Abstract
Purpose
We investigated the molecular types of uropathogenic Escherichia coli (UPEC) by using conventional phylogrouping, multilocus sequence typing (MLST), and fimH genotyping.
Methods
Samples of patients younger than 18 years of age were collected from the Chung-Ang University Hospital over 2 years. Conventional phylogenetic grouping for UPEC strains was performed by polymerase chain reaction (PCR). Bacterial strain sequence types (STs) were classified on the basis of the results of partial sequencing of seven housekeeping genes. In addition, we analyzed nucleotide variations in a 424-base pair fragment of fimH, a major virulence factor in UPEC.
Results
Sixty-four UPEC isolates were analyzed in this study. Phylogenetic grouping revealed that group B2 was the most common type (n=54, 84%). We identified 16 distinctive STs using MLST. The most common STs were ST95 (35.9%), ST73 (15.6%), ST131 (12.5%), ST69 (7.8%), and ST14 (6.3%). Fourteen fimH allele types were identified, of which 11 had been previously reported, and the remaining three were identified in this study. f1 (n=28, 45.2%) was found to be the most common allele type, followed by f6 and f9 (n=7, 11.3% each). Comparative analysis of the results from the three different molecular typing techniques revealed that both MLST and fimH typing generated more discriminatory UPEC types than did PCR-based phylogrouping.
Conclusion
We characterized UPEC molecular types isolated from Korean children by MLST and fimH genotyping. fimH genotyping might serve as a useful molecular test for large epidemiologic studies of UPEC isolates.
Keywords: Uropathogenic Escherichia coli, Phylogeny, Multilocus sequence typing, fimH protein
Introduction
Urinary tract infection (UTI) is the most common bacterial infection during childhood; many affected infants have severe symptoms and lobar nephronia. Kidney scarring related to UTI has been linked to long-term morbidity1,2). Uropathogenic Escherichia coli (UPEC) is the most common causative agent of UTI in children. Extended-spectrum beta-lactamase (ESBL)-producing UPEC strains have an appreciable deleterious impact on the clinical management of UTIs3,4). Virulence factors, including ESBL, seem to be correlated with certain UPEC strains. Knowledge of the distinguishing characteristics of various UPEC strains could conceivably lead to the development of strategies for the prevention and management of UTI in children; therefore, the molecular epidemiology of UPEC strains has been the subject of intensive research worldwide5,6).
In 2000, Clermont et al.7) described a triplex polymerase chain reaction (PCR) strategy for the rapid assignment of Escherichia coli isolates to one of four phylogroups: A, B1, B2, and D; this technique has been used extensively. Multilocus sequence typing (MLST) is a standard molecular subtyping technique that may be used to determine the genetic relatedness between strains and identify a certain strain with high discriminatory power for various bacterial pathogens. This method has been standardized and is now widely used for the identification and classification of UPEC8,9). Furthermore, the digitalization of MLST data has facilitated the establishment of global, web-accessible databases for a variety of organisms and is rapidly contributing to our understanding of the clonal distribution of infectious disease agents. Single nucleotide polymorphisms (SNPs) occur in several different MLST loci and represent neutral genetic variation; they presumably exhibit minimal autocorrelation, and could potentially confound epidemiologic conclusions. On the other hand, a genotyping method for fimH, which encodes the P-fimbriae adhesion protein, was suggested as a simple screening test for epidemiological studies of UPEC and was used to evaluate a large population of E. coli isolates10,11).
In this study, we aimed to identify the epidemiologic features of UPEC isolated from Korean children using the three different molecular typing methods that are widely used for E. coli identification.
Materials and methods
1. Subjects and UPEC isolates
Sixty-four UPEC isolates were obtained from children younger than 18 years of age, admitted to the Chung-Ang University Hospital between March 2011 and February 2013. All subjects were diagnosed with community-acquired UTI. Urine samples were collected from midstream urine in toilet-trained children and by catheterization in others. Clinical data were obtained from the medical records of the patients and entered into standardized case report forms. All UPEC isolates were identified by typical morphology, lactose fermentation, positive spot indole test, and by using VITEK 2-GN cards (bioMérieux, Hazelwood, MO, USA). Only one isolate per patient was examined. The isolates were stored at -80℃ until further use.
2. Antimicrobial susceptibility test
Antimicrobial susceptibility testing of all isolates to ampicillin, gentamicin, piperacillin, trimethoprim/sulfamethoxazole, tetracycline, amoxicillin/clavulanic acid, cefazolin, aztreonam, ciprofloxacin, cefepime, cefotaxime, tobramycin, piperacillin/tazobactam, and levofloxacin was performed using a VITEK 2 automated system (bioMérieux). In addition, each isolate was tested using a VITEK 2 system with the ESBL test panel (bioMérieux) contained in the NO45 card (bioMérieux). In vitro antimicrobial susceptibility testing was performed by the broth microdilution method and the results were interpreted using the 2010 Clinical Laboratory Standard Institute breakpoints. Isolates resistant to antibiotics of three or more different classes were classified as multidrug-resistant (MDR).
3. Conventional phylogenetic grouping
Extraction and purification of bacterial whole-cell DNA from UPEC colonies were performed as described in the instruction manual of the QIAamp Kit (QIAGEN GmbH, Hilden, Germany). We modified the most recent version of the phylogenetic grouping method for UPEC12), and performed multiple PCRs for the gadA, chuA, and yjaA genes and the TSPE4.C2 DNA fragment. All primers used in this study are listed in Table 1.
Table 1. Primer sequences used for genotyping of uropathogenic Escherichia coli isolates.
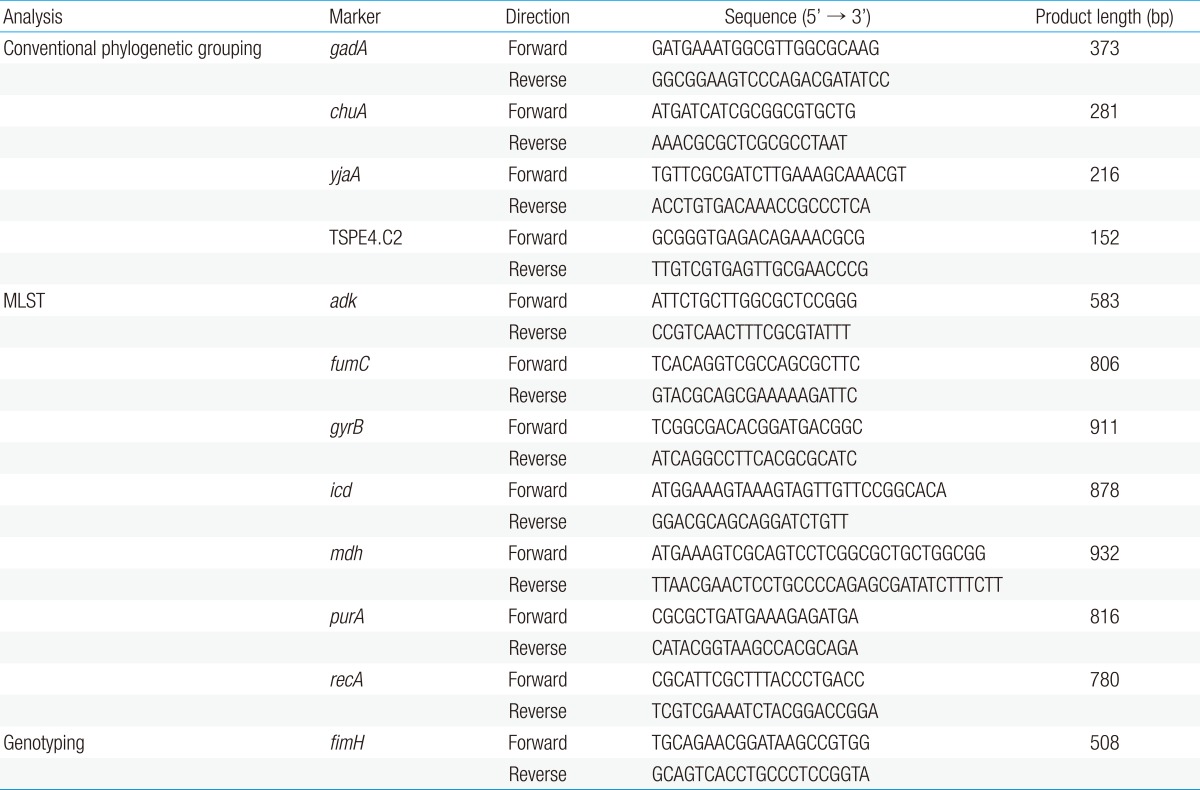
MLST, multilocus sequence typing.
The PCRs were performed in 20-µL reaction volumes, with each reaction mixture containing 3.0 µL of 10× Tris-HCl buffer (100mM, pH 8.3, Mg2+-free), 1.4 µL of 2.5mM dNTPs, 1.4 µL of MgCl2, 0.2 µL of 5.0 U/µL Taq DNA polymerase (Takara Bio Inc., Otsu, Japan), 0.5 µL of each primer, and distilled water to a final volume of 17 µL. Finally, 3.0 µL of the DNA extract from the UPEC isolates was added to each reaction mixture. Thermal cycling was performed in a PTC-200 Peltier Thermal Cycler DNA engine (MJ Research, Watertown, MA, USA) under the following conditions: denaturation for 5 minutes at 95℃; 35 amplification cycles of 1 minute at 95℃, 1 minute at 58℃, and 2 minutes at 72℃; and a final extension of 10 minutes at 72℃. PCR products were electrophoresed on agarose gels and photographed under ultraviolet trans-illumination. We could identify the 373-, 281-, 216-, and 158-base pair (bp) bands for gadA, chuA, yjaA, and TSPE4.C2, respectively.
On the basis of the results of the PCRs, chuA- TSPE4.C2-, chuA- TSPE4.C2+, chuA+ yjaA-, and chuA+ yjaA+ strains were classified as belonging to groups A, B1, D, and B2, respectively. The glutamate decarboxylase-alpha gadA gene of E. coli was used as an internal amplification control.
4. Multilocus sequence typing
MLST was performed on all the UPEC isolates (n=64) using a previously standardized MLST protocol for E. coli8). The scheme used the following seven housekeeping genes: adk (adenylate kinase), fumC (fumarate hydratase), gyrB (DNA gyrase), icd (isocitrate dehydrogenase), mdh (malate dehydrogenase), purA (adenylosuccinate synthetase), and recA (adenosine triphosphate/guanosine triphosphate binding motif). PCR amplification for each gene was performed using the same reaction mixture and conditions described for the phylogenetic grouping in the previous section. The primer sequences used for the MLST are listed in Table 1.
The sequencing reactions were carried out using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems Inc., Foster City, CA, USA) with each forward primer. The reaction products were separated and detected using an Applied Biosystems 3730xl automated sequencer (Applied Biosystems Inc.), and the data were analyzed using the Sequencing analysis v3.3 software (Applied Biosystems Inc.). The allelic profile was summarized by assigning a sequence type (ST) via a web database (http://mlst.ucc.ie/mlst/dbs/Ecoli). The eBURST v3 software (available at http://www.mlst.net) was used to estimate the relationships between the isolates and to assign the strains to a clonal complex (CC)13).
5. fimH genotyping
All isolates were tested for the presence of the fimH gene as described previously14). The primers were designed to amplify a 508-bp fragment of the 903-bp fimH gene (Table 1). The PCR and sequencing procedures were performed as described for the MLST analysis. Forward and reverse PCR primers were used for the sequencing of the fimH genes. Within the 508-bp amplicon, the region between nucleotides 401 and 824 (424-bp) of the fimH gene was selected for genotyping, as described previously11). The fimH sequence of the K12 strain (GenBank accession number GQ487190) was used as the reference sequence in this study. These data were compared to fimH allele types determined previously, and the newly identified allele types were numbered in order. The fimH gene partial sequences were analyzed and deposited in the GenBank database under accession numbers KJ190185 to KJ190246.
For the phylogenetic analysis of the fimH gene, the sequences of all known fimH allele types and those identified in this study were aligned and subjected to neighbor-joining analysis. All evolutionary trees were drawn using MEGA515).
Results
1. Subject characteristics
The median age of the subjects was 5.3 months (range, 1.1-159.4 months) and the ratio of males to females was 1.7:1. Among the 64 subjects, 57 (89.1%) had a fever and 10 (15.6%) had at least one of the following urologic symptoms: dysuria, frequency, and flank pain. All subjects were tested with ultrasonography (US) and 99mTc-dimercaptosuccinic acid (DMSA) scans. Eight subjects were further evaluated by voiding cystourethrography (VCUG). Radiologic abnormality was detected in 17 (26.6%), 16 (25.0%), and 3 (4.7%) subjects by US, DMSA scan, and VCUG testing, respectively.
2. Antimicrobial susceptibility
The susceptibility rates of UPEC isolates for all antimicrobial agents tested were as follows: piperacillin, 20.7%; ampicillin, 39.1%; trimethoprim-sulfamethoxazole, 64.1%; tobramycin, 65.5%; gentamycin, 73.4%; amoxicillin-clavulanate, 84.4%; ciprofloxacin, 85.7%; levofloxacin, 86.2%; aztreonam, 90.6%; cefepime, 90.6%; ceftazidime, 92.2%; cefotaxime, 92.2%; piperacillin-tazobactam, 97.1%; amikacin, 100%; ertapenem, 100%; meropenem, 100%; imipenem, 100%; and tigecycline, 100%. A total of 21 isolates (32.8%) were susceptible to all antibiotics, while 14 isolates (21.9%) were classified as MDR pathogens, which included 5 (7.8%) ESBL-producing isolates.
3. UPEC typing based on three different molecular methods
In the PCR-based phylogrouping method, the gadA gene was amplified in all the UPEC isolates. On the basis of the classification guidelines suggested previously7), we assigned a phylogenetic group to each isolate by analyzing the PCR amplification patterns of the chuA, yjaA, and TSPE4.C2 genes (data not shown). Group B2 was the most common at 84% (n=54), followed by groups D (11%, n=7), A (3%, n=2), and B1 (2%, n=1). All isolates could be classified on the basis of this conventional phylogenetic grouping.
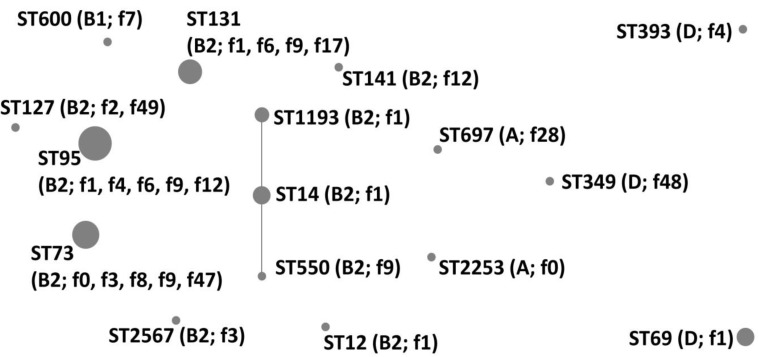
The MLST analysis identified 16 unique STs among the 64 UPEC isolates: ST95 (n=23), ST73 (n=10), ST131 (n=8), ST69 (n=5), ST14 (n=4), ST1193 (n=3), ST127 (n=2), ST12 (n=1), ST141 (n=1), ST349 (n=1), ST393 (n=1), ST550 (n=1), ST600 (n=1), ST697 (n=1), ST2253 (n=1), and ST2567 (n=1) (Fig. 1). ST550 and ST1193 were single-locus variants of ST14 (CC14).
Fig. 1. eBURST analysis of 64 uropathogenic Escherichia coli isolates. Circle size correlates with the number of isolates for each sequence type (ST). The lengths and characteristics of the lines connecting the circles correspond to the relationship between the STs. Phylogroups and fimH allele types included in each ST are indicated in parentheses.
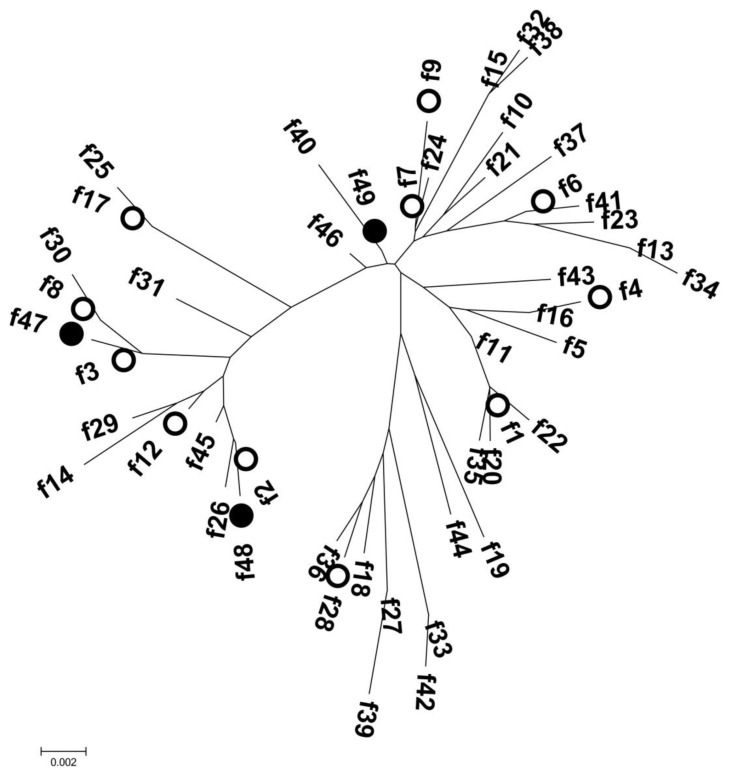
The fimH gene was detected in 62 isolates (96.9%), and 14 allele types were identified by analyzing nucleotide differences in the targeted region (Table 2). The fimH sequences of 58 UPEC isolates corresponded to fimH allele types that had been reported previously; however, four fimH sequences were identified for the first time in this study and were assigned as f47 (n=2), f48 (n=1), and f49 (n=1). These three allele types were separated from each other in the phylogenetic tree (Fig. 2). Alleles f47 and f48 shared common ancestors with allele types identified in this study (f3 and f2, respectively). Although allele f49 had maximum sequence similarity with allele f7 (identified in this study), the two did not share a common ancestor. Among the fimH allele types identified in this study, the f1 type was most common (n=28, 45.2%), followed by the f6 and f9 types (n=7, 11.3%; each). Allele types f2 (n=1), f3 (n=2), f4 (n=2), f7 (n=1), f8 (n=4), f12 (n=3), f17 (n=2), and f28 (n=1) were also identified.
Table 2. Allele types and their corresponding variations in fimH nucleotide sequences.
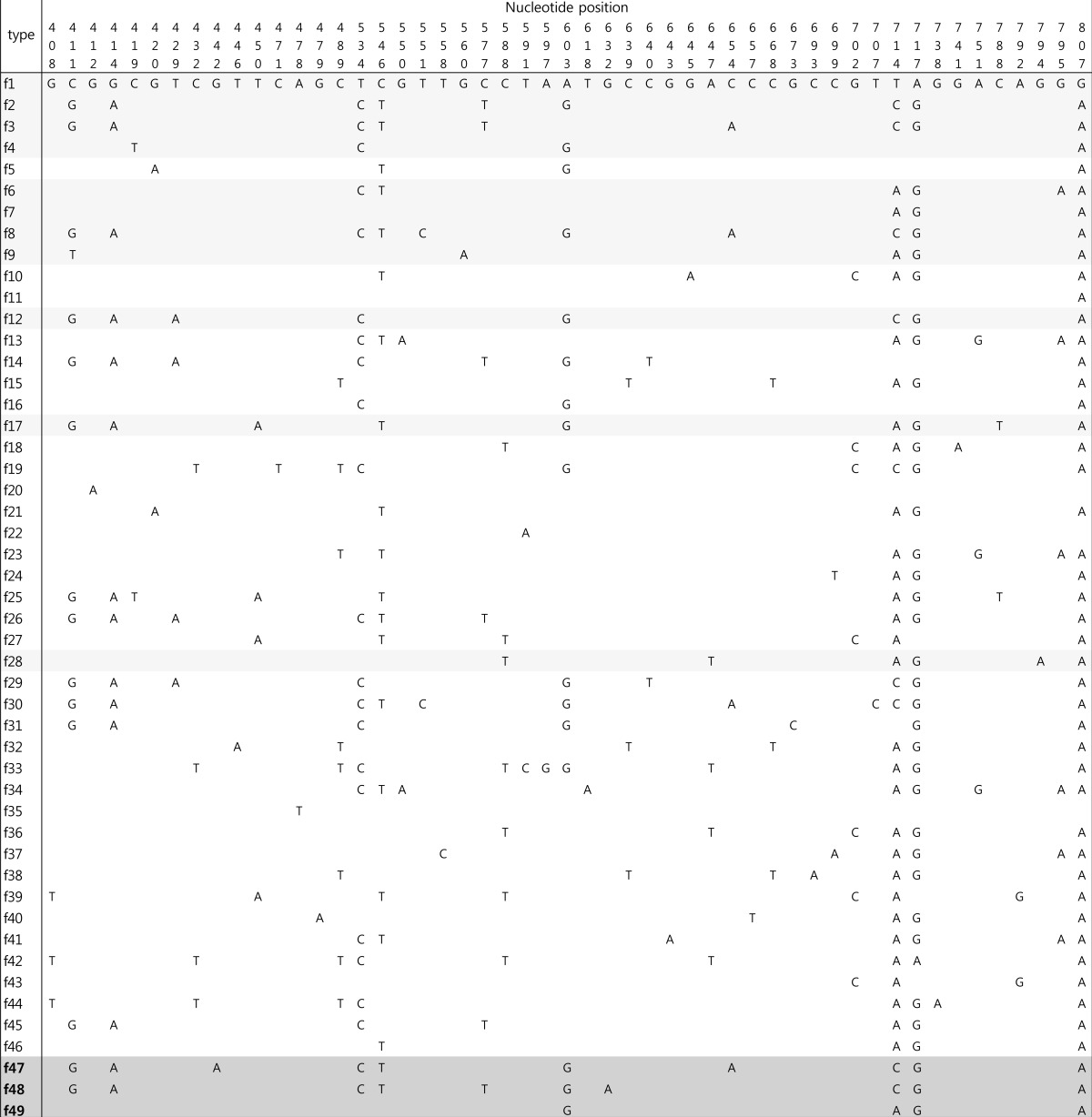
The fimH alleles observed in this study are indicated by tinted rows. Novel alleles are highlighted in dark grey, while the previously identified alleles are underscored in light grey.
Fig. 2. Phylogenetic tree derived from all fimH sequence variants, including those established in the 62 clinical uropathogenic Escherichia coli isolates from this study (denoted by circles; ○ or ●). Three novel variants (f46, f47, and f48) were identified in this study (denoted by closed circles; ●).
4. Comparison of UPEC strains classified on the basis of molecular typing
Phylogroups A, B1, and D were divided into two (ST697 and ST2253), one (ST600), and three (ST69, ST349, and ST393) STs, respectively (Fig. 2, Table 3). Each of the six STs (ST69, ST349, ST393, ST600, ST697, and ST2253) could be matched to a fimH type (f1, f48, f4, f7, f28, and f0, respectively). There were 10 STs and 12 fimH types in phylogroup B2. Among them, ST12, ST141, ST550, and ST2567 matched to a fimH type (f1, f12, f9, and f3, respectively). On the other hand, ST73 and ST95 were divided into five fimH types each, and ST131 was divided into four fimH types. The B2-ST95-f1 genotype was the most commonly identified genotype in this study (n=14, 21.9%).
Table 3. Comparison of results of the three different molecular typing techniques and antimicrobial susceptibility testing of uropathogenic Escherichia coli isolates.
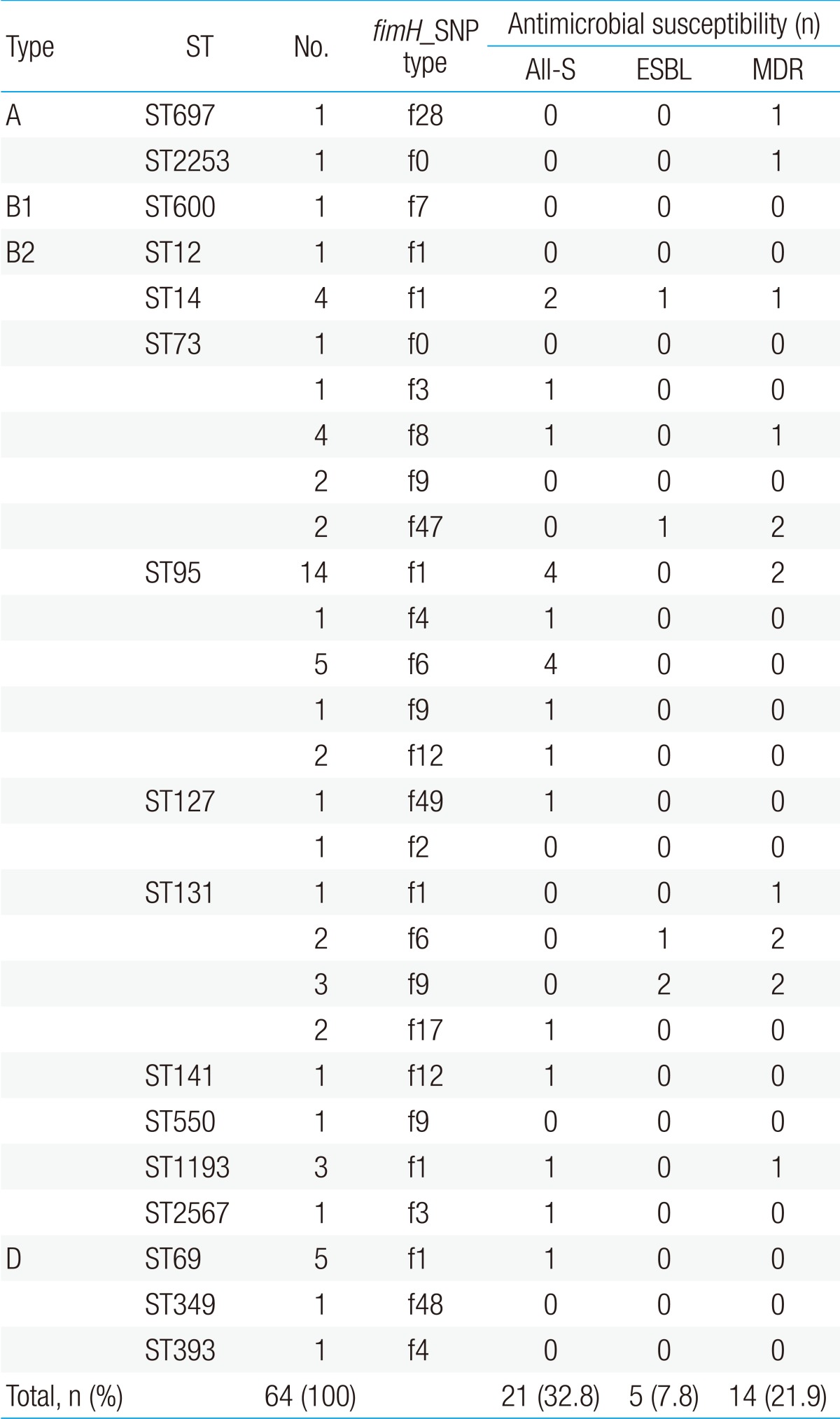
ST, sequence type; SNP, single nucleotide polymorphism; All-S, all susceptible; ESBL, extended spectrum beta-lactamase; MDR, multidrug resistant.
The number of isolates with ESBL production and MDR pattern were low (0/23 [0%] and 2/23 [8.7%], respectively); the number of all-susceptible isolates was highest (11/23 [47.8%]) in ST95. However, a higher number of ST131 and ST73 isolates showed ESBL production (3/8 [37.5%] and 1/10 [10.0%], respectively) and MDR (5/8 [62.5%] and 3/10 [30.0%], respectively). The number of ST131 (12.5%, n=1) and ST73 (20%, n=2) isolates susceptible to all antibiotics tested was lower, relative to the total number of isolates (Table 3). Specifically, the ST131 strains were isolated mostly from infants younger than 12-months old (7/8, 87.5%), in whom the only symptom was fever, in the absence of any urologic symptoms. Among the subjects infected with the ST131 strains, radiologic abnormality was detected in four (50.0%), three (37.5%), and one subjects (12.5%) by US, DMSA scan, and VCUG testing, respectively. One subject had abnormalities in both US and DMSA scan and one subject had abnormalities in all three radiologic testing. Any radiologic abnormality was more frequently detected in UTI patients infected with ST131 strains (5/8, 62.5%) than in those infected with other strains (22/56, 39.3%), but statistically not significant (P=0.51).
Discussion
This study investigated the UPEC molecular types using three genotypic methods: PCR-based conventional phylogrouping, MLST, and fimH genotyping. The B2 phylogroup, ST95, and the f1 allele type were most commonly identified using the individual methods, respectively; three new fimH allele types were identified for the first time. To the best of our knowledge, this was the first study to determine the molecular epidemiologic data for UPEC clinical isolates of pediatric UTIs using MLST and fimH genotyping.
Approximately 7% of girls and 2% of boys have a UTI during the first eight years of life. Febrile UTI occurs most frequently during the first year of life in both sexes, and is associated with a high probability of kidney involvement and consequent renal scarring. Antibiotic administration is the cornerstone of treatment for acute UTIs and is important for the prevention of parenchymal localization of the infection1).
Certain bacteria have virulence factors that favor the establishment of infections like UTIs. In particular, UPEC is the most common pathogen causing UTIs in children; UPEC have various virulence factors that render them more competent for survival in the human urinary tract. These virulence factors are frequently confined to distinct UPEC strains, and are more likely to be changed or modified under selective pressure. Thus, genotypic characterization of pathogens and their virulence factors has become an important objective in the epidemiologic investigation of infectious agents. In addition, genotyping tests have enhanced our understanding of the epidemiology of UTIs caused by UPEC and have helped characterize the modes of transmission, sources, and risk factors for infection5,6,11).
Sequence-based genotyping methods are becoming increasingly popular in epidemiological studies of infectious diseases such as UTIs. However, so far, only the conventional phylogrouping method have been used for analyzing clinical UPEC isolates from children with UTI in Korea16,17).
Phylogenetic grouping analysis has shown that E. coli isolates fall into four main phylogenetic groups-A, B1, B2, and D7). The phylogenetic strategy involved the use of three phylogenetic group markers, the chuA and yjaA genes that encode hypothetical proteins and the TSPE4.C2 DNA sequences that are situated within a gene that encodes a putative lipase esterase. The groups were assigned on the basis of different combinations of the presence and/or absence of these three amplicons. Recently, using data from whole-genome projects, an updated multiplex PCR strategy was developed for the rapid assignment of E. coli isolates to major phylogenetic groups12).
This phylogrouping strategy has been used extensively worldwide. In many studies, phylogroup B2 has been shown to be the most prevalent UPEC isolate in pediatric UTIs. For example, in a study involving Korean children, phylogroup B2 (68.1%) was found to be the most common, followed by phylogroups B1 and D (12.8% each)17). In addition, a recent study on Korean children with UTIs revealed that B2 (61.6%) and D (26.8%) comprised the majority of all isolated strains17). The B2 phylogroup was the most common (84%) type and the B2 and D phylogroups comprised the majority (95%) of the UPEC isolates in our study as well.
Nevertheless, we encountered inconsistent PCR amplification fidelities or low amplification efficiencies that led to some isolates being assigned to seemingly anomalous phylogroups. Another study has also reported unusual PCR profiles and incongruence between MLST results and the phylogenetic group assigned for some isolates12). Therefore, a more reliable, relevant, and consistent typing method using sequence analysis, such as MLST, is required for epidemiologic investigations.
MLST was developed as an attractive sequence-based genotyping technique because it provides reproducibility, comparability, and transferability between laboratories. Therefore, MLST is a powerful tool for the global and long-term surveillance of pathogenic bacteria8). In other studies on UPEC isolate using MLST data, ST14, ST69, ST73, ST95, and ST131 were the main STs identified8,9,18). We identified all these STs in our isolates as well; these were the five most common STs that comprised 78% of the isolates. In particular, ST131 is known to be a major ESBL-producing MDR pathogen19,20); we identified eight UPEC isolates of ST131 in this study. Also in this study, ST131 strains were more resistant to antimicrobials and, furthermore, related to radiologic abnormalities. However, further analysis with large amount of the strains will be needed to confirm these findings statistically.
Although MLST appears to be an excellent technique for sequence-based genotyping, it is still impractical for large-scale epidemiological studies. In addition, as mentioned above, MLST is based on sequencing housekeeping genes, which are under stabilizing selection. Therefore, this system may not be optimal for distinguishing closely related strains10). On the other hand, virulence factors are frequently under selective pressure driven by host innate and acquired immunity. Therefore, genes encoding such factors are more likely to undergo mutations over a shorter period than the housekeeping genes10). In previous studies, fimH SNP analysis has been explored as a possible screening tool for the sequence-based typing of UPEC10,11).
FimH is a mannose-binding subunit protein located at the tip of type 1 fimbriae. Phenotypic variants of FimH are predominantly the products of SNPs in the fimH gene. FimH is a critical determinant of tropism in the urinary tract and vaginal epithelium for extraintestinal E. coli. The urovirulent phenotype is associated with genetic variants of this protein and, therefore, might be clinically relevant. A vast majority of both intestinal and extraintestinal E. coli strains express type 1 fimbriae. Previous studies have shown that the fimH gene is the most commonly expressed virulence gene in UPEC5,6,14). Furthermore, many fimH sequences from a variety of E. coli isolates have been deposited in the nucleic acid sequence database for comparison. The fimH gene is known to be under strong selective pressure and is thus likely to show a high degree of sequence heterogeneity. It might be able to discriminate strains beyond those discriminated by the standardized MLST procedure. A previous study revealed that the two techniques (MLST and fimH SNP analysis) showed similar discriminatory powers, and in some of the strains, fimH SNP analysis provided more information than MLST10).
In this study, analysis of the fimH gene identified 11 allele types of 46 known sequences and three additional allele types. Allele type f1, which corresponded to the sequence of the K12 reference strain, was most the commonly identified (45.2%) type and was distributed through the isolates with various STs (ST12, ST14, ST69, ST95, ST1313, and ST1193).
A comparison of the results of the three molecular typing methods revealed that MLST and fimH typing provided more discriminatory results than phylogenetic phenotyping. Isolates in ST73 and ST131 could be divided into several fimH allele types and, showed a more resistant antimicrobial susceptibility pattern. We postulated that they were under greater selective pressure in the host and in the environment. Although ST95 could be divided into five fimH types, it showed a less resistant antimicrobial susceptibility pattern; this observation could not be explained by the results obtained in this study.
This study was limited by including a relatively small number of isolates from a single tertiary-care hospital. Therefore, the results of this study are not representative of all UPEC isolates in Korean children. In addition, we analyzed only one virulence gene (fimH) and did not focus on the clinical aspects of UPEC strains. However, we identified new molecular epidemiologic data for UPEC that is especially valuable because this is the first example of the application of these methods for the analysis of clinical UPEC isolates from Korean children. We expect to conduct other molecular epidemiologic studies using these comprehensive and reliable methods in the future.
To the best of our knowledge, this was the first study that involved an MLST analysis of clinical UPEC isolates from pediatric UTIs in Korea; the results have improved upon the conventional phylogenetic groupings. This study was also the first to apply fimH genotyping to UPEC isolates in Korea, and led to the identification of three new allele types. The fimH genotyping method might serve as a relatively simple, sequence-based screening test that could be applied to a large number of UPEC isolates for the characterization of recent epidemiological events.
Acknowledgment
This study was supported by a 2012 research grant from the Korean Pediatric Society (MSD Award).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Montini G, Tullus K, Hewitt I. Febrile urinary tract infections in children. N Engl J Med. 2011;365:239–250. doi: 10.1056/NEJMra1007755. [DOI] [PubMed] [Google Scholar]
- 2.Park YS. Renal scar formation after urinary tract infection in children. Korean J Pediatr. 2012;55:367–370. doi: 10.3345/kjp.2012.55.10.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 4.Dayan N, Dabbah H, Weissman I, Aga I, Even L, Glikman D. Urinary tract infections caused by community-acquired extended-spectrum β-lactamase-producing and nonproducing bacteria: a comparative study. J Pediatr. 2013;163:1417–1421. doi: 10.1016/j.jpeds.2013.06.078. [DOI] [PubMed] [Google Scholar]
- 5.Ramos NL, Dzung DT, Stopsack K, Janko V, Pourshafie MR, Katouli M, et al. Characterisation of uropathogenic Escherichia coli from children with urinary tract infection in different countries. Eur J Clin Microbiol Infect Dis. 2011;30:1587–1593. doi: 10.1007/s10096-011-1264-4. [DOI] [PubMed] [Google Scholar]
- 6.Kudinha T, Johnson JR, Andrew SD, Kong F, Anderson P, Gilbert GL. Genotypic and phenotypic characterization of Escherichia coli isolates from children with urinary tract infection and from healthy carriers. Pediatr Infect Dis J. 2013;32:543–548. doi: 10.1097/INF.0b013e31828ba3f1. [DOI] [PubMed] [Google Scholar]
- 7.Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000;66:4555–4558. doi: 10.1128/aem.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tartof SY, Solberg OD, Manges AR, Riley LW. Analysis of a uropathogenic Escherichia coli clonal group by multilocus sequence typing. J Clin Microbiol. 2005;43:5860–5864. doi: 10.1128/JCM.43.12.5860-5864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau SH, Reddy S, Cheesbrough J, Bolton FJ, Willshaw G, Cheasty T, et al. Major uropathogenic Escherichia coli strain isolated in the northwest of England identified by multilocus sequence typing. J Clin Microbiol. 2008;46:1076–1080. doi: 10.1128/JCM.02065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tartof SY, Solberg OD, Riley LW. Genotypic analyses of uropathogenic Escherichia coli based on fimH single nucleotide polymorphisms (SNPs) J Med Microbiol. 2007;56(Pt 10):1363–1369. doi: 10.1099/jmm.0.47262-0. [DOI] [PubMed] [Google Scholar]
- 11.Dias RC, Moreira BM, Riley LW. Use of fimH single-nucleotide polymorphisms for strain typing of clinical isolates of Escherichia coli for epidemiologic investigation. J Clin Microbiol. 2010;48:483–488. doi: 10.1128/JCM.01858-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doumith M, Day MJ, Hope R, Wain J, Woodford N. Improved multiplex PCR strategy for rapid assignment of the four major Escherichia coli phylogenetic groups. J Clin Microbiol. 2012;50:3108–3110. doi: 10.1128/JCM.01468-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol. 2004;186:1518–1530. doi: 10.1128/JB.186.5.1518-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yun KW, Kim HY, Park HK, Kim W, Lim IS. Virulence factors of uropathogenic Escherichia coli of urinary tract infections and asymptomatic bacteriuria in children. J Microbiol Immunol Infect. 2013 Sep 21; doi: 10.1016/j.jmii.2013.07.010. [Epub]. http://dx.doi.org/10.1016/j.jmii.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JE, Lee YH, Nam CH, Kwak GY, Lee SY, Kim JH, et al. Clinical and phylogenetic characteristics of Escherichia coli urinary tract infections. Korean J Pediatr Infect Dis. 2010;17:16–22. [Google Scholar]
- 17.Choi UY, Han SB, Lee SY, Kang JH, Kim SM, Ma SH. Regional differences in phylogenetic group of Escherichia coli strains isolated from children with urinary tract infection in Korea. Korean J Pediatr. 2012;55:420–423. doi: 10.3345/kjp.2012.55.11.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, et al. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol. 2006;60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Totsika M, Beatson SA, Sarkar S, Phan MD, Petty NK, Bachmann N, et al. Insights into a multidrug resistant Escherichia coli pathogen of the globally disseminated ST131 lineage: genome analysis and virulence mechanisms. PLoS One. 2011;6:e26578. doi: 10.1371/journal.pone.0026578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavigne JP, Vergunst AC, Goret L, Sotto A, Combescure C, Blanco J, et al. Virulence potential and genomic mapping of the worldwide clone Escherichia coli ST131. PLoS One. 2012;7:e34294. doi: 10.1371/journal.pone.0034294. [DOI] [PMC free article] [PubMed] [Google Scholar]