) which forms foraging trails of dozens of individuals, most protein‐deficient individuals initiate collective departure and lead the group towards new feeding sites, whereas protein‐satiated individuals follow behind (McClure et al. 2011). In these groups, differences in nutritional state among individuals thus regulate the emergence of temporary roles, which are essential for group synchronisation (Sumpter 2010).
Reproductive skews
In addition to affecting behaviour, the amount and blend of nutrients that individuals ingest can have direct consequences on their fitness, by acting upon development, lifespan and fecundity (Lee et al. 2008; Solon‐Biet et al. 2014). In a social context, differential access to key nutrients between individuals can lead to considerable variation in their reproductive success, and the reproductive division of labour that characterises the most advanced stages of sociality (Wilson 1975; Bourke 2011).
In cooperative breeders, where only a subset of individuals reproduce (the ‘breeders’) while others engage in alloparental brood care (the ‘helpers’), contest competition for nutrients can generate reproductive skews. In the burying beetle (Nicrophorus vespilloides), for instance, the best‐fed females tend to become dominant and monopolise reproduction on shared carcasses (Hopwood et al. 2013). When artificially supplemented with protein, subordinate females increase their fecundity laying as many eggs as the dominants (Eggert et al. 2008). In these necrophageous insects, reproductive skews thus simply arise from limited access by subordinates to protein due to intense competition with dominants, which ultimately constrain their ovarian development and reproduction. A similar mechanism explains transitions from shared reproduction to cooperative breeding in social spiders of the genus Stegodyphus (Salomon et al. 2008). S. dumicola females typically share large webs, allowing them to capture larger and more diverse prey than solitary conspecifics while setting the stage for competition. When artificially supplemented with lipid‐rich prey items, colonies produce higher proportions of breeders relative to helpers, indicating that the amount and nature of nutrients available in food determines the magnitude of reproductive skews (Salomon et al. 2008). Although a direct demonstration of such nutritional control of reproductive skews is still lacking in cooperative breeding vertebrates, correlative studies in mongooses (Nichols et al. 2012) and meerkats (Clutton‐Brock et al. 2001) show that subordinate females breed more frequently in periods of food abundance, suggesting that competition for nutrients also influences social organisation in these animals.
In societies where adults feed juveniles, differential nourishment can also mediate reproductive division of labour. This mechanism is particularly important in colonies of eusocial insects where selective allocation of nutrients by workers to the larvae influences their future developmental trajectories (Schwander et al. 2010). In the honeybee (A. mellifera), larvae fed a mix of glandular secretions (royal jelly), honey and pollen develop into sterile workers, while larvae exclusively fed royal jelly develop into queens capable of reproduction (Wheeler 1986). Compounds in royal jelly trigger an epigenetic regulation of gene expression involved in the development and differentiation of reproductive castes by mediating DNA methylation (Kucharski et al. 2008). A single protein (royalactin) is responsible for a significant increase in body size, prolongation of ovary development and shortening of development time in the future queens in comparison to workers (Kamakura 2011). Studies in harvester ants (Pogonomyrmex badius) suggest that variation in larval diet also contributes to major morphological, physiological and behavioural differences among non‐reproductive worker castes (Smith & Suarez 2010). In these integrated societies, interactions between individuals (competition, nourishment) thus generate strong differences in the nutritional states of the juvenile stages that have long‐term consequences on social organisation. According to the ‘reproductive ground plan’ hypothesis, such nutrition‐dependent fertility responses predisposed solitary Hymenoptera to the evolution of division of labour and eusociality (West‐Eberrhard 2003; Amdam et al. 2006; Hunt et al. 2007; Amdam & Page 2010).
A Conceptual Framework for Integrating Nutrition and Social Interactions
In the previous section, we have presented examples illustrating how variance in the nutritional needs and food collecting potential among individuals influences collective dynamics, and provides the basis for the emergence of complex social organisations. Here, we develop these ideas and propose a framework integrating concepts of social biology (the framework of collective animal behaviour; Box 1) and nutritional ecology (the framework of NG; Box 2) to study the mechanisms and evolution of social interactions, using theoretical and empirical approaches.
Modelling interactions between individual‐ and collective‐level nutritional phenotypes
Central to NG is the notion of the intake target (Box 2). This represents the amounts and balance of nutrients, which if ingested, will optimise the fitness of an organism. Just as an individual can be represented by the position of its nutritional state relative to its intake target in a nutrient space (Fig. 1), a group can be depicted as a collection of individuals, each attempting to reach its own intake target (Fig. 2). In this approach, each individual's decision to eat a food not only depends on its nutrient needs but also on the nature and frequency of its interactions with all other group members (e.g. cannibalism, social attraction, contest competition, parental nourishment). Therefore, patterns of distribution of the nutritional states and intake targets of all individuals in the nutrient space can help reveal how these trade‐offs are resolved by individuals and predict their impact on collective‐level phenomena, such as collective dynamics and social organisation (see theoretical examples in Fig. 2).
Figure 2.
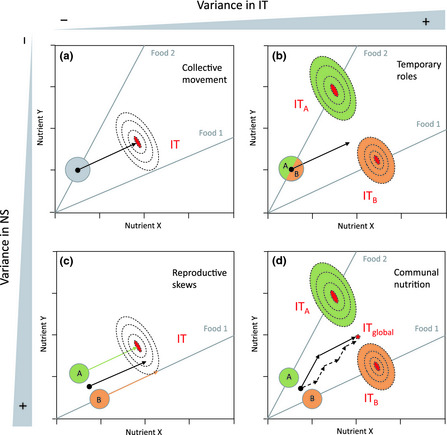
Nutritional Geometry models for hypothetical groups. Nutritional rails (grey lines) represent the ratio of nutrients X and Y in foods. Black dots are the average nutritional states (NS), plain circles are the variance of NS and dashed lines are fitness levels around the intake target (IT, red surface). (a) Gregarious individuals whose NS are distributed in one cluster. The average NS is at a critical switching point to reach the IT, predicting a collective movement from Food 2 to Food 1 (arrow). (b) Gregarious individuals with different IT. ‘B’ individuals have the greatest need for Food 1 and lead the collective movement. (c) Cooperative breeders whose NS are in two clusters. Only ‘A’ individuals can reach their IT (become breeders) by eating Food 1. (d) Eusocial group. ‘A’ individuals provision ‘B’ individuals. ‘A’ individuals must reach a group‐level (global) IT to simultaneously address their needs and those of ‘B’ individuals..
Two nutritional factors, both of which can be accounted for and modelled by NG, are central to interpreting these patterns of individual variation and their social consequences. The first is the average nutritional state of all individuals, relative to their intake targets. In groups with a simple social structure, such as a cohort of gregarious animals, all individuals are expected to have similar nutritional states and similar intake targets, due to their synchronous feeding and development (Fig. 2a). In such homogeneous groups, where it is expected that all nutritional states are distributed in one cluster around the mean, the average value reflects the needs of the majority of the individuals. It should thus have a strong influence on the outcome of collective dynamics, such as the collective decision to stay on a food patch or to leave. For example, when a population of Mormon crickets is protein‐deprived, their intake target is higher in protein than the vegetation in their environment provides. The resulting cannibalistic interactions that arise as a means to redress this protein imbalance for individuals provide a mechanism to synchronise their behaviour and drive mass migrations (Simpson et al. 2006).
However, in groups with a more complex social structure, for instance a family group where adults and juveniles eat different foods, the predictive power of the average nutritional state may be much reduced. In such heterogeneous groups, the nutritional states of all individuals are expected to be distributed in two or more clusters (Fig. 2c) and may also point towards separate intake targets (Fig. 2d). Even if all group members have the same intake target, there can be some degree of variance in the extent to which they will reach this target, depending on nutrient availability and social interactions (Fig. 2b). Therefore, a second important nutritional factor to take into account is the variance in nutritional states and intake targets among individuals. The shape and amplitude of this variance will reflect the direction and strength of the influence of individual nutrition on collective dynamics and social organisations. In schools of juvenile roaches, for instance, fluctuations in the dynamics of nutrient acquisition among fish induce slight variation in nutritional states that affects the initiation of behavioural roles, where the most nutritionally deficient individuals temporarily lead group movements (Krause et al. 1992). In social spiders, variability in lipid acquisition among competing females generates reproductive skews and, ultimately, cooperative breeding (Salomon et al. 2008). In the colonies of eusocial insects, where members of different castes have distinct nutrient states and intake targets (i.e. nutritional subgroups), social interactions are pivotal for achieving communal nutrition. This is the case for the nutritional decisions of ant foragers that must integrate the needs of all other colony members, including the reproductive queens and growing larvae whose intake target is more protein‐biased (Dussutour & Simpson 2009).
Simulating social interactions in nutritionally explicit individual‐based models
Expanding the framework of NG to socially interacting organisms involves examining nutritional processes at two different organisational levels, the individual and the group (Fig. 2). In this approach, groups can be regarded as complex nutritional systems in which global properties (e.g. social phenomena) emerge from interactions at lower organisation levels (e.g. nutritional interactions between individuals and the nutritional environment or social interactions between individuals). Research on collective behaviour (Box 1) has been remarkably successful in applying individual‐based modelling approaches to the study of multi‐level interactions in animal groups, whose consequences are often hard to predict due to their stochastic, self‐organising and non‐linear nature (Camazine et al. 2001; Couzin 2009; Sumpter 2010). Incorporating concepts of NG into such models thus holds considerable promise to test new hypotheses and generate predictions about the role of nutrition in the development and maintenance of social interactions. To illustrate the potential of this approach, we developed examples of nutritionally explicit individual‐based models (see details of the models in Box 3) and used them to derive specific predictions about how variation in the nutritional states and intake targets among individuals can influence three collective phenomena that have historically been studied from a one‐dimension nutritional perspective (e.g. influence of food, energy or single nutrients): collective decision‐making, the alternation of behavioural roles and the emergence of a social structure (Fig. 3).
Box 3. Examples of nutritionally explicit individual‐based model.
We implemented concepts of Nutritional Geometry (Box 2) in an individual‐based model structure derived from collective animal behaviour studies (Box 1). In our model, individuals are defined by their nutritional state (NS) and intake target (IT) in a two‐dimensional nutrient space. Food rails are defined by their ratio of nutrients X and Y. At each step, individuals can eat food up to the point where their NS reaches the shortest Euclidean distance to their IT (see closest distance rule of compromise in Fig. 1c). An individual's appetite is:
where β is the angle between (vector joining the NS of individual i to the nearest point to its IT along the selected food rail) and (the vector joining its NS to its IT). If the quantity of food required to reach the IT is higher than the maximal quantity the individual can eat in one step (φ), its NS increases by φ along the food rail (0.0028 in all simulations). The distance D N between NS and IT is a measure of the individual's fitness:
Unless otherwise specified, all individuals’ IT are set along a chosen rail at a distance D IT = 1 and their initial NS at coordinates (0,0). Below we illustrate how variants of this general model can be used to explore how individuals can satisfy their nutritional needs while interacting with each other.
Variant 1: Social foraging. Individuals decide to leave a food based on an individual component P ind (the closer the food rail is to the ideal rail pointing to the IT, the lower the probability to leave) and a social component P soc (the more individuals present on the food, the lower the probability to leave):
and
where α ideal and α f are the angles associated with the ideal rail and the food rail (f). λ is a constant (7 in all simulations), N f the number of individuals on f and N the total number of individuals. The minimum P leave is 0.05. The relative weight of both components varies according to a constant K soc . Before visiting a new food, the individual waits a time T t (2 in our simulations). Fig. 3a shows examples of simulation runs for groups of 100 individuals with the same IT presented two nutritionally complementary foods for 675 time steps. Moderate levels of social influence on leaving decisions (K soc ) synchronise the foraging choices of individuals, resulting in an optimal collective regulation of nutrient intake. Fig. 3b shows how socially interacting individuals from two subgroups of 50 individuals with a different IT may compromise between tracking their own IT and maintaining social cohesion. Small differences in the distance between NS and IT among individuals favour the emergence of temporary ‘leaders’, whereby the most nutritionally deficient individuals initiate the collective switch from one food to the other.
Variant 2: Contest competition. Individuals share F food items across n food types in their environment. Only C = F/n food items are available on a given food at any time. At each step, an individual selects a random food. If the number of individuals already present on that food equals C, foragers engage in a contest (similar to what is described in Bonabeau et al. (1996), except that the ‘force’ is fitness):
where f i and f j is the fitness of individual i and j and η is a constant (25 in all simulations). The winner can eat from the food, whereas the loser will search for another food on its next step. An individual spontaneously leaves a food based on the quantity consumed and the angular difference between the food rail and the ideal rail pointing to its IT:
where S is a constant reflecting food selectivity (when S = 1, individuals do not stay long on imbalanced foods). The faster individuals increase their fitness relative to competitors, the higher their chances to win future contests and monopolise the required nutrients. Fig. 3c shows how variation in competition strength (inversely proportional to C) and food selectivity (S) can lead to non‐uniform distributions of NS in groups of 150 individuals with the same initial NS and the same IT (simulations stop when an individual reaches a fitness of 0.99). High levels of competition stretch the distribution of NS so that only few individuals reach their IT. Low levels of food selectivity enable individuals to feed for longer on imbalanced foods and get closer to their IT, further amplifying group nutritional differentiation.
Figure 3.
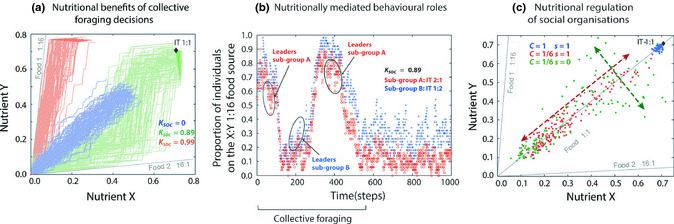
Examples of simulation models (see Box 3). (a) Trajectories of the nutritional states (NS) of individuals with the same intake target (IT) presented two complementary foods (grey lines). With moderate social attraction (green), individuals reach their IT by synchronously switching between foods. Without attraction (blue), individuals asynchronously alternate between foods, reaching their IT at a slower rate. With strong attraction (red), individuals stay on the first food they choose. (b) Foraging dynamics of two subgroups of individuals with different IT and moderate attraction in the same environment as (a). During the initial phase of collective foraging, individuals of the subgroup deviating the most from their IT lead the collective switches between foods. (c) Group of individuals with the same IT exploiting three foods. Under no competition, all individuals reach their IT (blue). Competition stretches the NS along the balanced food rail (red). Low food selectivity stretches the NS transversely (green).
Example 1: Nutritional benefits of collective foraging decisions
While most studies on collective decisions have involved the ability of animal groups to choose between two identical food patches or between several food patches of different profitability (e.g. size, energetic value) (Sumpter 2010; Jeanson et al. 2012), we used a nutritionally explicit individual‐based model to explore the foraging efficiency of groups in more complex and ecologically relevant nutritional contexts where animals attempt to maintain nutrient balance (see model variant 1 in Box 3). We investigated a situation where only nutritionally imbalanced foods are available. In such conditions, individuals must alternate between feeding on multiple foods in order to reach their intake target (Fig. 1b). Simulations of our model predict that moderate levels of social attraction among foraging individuals can lead to collective dynamics whereby groups exploit a given food until a critical number of individuals (quorum; Box 1), when their nutritional state deviates too much from their intake target, initiates collective switching to an alternate food (Fig. 3a). Collective foraging minimises the time spent finding a new complementary food, thus enabling socially foraging individuals to reach their intake target faster than solitary foragers (Fig. S1). Such a model of collective foraging could be expanded to explore how animals exhibiting distinct types of social interactions (e.g. remote recruitment: Seeley et al. 1991; pheromone trails: Detrain & Deneubourg 2008; social retention: Lihoreau et al. 2010) may perform in different nutritional environments, for instance, by experimentally manipulating numbers, combinations and temporal accessibility of food types available to groups.
Example 2: Nutritionally mediated behavioural roles
Using the same model structure (model variant 1 in Box 3), we addressed the question of how collective foraging dynamics are affected in heterogeneous groups composed of individuals with different intake targets (Fig 3b). While many previous studies have investigated the problem of how differences in satiation levels (Krause et al. 1992; Rands et al. 2003; Conradt et al. 2009) or specific nutrient acquisition (McClure et al. 2011) may regulate behavioural roles in animal groups, none of them have explored the possibility of a more sophisticated control of division of labour by nutritional imbalance. For instance, in a group of socially foraging individuals composed of two nutritional subgroups (classes of individuals with distinct intake targets) with access to two imbalanced foods, our model predicts that individuals with nutrient requirements diverging the most from the composition of the food they are on will act as leaders, initiating the collective decision to leave the food for an alternative one. Therefore, it is the amount and balance of the nutrients in food, not just the deprivation of one of them, that are critical to explain the collective dynamics. Varying the level of social cohesion among foragers shows that changes in the spatiotemporal availability of nutrients itself can drive the emergence or absence of collective foraging (Fig. S2). These interactive effects of nutritional needs and nutrient distribution on group cohesion could be experimentally explored in gregarious species, where males and females with distinct intake targets forage collectively, for instance, in fusion–fission societies of ungulates and primates (Couzin & Krause 2003).
Example 3: Nutritional regulation of social organisations
We explored how contest competition for nutrient acquisition may encourage the emergence of complex group structures (see model variant 2 in Box 3; Fig. 3c). In this model, individuals that are successful in maintaining a nutritional state close to their intake target develop better and are more likely to gain further access to a limited amount of nutrients by displacing less successful conspecifics from foods. As less food is available in the environment, the more successful individuals benefit from this positive feedback loop (Box 1), where winning contests reinforces their chances of winning even more in the future. As a result, resource scarcity increases variance in the distribution of the nutritional states of individuals, potentially leading to the emergence of social structures (Eggert et al. 2008; Salomon et al. 2008). This model can be used to precisely predict changes in the dominance or reproductive status of given group members in response to spatiotemporal changes in nutrient availability (Fig. S3).
Testing model predictions with diet manipulation experiments
Importantly, the predictions of such nutritionally explicitly individual‐based models are based on quantitative nutritional and behavioural data (Box 3). These predictions are empirically testable and provide the basis for a powerful dialogue between simulations and observations (see examples in Figs S1–S3). While classical NG experiments involve measuring the nutritional responses of isolated individuals in the presence of one or multiple chemically defined foods for which the balance and concentration of nutrients are tightly controlled (Raubenheimer & Simpson 1993; Simpson & Raubenheimer 2012), studying socially interacting organisms involves quantifying the nutrient intake and performance of all (or at least some fraction) of the individuals in the group, without separating them out. Several technological advances in behavioural tracking now enable experimenters to accurately monitor the behaviour of individually marked animals engaging in social interactions, both in the laboratory and in the wild (e.g. radio frequency identification: Gill et al. 2012; harmonic radar: Lihoreau et al. 2012; barcode labels: Mersch et al. 2013; computer vision: Pérez‐Escudero et al. 2014). Modern approaches in nutrition research have also been developed to quantify and trace nutrient intake by individual animals, for instance using food dye (e.g. Wong et al. 2009), radioactively labelled nutrients (e.g. Buffin et al. 2009) or bioluminescent transgenic animals (e.g. Itskov et al. 2014). Combining these methods and other advances will enable the reconstruction of the complete foraging histories of interacting individuals, detailing their behaviour, nutrient intake and absorption, with high spatial and temporal resolution, thus providing all the necessary data to put model predictions to the test. Although these approaches have been developed in several biological systems, social insects in particular are very promising models to empirically explore the nutritional basis of social interactions, due to their well‐documented nutritional ecology, their extreme diversity of social behaviour, and the long tradition of studying their societies as self‐organised complex systems (Lihoreau et al. 2014).
Evolutionary implications
Several hypotheses have been put forward to emphasise the importance of nutrition in social evolution (Wheeler 1928; Rubenstein & Wrangham 1986; Hunt & Nalepa 1994; West‐Eberrhard 2003; Amdam et al. 2006; Hunt et al. 2007; Amdam & Page 2010). So far, however, tests of these hypotheses have been piecemeal and incomplete. In addition to providing new insights into the nutritional mechanisms of social interactions (Figs 2, 3, S1–S3), our analytical approach will offer new opportunities to explore their potential impact on processes of social evolution. As an example, we discuss a simple scenario showing how some of the nutritional mechanisms identified above may drive the series of steps that lead to key social transitions, by acting upon the physiology, behaviour and performance of individuals. In this hypothetical scenario, causal relationships between changes in variance in intake targets among individuals and changes in social organisation are envisaged (Fig. 4).
Figure 4.
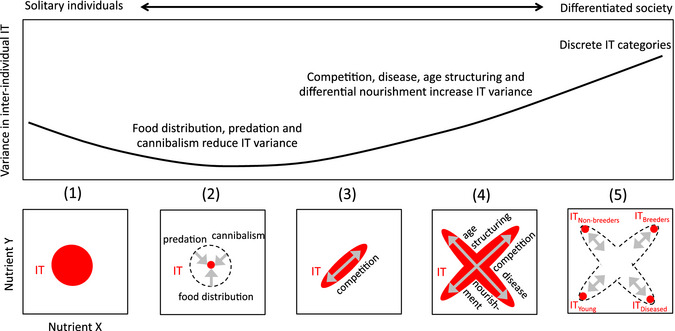
Conceptual diagram depicting the expected variance of intake targets (IT) in groups of increasing organisational complexity. The variance (black line) is affected by social and ecological factors (grey arrows). Theoretical IT (red surfaces) are regions in a two‐dimensional space for nutrients X and Y. Their shape determines how selection might influence social interactions by acting upon individual nutrition. Transition from solitary‐ to group‐living (1–2) is accompanied by a reduction in variance through the synchronising influences of food distribution, predation and cannibalism. With increasing levels of social complexity, IT surfaces differentiate (3–4) and split (4–5) into clusters through the action of additional factors, leading to nutritional niche partitioning. (5) In societies with an age structure and a division of labour, adults require more nutrients than the young. Non‐breeders need more of Y for energy‐consuming tasks; diseased individuals need more of X to combat infections; breeders need more of both to reproduce.
At the most basal level of social phenomena, individuals congregate in response to nutrient scarcity or high predation risk (Reynolds et al. 2009; Gregor et al. 2010). If grouping provides substantial benefits, individuals should synchronise their activities (Buhl et al. 2006). The resulting synchronisation of nutrient acquisition, based on quorum responses and feedback loops (Box 1), is expected to decrease the variance in the nutritional states and intake targets among individuals in comparison to populations of isolated (unsynchronised) foragers. The decrease might be greater still if individuals also synchronise their development and reproduction, for example to minimise risks of being cannibalized while in vulnerable life stages (Crossland et al. 2011).
Homogenisation of nutritional needs among individuals during these early stages of socialisation is expected to act against the structuring of societies. However, other selection pressures could increase the variance in nutritional states and intake targets, to favour the emergence of division of labour, where individuals with different needs take different behavioural roles (Krause et al. 1992; McClure et al. 2011). Several social and ecological factors might act in concert to generate this variance and determine its shape. For instance, the settlement of a group in a stable nesting site ensures long‐term cohesion of the groups, which might ultimately reduce the need for behavioural synchronisation. In environments where foods are nutritionally imbalanced or low in nutrient density, intense competition over nutrient acquisition might encourage variation in reproductive performance and lifespan at the origin of cooperative breeding (Eggert et al. 2008; Salomon et al. 2008). An increased rate of disease transmission is also a potential source of variance, forcing infected individuals to modulate the composition of their diet to combat parasites and pathogens (Lee et al. 2006; Povey et al. 2009).
In the most advanced social forms, additional factors such as age structuring (i.e. generation overlaps of young and adults) and differential nourishment would further amplify the variance in nutrient states and intake targets among group members. Ultimately, partitioning of nutritional niches, by which classes of individuals with fully differentiated intake targets emerge (e.g. breeders, helpers), may favour transitions to reproductive division of labour and cooperative brood care that characterise eusociality. In this process, the role of nutrition can broaden from a constraint defining the selective environment, to a proximate mechanism that promotes phenotypic diversity by influencing the developmental trajectories of specialised castes. In the Hymenoptera, for example ‘nutritional castration’ by which colony members receive different amounts and ratios of nutrients may have favoured the evolution of reproductive division of labour (Wilson 1975; Hunt & Nalepa 1994). In termites, the acquisition of a wood‐feeding habit may have caused an increasing interdependence between adults and their progeny based on regular exchanges of obligate hindgut symbionts for cellulose digestion, ultimately favouring the evolution of the worker castes (Nalepa et al. 2001).
Conclusion
Many organisms interact socially around food resources or during the process of nutrient acquisition, yet the causal relationships between variations in nutrition and social interactions are still poorly understood (Simpson & Raubenheimer 2012). Here. we have introduced a general framework integrating concepts of nutritional geometry and collective animal behaviour to study these relationships both theoretically and empirically across a taxonomically and ecologically diverse range of organisms. We hope that our attempt to position our conceptual framework in an evolutionary context (Fig. 4) will serve as a starting point for a further examination of potential evolutionary pathways through which nutritional constraints may lead to key social transitions. We have identified four major themes of research that will guide future studies along these lines (Box 4). Ultimately, a broader integration of the concepts of nutritional ecology into research in social biology will provide a major step ahead towards a general understanding of the role of the ecological environment in both the expression and evolution of social interactions.
Box 4. Future research areas.
Four themes for investigating the mechanisms and evolution of social interactions from a nutritional perspective, using simulation models and manipulative experiments.
Characterising the nutritional ecology of social organisms. Nutrition research has traditionally focused on individual organisms and only recently have studies begun to investigate these processes in a more complex, group‐level context. A detailed exploration of the nutritional strategies of social organisms will thus considerably expand the scope of nutritional ecology. For instance, the extreme levels of cooperation for nutrient regulation observed in eusocial insects (Dussutour & Simpson 2009; Cook et al. 2010) raise the question of whether and how collective nutritional balance is achieved in simpler animal groups. Do similar processes apply to gregarious species or to parent–offspring nutritional interactions? Answering these questions by comparing quantitative nutritional and behavioural data across a range of species will necessitate identifying how the trade‐offs between optimising nutrition and responding to social interactions are resolved by individuals, and how this varies across taxa, social and ecological contexts.
Modelling nutritional and social interactions in spatial environments. Our modelling framework integrates state‐space models from nutritional ecology and individual‐based models from collective animal behaviour studies (see examples in Box 3, Figs 3, S1–S3). Further development of these models, for instance using multi‐scale modelling approaches of landscape ecology (Levin 1992; Viswanathan et al. 1999), will make it possible to simulate the movements and interactions of foragers in more complex, spatial and dynamic nutritional environments. Questions related to how features of the environment (e.g. type, amount and spatio‐temporal distribution of nutrients) encourage social interactions could be explored. This will also allow assessing the role of nutrient availability in regulating complex population dynamics (e.g. metapopulations, fusion–fission societies) and addressing problems of conservation ecology in the context large‐scale environmental changes.
Identifying the nutritional drivers of social evolution. A major goal of research on nutrition and social interactions is to identify potential pathways through which nutrition may have favoured the evolution of social life (Hunt & Nalepa 1994; West‐Eberrhard 2003; Amdam et al. 2006; Hunt et al. 2007; Amdam & Page 2010). Our models already incorporate fitness outcomes in the form of performance consequences of excesses and deficits of nutrients on key life history traits (Boxes 2 and 3), which are the bases for selection to operate (Simpson et al. 2004). The challenge for future studies will be to identify and put to the test evolutionary relationships between nutritional strategies (distributions of nutritional states and intake targets among individuals) and social systems (e.g. Fig. 4). Phylogenetic meta‐analyses (Rosas‐Guerrero et al. 2014), evolution experiments (Kawecki et al. 2012) and evolutionary modelling (Ashlock 2006) are all approaches that will allow these questions to be addressed.
Investigating nutritional interactions beyond social systems. Our modelling and empirical approach aims at investigating interactions between nutritional processes at the individual and collective levels, within groups and societies (Box 3). Importantly, this conceptual framework can be extended to nutritional processes at higher levels of biological organisations, within populations and between species (e.g. prey–predator interactions, competing species, host–microbiota) at the levels of communities and ecosystems. For instance, growing evidence shows that the nutritional intake of individuals can directly influence the immune response efficiency of groups (social immunity) against infections by parasites (Kay et al. 2014). The ultimate aim is to develop an understanding of nutritional processes and their interactions across organisational levels, from cells to ecosystems. Achieving this integrative synthesis in nutritional ecology will help in more realistically addressing fundamental questions in ecology and evolution such as how species assemblages and food webs emerge (Simpson et al. 2015).
Authorship
ML, CB, MAC, GAS, DR and SJS wrote the manuscript. CB and MAC performed the computer simulations.
Supporting information
Acknowledgements
We thank Micky Eubanks, Adam Kay and two anonymous referees for very useful comments on an earlier version of the manuscript. This work was funded by an IDEX – University of Toulouse Starting Grant to ML, Australian Research Council (ARC) Discovery Project and Future Fellowship grants to CB, ARC Discovery and Linkage Projects to GAS and SJS and an ARC Laureate Fellowship to SJS.
Ecology Letters (2015) 18: 273–286
References
- Aanen, D.K. , De Fine Licht, H.H. , Debets, A.J.M. , Kerstes, N.A.G. , Hoekstra, R.F. & Boomsma, J.J. (2009). High symbiont relatedness stabilizes mutualistic cooperation in fungus‐growing termites. Science, 326, 1103–1106. [DOI] [PubMed] [Google Scholar]
- Amdam, G.V. & Page, R.E. (2010). The developmental genetics and physiology of honeybee societies. Anim. Behav., 79, 973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam, G.V. , Csondes, A. , Fonrk, M.K. & Page, R.E. (2006). Complex social behaviour derived from maternal reproductive traits. Nature, 439, 76–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amé, J.M. , Halloy, J. , Rivault, C. , Detrain, C. & Deneubourg, J.L. (2006). Collegial decision making based on social amplification leads to optimal group formation. Proc. Natl Acad. Sci. USA, 103, 5835–5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashlock, D. (2006). Evolutionary Computation for Modeling and Optimization. Springer‐Verlag, New‐York, NY. 572 pp. [Google Scholar]
- Bazazi, S. , Romanczuk, P. , Thomas, S. , Schimansky‐Geier, L. , Hale, J.J. , Miller, G.A. et al. (2011). Nutritional state and collective motion: from individuals to mass migration. Proc. R. Soc. B, 278, 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behmer, S.T. (2009). Insect herbivore nutrient regulation. Ann. Rev. Entomol., 54, 165–187. [DOI] [PubMed] [Google Scholar]
- Bonabeau, E. , Theraulaz, G. & Deneubourg, J.L. (1996). Mathematical model of self‐organizing hierarchies in animal societies. Bull. Math. Biol., 58, 661–717. [Google Scholar]
- Bourke, A.F.G. (2011). Principles of Social Evolution. Oxford University Press, Oxford, UK. 288 pp. [Google Scholar]
- Buffin, A. , Denis, D. , Van Simaeys, G. , Goldman, S. & Deneubourg, J.L. (2009). Feeding and stocking up: radio‐labelled food reveals exchange patterns in ants. PLoS ONE, 4, e5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl, C. , Sumpter, D.J.T. , Couzin, I.D. , Hale, J.J. , Despland, E. , Miller, E.R. et al. (2006). From disorder to order in marching locusts. Science, 312, 1402–1406. [DOI] [PubMed] [Google Scholar]
- Bumann, D. & Krause, J. (1993). Front individuals lead in shoals of three‐spined sticklebacks (Gasterosteus aculeatus) and juvenile roach (Rutilus rutilus). Behaviour, 125, 189–198. [Google Scholar]
- Camazine, S. , Deneubourg, J.L. , Franks, N. , Sneyd, J. , Theraulaz, G. & Bonabeau, E. (2001). Self‐Organization in Biological Systems. Princeton University Press, Princeton, NJ. 560 pp. [Google Scholar]
- Clutton‐Brock, T.H. , Brotherton, P.N.M. , Russell, A.F. , O'Riain, M.J. , Gaynor, D. , Kansky, R. et al. (2001). Cooperation, control, and concession in meerkat groups. Science, 291, 478–481. [DOI] [PubMed] [Google Scholar]
- Collett, M. , Despland, E. , Simpson, S.J. & Krakauer, D.C. (1998). Spatial scales of desert locust gregarization. Proc. Natl Acad. Sci. USA, 95, 13052–13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, L.M. & Sumpter, D.J.T. (2007). The feeding dynamics of broiler chickens. J. R. Soc. Interface, 4, 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt, L. & Roper, T.J. (2005). Consensus decision making in animals. Trends Ecol. Evol., 20, 449–456. [DOI] [PubMed] [Google Scholar]
- Conradt, L. , Krause, J. , Couzin, I.D. & Roper, T.J. (2009). ‘Leading according to need’ in self‐organizing groups. Am. Nat., 173, 304–312. [DOI] [PubMed] [Google Scholar]
- Cook, S.C. , Eubanks, M.D. , Gold, R.E. & Behmer, S.T. (2010). Colony‐level macronutrient regulation in ants: mechanisms, hoarding and associated costs. Anim. Behav., 79, 429–437. [Google Scholar]
- Couzin, I.D. (2009). Collective cognition in animals. Trends Cognit. Sci., 13, 36–43. [DOI] [PubMed] [Google Scholar]
- Couzin, I.D. & Krause, J. (2003). Self‐organization and collective behavior in vertebrates. Adv. Stud. Behav., 32, 1–75. [Google Scholar]
- Crossland, M.R. , Hearnden, M.N. , Pizzatto, L. , Alford, R.A. & Shine, R. (2011). Why be a cannibal? The benefits to cane toad, Rhinella marina [=Bufo marinus], tadpoles of consuming conspecific eggs. Anim. Behav., 82, 775–782. [Google Scholar]
- Danchin, E. , Giraldeau, L.A. , Valone, T.J. & Wagner, R.H. (2004). Public information: from nosy neighbors to cultural evolution. Science, 305, 487–491. [DOI] [PubMed] [Google Scholar]
- Deisboeck, T.S. & Couzin, I.D. (2009). Collective behaviour in cancer cell populations. BioEssays, 31, 190–197. [DOI] [PubMed] [Google Scholar]
- Despland, E. & Simpson, S.J. (2000). Small‐scale vegetation patterns in the parental environment influence the phase state of hatchlings of the desert locust. Physiol. Entomol., 25, 74–81. [Google Scholar]
- Detrain, C. & Deneubourg, J.L. (2008). Collective decision‐making and foraging patterns in ants and honeybees. Adv. Insect. Physiol., 35, 123–173. [Google Scholar]
- Dussutour, A. & Simpson, S.J. (2009). Communal nutrition in ants. Curr. Biol., 19, 740–744. [DOI] [PubMed] [Google Scholar]
- Dussutour, A. & Simpson, S.J. (2012). Ant workers die young and colonies collapse when fed a high‐protein diet. Proc. R. Soc. B, 279, 2402–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussutour, A. , Nicolis, S.C. , Despland, E. & Simpson, S.J. (2008). Individual differences influence collective behaviour in social caterpillars. Anim. Behav., 76, 5–16. [Google Scholar]
- Eggert, A.‐K. , Otte, T. & Müller, J.K. (2008). Starving the competition: a proximate cause of reproductive skew in burying beetles (Nicrophorus vespilloides). Proc. R. Soc. B, 275, 2521–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischhoff, I.R. , Sundaresan, S. , Cordingley, J. , Larkin, H.M. , Sellier, M.J. & Rubinstein, D. (2007). Social relationships and reproductive state influence leadership roles in movements of plains zebra, Equus burchellii . Anim. Behav., 73, 825–831. [Google Scholar]
- von Frisch, K. (1967). The Dance Language and Orientation of Bees. Harvard University Press, Cambridge, MA. 592 pp. [Google Scholar]
- Gautrais, J. , Michelena, P. , Sibbald, A. , Bon, R. & Deneubourg, J.L. (2007). Allomimetic synchronization in Merino sheep. Anim. Behav., 74, 1443–1454. [Google Scholar]
- Gill, R.J. , Ramos‐Rodriguez, O. & Raine, N.E. (2012). Combined pesticide exposure severely affects individual‐ and colony‐level traits in bees. Nature, 491, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldeau, L.A. & Caraco, T. (2000). Social Foraging Theory. Princeton University Press, Princeton, NJ. 376 pp. [Google Scholar]
- Gordon, D.M. (2014). The ecology of collective behavior. PLoS Biol., 12, e1001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss, S. , Aron, S. , Deneubourg, J.L. & Pasteels, J.M. (1989). Self‐organized shortcuts in the Argentine ant. Naturwissenschaften, 76, 579–581. [Google Scholar]
- Gregor, T. , Fujimoto, K. , Masaki, N. & Sawai, S. (2010). The onset of collective behavior in social amoebae. Science, 328, 1021–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover, C.D. , Kay, A.D. , Monson, J.A. , Marsh, T.C. & Holway, D.A. (2007). Linking nutrition and behavioural dominance: carbohydrate scarcity limits aggression and activity in Argentine ants. Proc. R. Soc. B, 274, 2951–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüter, C. & Leadbeater, E. (2014). Insights from insects about adaptive social information use. Trends Ecol. Evol., 29, 177–184. [DOI] [PubMed] [Google Scholar]
- Guttal, V. , Romanczuk, P. , Simpson, S.J. , Sword, G.A. & Couzin, I.D. (2012). Cannibalism can drive the evolution of behavioural phase polyphenism in locusts. Ecol. Lett., 15, 1158–1166. [DOI] [PubMed] [Google Scholar]
- Hewson‐Hughes, A.K. , Hewson‐Hughes, V.L. , Miller, A.T. , Hall, S.R. , Simpson, S.J. & Raubenheimer, D. (2011). Geometric analysis of macronutrient selection in the adult domestic cat, Felis catus . J. Exp. Biol., 214, 1039–1051. [DOI] [PubMed] [Google Scholar]
- Hofmann, H.A. , Beery, A.K. , Blumstein, D.T. , Couzin, I.D. , Earley, R.L. , Hayes, L.D. et al. (2014). An evolutionary framework for studying the mechanisms of social behavior. Trends Ecol. Evol., 29, 581–589. [DOI] [PubMed] [Google Scholar]
- Hölldobler, B. & Wilson, E.O. (2009). The Superorganism: The Beauty, Elegance, and Strangeness of Insect Societies. Norton & Co, New York, NY. 522 pp. [Google Scholar]
- Hopwood, P.E. , Moore, A.J. & Royle, N.J. (2013). Nutrition during sexual maturation affects competitive ability but not reproductive productivity in burying beetles. Funct. Ecol., 27, 1350–1357. [Google Scholar]
- Hunt, J.H. & Nalepa, C.A. (1994). Nourishment and Evolution in Insect Societies. Westview press, Boulder, CO. 450 pp. [Google Scholar]
- Hunt, J.H. , Kensinger, B.J. , Kossuth, J.A. , Henshaw, M.T. , Norberg, K. , Wolschin, F. et al. (2007). A diapause pathway underlies the gyne phenotype in Polistes wasps, revealing an evolutionary route to caste‐containing insect societies. Proc. Natl Acad. Sci. USA, 104, 14020–14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskov, P.M. , Moreira, J.M. , Vinnik, E. , Lopes, G. , Safarik, S. , Disckinson, M.H. et al. (2014). Automated monitoring and quantitative analysis of feeding behaviour in Drosophila . Nat. Commun., 5, 4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanson, R. , Dussutour, A. & Fourcassié, V. (2012). Key factors for the emergence of collective decision in invertebrates. Front Neurosci., 6, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakura, M. (2011). Royalactin induces queen differentiation in honeybees. Nature, 473, 478–483. [DOI] [PubMed] [Google Scholar]
- Kawecki, T.J. , Lenski, R.E. , Ebert, D. , Hollis, B. , Oliveri, I. & Whitlock, M.C. (2012). Experimental evolution. Trends Ecol. Evol., 27, 547–560. [DOI] [PubMed] [Google Scholar]
- Kay, A.D. , Bruning, A.J. , van Alst, A. , Abrahamson, T.T. , Hughes, W.O.H. & Kaspari, M. (2014). A carbohydrate‐rich diet increases social immunity in ants. Proc. R. Soc. B, 281, 0132374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause, J. & Ruxton, G.D. (2002). Living in Groups. Oxford University Press, Oxford, UK. 210 pp. [Google Scholar]
- Krause, J. , Bumann, D. & Todt, D. (1992). Relationship between the position preference and nutritional state of individuals in schools of juvenile roach (Rutilus rutilus). Behav. Ecol. Sociobiol., 30, 177–180. [Google Scholar]
- Kucharski, R. , Maleszka, J. , Foret, S. & Maleszka, R. (2008). Nutritional control of reproductive status in honeybees via DNA methylation. Science, 319, 1827–1830. [DOI] [PubMed] [Google Scholar]
- Lee, K.P. , Cory, S.J. , Wilson, K. , Raubenheimer, D. & Simpson, S.J. (2006). Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proc. R. Soc. B, 273, 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K.P. , Simpson, S.J. , Clissold, F.J. , Brooks, R. , Ballard, J.W.O. , Taylor, P.W. et al. (2008). Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc. Natl Acad. Sci. USA, 105, 2498–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, S.A. (1992). The problems of pattern and scale in ecology. Ecology, 73, 1943–1967. [Google Scholar]
- Li, S.I. & Purugganan, M.D. (2011). The cooperative amoeba: Dictyostelium as a model for social evolution. Trends Genet., 27, 48–54. [DOI] [PubMed] [Google Scholar]
- Lihoreau, M. , Deneubourg, J.L. & Rivault, C. (2010). Collective foraging decision in a gregarious insect. Behav. Ecol. Sociobiol., 64, 1577–1587. [Google Scholar]
- Lihoreau, M. , Raine, N.E. , Reynolds, A.M. , Stelzer, R.J. , Lim, K.S. , Smith, A.D. et al. (2012). Radar tracking and motion‐sensitive cameras on flowers reveal the development of pollinator multi‐destination routes over large spatial scales. PLoS Biol., 10, e1001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lihoreau, M. , Buhl, C. , Charleston, M.A. , Sword, G.A. , Raubenheimer, D. & Simpson, S.J. (2014). Modeling nutrition across organizational levels: from individuals to superorganisms. J. Insect Physiol., 69, 2–11. [DOI] [PubMed] [Google Scholar]
- Maynard‐Smith, J. & Szathmary, L. (1995). The Major Transitions in Evolution. Oxford University Press, Oxford, UK. 360 pp. [Google Scholar]
- McClure, M. , Ralph, M. & Despland, E. (2011). Group leadership depends on energetic state in a nomadic collective foraging caterpillar. Behav. Ecol. Sociobiol., 65, 1573–1579. [Google Scholar]
- Mersch, D.P. , Crespi, A. & Keller, L. (2013). Tracking individuals shows spatial fidelity is a key regulator of ant social organization. Science, 340, 1090–1093. [DOI] [PubMed] [Google Scholar]
- Nalepa, C.A. , Bignell, D.E. & Bandi, C. (2001). Detritivory, coprophagy, and the evolution of digestive mutualisms in Dictyoptera. Insectes Soc., 48, 194–201. [Google Scholar]
- Nichols, H.J. , Bell, M.B.V. , Hodge, S.J. & Cant, M.A. (2012). Resource limitation moderates the adaptive suppression of subordinate breeding in a cooperatively breeding mangoose. Behav. Ecol., 23, 635–642. [Google Scholar]
- Nie, Y. , Zhang, Z. , Raubenheimer, D. , Elser, J.J. , Wei, W. & Wei, F. (2014). Obligate herbivory in an ancestrally carnivorous lineage: the giant panda and bamboo from the perspective of nutritional geometry. Funct. Ecol. DOI: 10.1111/1365-2435.12302. [DOI] [Google Scholar]
- Paoli, P.P. , Donley, D. , Stabler, S. , Saseendranath, A. , Nicolson, S.W. , Simpson, S.J. et al. (2014). Nutritional balance of essential amino acids and carbohydrates of the adult worker honeybee depends on age. Amino Acids, 46, 1449–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez‐Escudero, A. , Vicente‐Page, J. , Hinz, R.C. , Arganda, S. & De Polavieja, G.G. (2014). idTracker: tracking individuals in a group by automatic identification of unmarked animals. Nat. Meth., 11, 743–748. [DOI] [PubMed] [Google Scholar]
- Povey, S. , Cotter, S.C. , Simpson, S.J. , Lee, K.P. & Wilson, K. (2009). Can the protein costs of bacterial resistance be offset by altered feeding behaviour? J. Anim. Ecol., 78, 437–446. [DOI] [PubMed] [Google Scholar]
- Rands, S.A. , Cowlishaw, G. , Pettifor, R.A. , Rowcliffe, J.M. & Johnstone, R.A. (2003). Spontaneous emergence of leaders and followers in foraging pairs. Nature, 423, 432–434. [DOI] [PubMed] [Google Scholar]
- Raubenheimer, D. & Simpson, S.J. (1993). The geometry of compensatory feeding in the locust. Anim. Behav., 45, 953–964. [Google Scholar]
- Raubenheimer, D. , Simpson, S.J. & Mayntz, D. (2009). Nutrition, ecology and nutritional ecology: toward an integrated framework. Funct. Ecol., 23, 4–16. [Google Scholar]
- Raubenheimer, D. , Simpson, S.J. & Tait, A. (2012). Match and mismatch: conservation physiology, nutritional ecology and the timescales of biological adaptation. Phil. Trans. R. Soc. B, 367, 1628–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, A.M. , Sword, G.A. , Simpson, S.J. & Reynolds, D.R. (2009). Predator percolation, insect outbreaks, and phase polyphenism. Curr. Biol., 19, 20–24. [DOI] [PubMed] [Google Scholar]
- Rosas‐Guerrero, V. , Aguillar, R. , Martén‐Rodriguez, S. , Ashworth, L. , Lopezaraiza‐Mikel, M. , Bastida, J.M. et al. (2014). A quantitative review of pollination syndromes: do floral traits predict effective pollinators? Ecol. Lett., 17, 388–400. [DOI] [PubMed] [Google Scholar]
- Rothman, J.M. , Raubenheimer, D. & Chapman, C.A. (2011). Nutritional geometry: gorillas prioritize non‐protein energy while consuming surplus protein. Biol. Lett., 7, 847–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein, D.I. & Wrangham, R.W. (1986). Ecological Aspects of Social Evolution. Princeton University Press, Princeton, NJ. 564 pp. [Google Scholar]
- Salomon, M. , Mayntz, D. & Lubin, Y. (2008). Colony nutrition skews reproduction in a social spider. Behav. Ecol., 19, 605–611. [Google Scholar]
- Schwander, T. , Lo, N. , Beekman, M. , Oldroyd, B.P. & Keller, L. (2010). Nature versus nurture in social insect caste differentiation. Trends Ecol. Evol., 25, 275–282. [DOI] [PubMed] [Google Scholar]
- Seeley, T.D. (1995). The Wisdom of the Hive: the Social Physiology of Honey Bee Colonies. Harvard University Press, Cambridge MA. 310 pp. [Google Scholar]
- Seeley, T.D. , Camazine, S. & Sneyd, J. (1991). Collective decision‐making in honey bees: how colonies choose among nectar sources. Behav. Ecol. Sociobiol., 28, 277–290. [Google Scholar]
- Seeley, T.D. , Visscher, P.K. , Schlegel, T. , Hogan, P.M. , Franks, N.R. & Marshall, J.A.R. (2012). Stop signals provide cross inhibition in collective decision‐making by honeybee swarms. Science, 335, 109–111. [DOI] [PubMed] [Google Scholar]
- Simpson, S.J. & Raubenheimer, D. (2012). The Nature of Nutrition: A Unifying Framework from Animal Adaptation to Human Obesity. Princeton University Press, Princeton, NJ. 239 pp. [Google Scholar]
- Simpson, S.J. & Sword, G.A. (2008). Locusts. Curr. Biol., 18, R364–R366. [DOI] [PubMed] [Google Scholar]
- Simpson, S.J. , Sibly, R.M. , Lee, K.P. , Behmer, S.T. & Raubenheimer, D. (2004). Optimal foraging when regulating intake of multiple nutrients. Anim. Behav., 68, 1299–1311. [Google Scholar]
- Simpson, S.J. , Sword, G.A. , Lorch, P.D. & Couzin, I.D. (2006). Cannibal crickets on a forced march for protein and salt. Proc. Natl Acad. Sci. USA, 103, 4152–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, S.J. , Clissold, F.J. , Lihoreau, M. , Ponton, F. , Wilder, S.M. & Raubenheimer, D. (2015). Recent advances in the integrative nutrition of arthropods. Annu. Rev. Entomol., doi: 10.1146/annurev-ento-010814-02091703, 4152‐4156. [DOI] [PubMed] [Google Scholar]
- Smith, C.R. & Suarez, A.V. (2010). The trophic ecology of castes in harvester ant colonies. Funct. Ecol., 24, 122–130. [Google Scholar]
- Sokolowski, M.B. (2010). Social interactions in ‘simple’ model systems. Neuron, 65, 780–794. [DOI] [PubMed] [Google Scholar]
- Solon‐Biet, S.M. , McMahon, A.C. , Ballard, J.W.O. , Ruohonen, K. , Wu, L.E. , Cogger, V.C. et al. (2014). The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum‐fed mice. Cell Metab., 19, 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter, D.J.T. (2010). Collective Animal Behaviour. Princeton University Press, Princeton, NJ. 302 pp. [Google Scholar]
- Sumpter, D.J.T. & Beekman, M. (2003). From nonlinearity to optimality: pheromone trail foraging by ants. Anim. Behav., 66, 273–280. [Google Scholar]
- Székely, T. , Moore, A.J. & Komdeur, J. (2010). Social Behaviour: Genes, Ecology and Evolution. Cambridge University Press, Cambridge, UK. 576 pp. [Google Scholar]
- Viswanathan, G.M. , Buldyrev, S.V. , Havlin, S. , Da Luz, M.G.E. , Raposo, E.P. & Stanley, H.E. (1999). Optimizing the success of random searches. Nature, 401, 911–914. [DOI] [PubMed] [Google Scholar]
- Ward, A.J.W. , Sumpter, D.J.T. , Couzin, I.D. , Hart, P.J.B. & Krause, J. (2008). Quorum decision‐making facilitates information transfer in fish shoals. Proc. Natl Acad. Sci. USA, 105, 6948–6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West‐Eberrhard, M.J. (2003). Developmental Plasticity and Evolution. Oxford University Press, New York, NY. 816 pp. [Google Scholar]
- Wheeler, W.M. (1928). The Social Insects, Their Origin and Evolution. Harcourt, New‐York, NY. 378 pp. [Google Scholar]
- Wheeler, D.E. (1986). Developmental and physiological determinants of caste in social Hymenoptera ‐ evolutionary implications. Am. Nat., 128, 13–34. [Google Scholar]
- Wilson, E.O. (1975). Sociobiology: The New Synthesis. Harvard University Press, Cambridge, MA. 720 pp. [Google Scholar]
- Wong, R. , Piper, M.D.W. , Wertheim, B. & Partridge, L. (2009). Quantification of food intake in Drosophila . PLoS ONE, 4, e6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


