Abstract
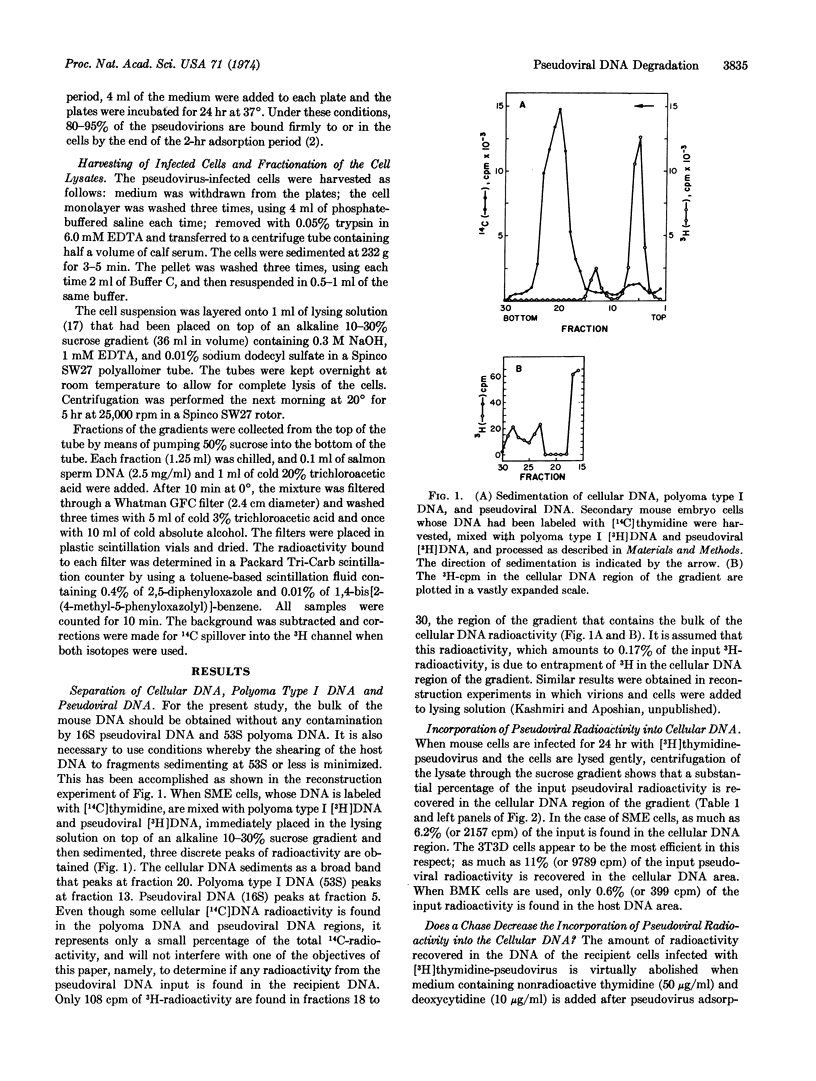
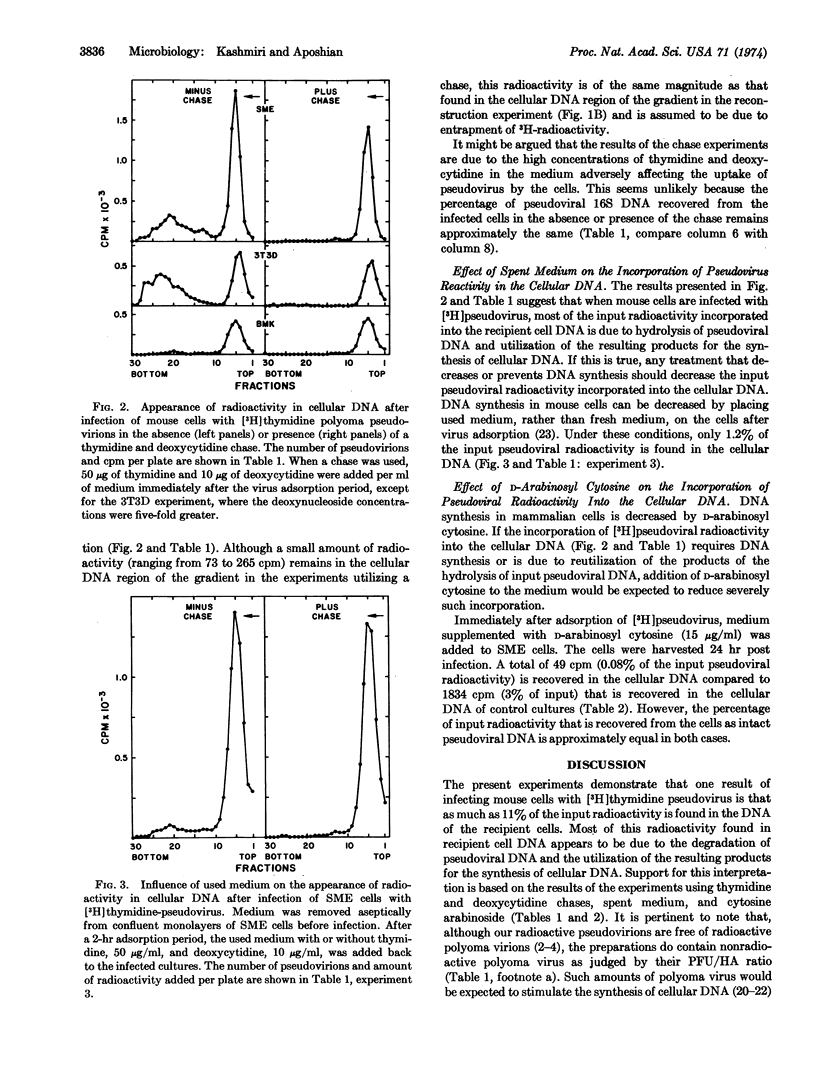
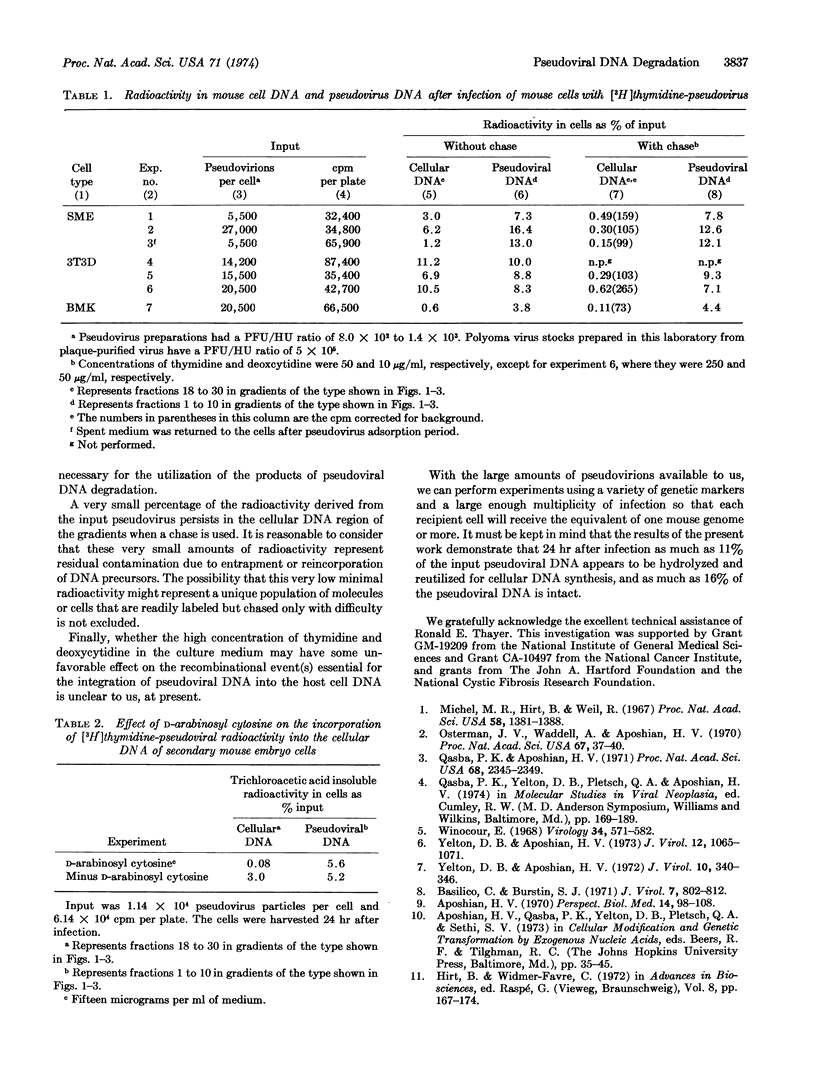
When mouse cells are infected with [3H]-thymidine-labeled polyoma pseudovirions, as much as 11% of the input pseudoviral radioactivity is found in the recipient cell DNA. However, the radioactivity found in the recipient cell DNA virtually disappears when nonradioactive thymidine and deoxycytidine, or D-arabinosyl cytosine, are added to the medium. The radioactivity found in the recipient cell DNA appears to be the result of the breakdown of pseudoviral DNA to precursors of DNA and the utilization of these precursors for the synthesis of cell DNA.
Keywords: mouse embryo cells, mouse kidney cells, 3T3D cells
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aposhian H. V., Qasba P. K., Yelton D. B., Pletsch Q. A., Sethi V. S. The polyoma pseudovirion--its properties, production, and use in transferring DNA to mouse and human cells. Johns Hopkins Med J Suppl. 1973;2:35–45. [PubMed] [Google Scholar]
- Aposhian H. V. The use of DNA for gene therapy--the need, experimental approach, and implications. Perspect Biol Med. 1970 Autumn;14(1):98–108. doi: 10.1353/pbm.1970.0011. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., Hudson J. B. 'Integration' of polyoma virus DNA into mammalian genomes. Biochem Biophys Res Commun. 1972 Apr 14;47(1):111–118. doi: 10.1016/s0006-291x(72)80017-2. [DOI] [PubMed] [Google Scholar]
- Basilico C., Burstin S. J. Multiplication of polyoma virus in mouse-hamster somatic hybrids: a hybrid cell line which produces viral particles containing predominantly host deoxyribonucleic acid. J Virol. 1971 Jun;7(6):802–812. doi: 10.1128/jvi.7.6.802-812.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD L. V., CRAWFORD E. M., WATSONDH The physical characteristics of polyoma virus. I. Two types of particle. Virology. 1962 Oct;18:170–176. doi: 10.1016/0042-6822(62)90002-8. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., HARTWELL L. H., VOGT M. INDUCTION OF CELLULAR DNA SYNTHESIS BY POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Feb;53:403–410. doi: 10.1073/pnas.53.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel-Tsipis J., Botstein D., Fox M. S. Generalized transduction by phage P22 in Salmonella typhimurium. I. Molecular origin of transducing DNA. J Mol Biol. 1972 Nov 14;71(2):433–448. doi: 10.1016/0022-2836(72)90361-0. [DOI] [PubMed] [Google Scholar]
- Ebel-Tsipis J., Fox M. S., Botstein D. Generalized transduction by bacteriophage P22 in Salmonella typhimurium. II. Mechanism of integration of transducing DNA. J Mol Biol. 1972 Nov 14;71(2):449–469. doi: 10.1016/0022-2836(72)90362-2. [DOI] [PubMed] [Google Scholar]
- Michel M. R., Hirt B., Weil R. Mouse cellular DNA enclosed in polyoma viral capsids (pseudovirions). Proc Natl Acad Sci U S A. 1967 Oct;58(4):1381–1388. doi: 10.1073/pnas.58.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman J. V., Waddell A., Aposhian H. V. DNA and gene therapy: uncoating of polyoma pseudovirus in mouse embryo cells. Proc Natl Acad Sci U S A. 1970 Sep;67(1):37–40. doi: 10.1073/pnas.67.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasba P. K., Aposhian H. V. DNA and gene therapy: transfer of mouse DNA to human and mouse embryonic cells by polyoma pseudovirions. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2345–2349. doi: 10.1073/pnas.68.10.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K., Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965 Dec;66(3):325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]
- Weil R., Michel M. R., Ruschmann G. K. Induction of cellular DNA synthesis by polyoma virus. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1468–1475. doi: 10.1073/pnas.53.6.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E. Further studies on the incorporation of cell DNA into polyoma-related particles. Virology. 1968 Apr;34(4):571–582. doi: 10.1016/0042-6822(68)90078-0. [DOI] [PubMed] [Google Scholar]
- Winocour E. Some aspects of the interaction between polyoma virus and cell DNA. Adv Virus Res. 1969;14:153–200. doi: 10.1016/s0065-3527(08)60559-x. [DOI] [PubMed] [Google Scholar]
- Yelton D. B., Aposhian H. V. Polyoma pseudovirions. I. Sequence of events in primary mouse embryo cells leading to pseudovirus production. J Virol. 1972 Sep;10(3):340–346. doi: 10.1128/jvi.10.3.340-346.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton D. B., Aposhian H. V. Polyoma pseudovirions. II. Influence of host cell on pseudovirus production. J Virol. 1973 Nov;12(5):1065–1071. doi: 10.1128/jvi.12.5.1065-1071.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


