Abstract
Retinol binding-protein 4 (RBP4), a recently identified adipokine and retinol transporter, has been shown to play a causative role in insulin resistance, an underpinning between obesity and colon neoplasia. Yet, the relationship between RPB4 and cancer, including colon neoplasia is largely unexplored. We carried out a cross-sectional study to determine the risk association between RBP4 and colon adenomas. We determined pre-diagnostic serum levels of RBP4 in 626 patients undergoing screening colonoscopies from January 2006 to March 2007. The cases had statistically significant higher levels of RBP4 than the controls (58.5µg/mL ± 38.2 vs. 51.9µg/mL ± 32.5, p=0.03). Multivariate logistic regression model revealed a statistically significant overall association of RBP4 with risk of colon adenoma (OR = 3.10 for each increment of 35µg/mL, CI = 1.15 – 8.66; p = 0.03). Stratified analysis by the median BMI showed that the risk association was largely limited to those with BMI < 27.8 kg/m2. Compared to those in the bottom tertile of RBP4, the ORs for the 2nd and 3rd tertiles were 1.84 (CI = 0.89–3.8) and 2.14 (CI = 1.08–4.23) respectively (p for trend = 0.03); there was little evidence for such an association among those with BMI ≥ 27.8 kg/m2. This is the first study to show colon adenoma risk association with high circulating levels of RBP4. Further study is merited to investigate the mechanism that underlies the RBP4-colon neoplasia link.
Key terms: retinol-binding protein 4, RBP4, colon adenoma, insulin resistance
Dear Editor,
Obesity is now considered as a convincing and probable cause for colorectal neoplasia (World Cancer Research Fund 2011). Insulin resistance is a key mechanism linking obesity to colon carcinogenesis (Giovannucci E 2001). Supporting evidence for this notion includes data from a number of epidemiological studies showing risk association of colon cancer and adenoma with insulin resistance-associated biomarkers, such as insulin, insulin-like growth factors (IGFs), adipokines (e.g., leptin, adiponectin) and inflammatory cytokines (e.g., IL-6, TNF-α) (Giovannucci E 2001; Sandhu MS, Dunger DB, et. al 2002; Ortiz AP, Thompson CL, et. al 2012).
Retinol-binding protein 4 (RBP4), a recently identified adipokine, has been shown to potentially have a causative effect on the development of insulin resistance (Yang Q, Graham TE, et al. 2005; Graham TE, Yang Q, et. al 2006). RBP4 increases expression of the glucose transporter, GLUT4, which facilitates the rate-limiting step in glucose uptake by skeletal muscle and adipocytes. In adipose tissue-specific GLUT4 knockout (adipose-GLUT4−/−) mice, secretion of RBP4 by adipocytes is greatly enhanced. Increased circulating levels of RBP4 suppress phosphoinositide-3-kinase (PI3K) in muscle cells, and in the liver, stimulate the expression of phosphoenolpyruvate carboxykinase (PEPCK), inducing systemic insulin resistance. Treatment of adipose-GLUT4−/− mice with rosiglitazone, an insulin sensitizer, reduced the elevated RBP4 mRNA levels in adipose tissue and completely normalized the serum RBP4 levels (Yang Q, Graham TE, et. al 2005). In humans, subcutaneous adipocyte GLUT4 protein levels correlated positively with the rate of glucose disposal and inversely with serum levels of RBP4 (Graham TE, Yang Q, et. al 2006).
Despite the emerging evidence for a causal link of RBP4 to insulin resistance, no previous study has investigated the relationship between RBP4 and colon neoplasia. Therefore, we carried out a cross-sectional study to examine the hypothesis that high levels of RBP4 are associated with an increased risk of colon adenoma.
Materials and Methods
The study population consisted of 626 patients who were referred for colonoscopy screening at the University Hospitals Health System (UHHS) from January 2006 to March 2007. Out of these, 196 patients who had histologically confirmed adenomatous polyps in the colon were defined as cases, and the remaining 430 patients with negative screening were defined as controls. We excluded those with: 1) a prior diagnosis of any cancer, colorectal adenoma or inflammatory bowel diseases; 2) known family history of Hereditary Non-Polyposis Colon Cancer (HNPCC), Familial Adenomatous Polyps (FAP), or other cancer syndromes; and 3) under the age of 30. Of the eligible participants, 64.9% agreed to participate.
Each participant completed a computer-assisted personal interview (CAPI) based on the Risk Factor Questionnaire (RFQ) developed by the NCI Colon Cancer Family Registry (http://epi.grants.cancer.gov/CFR/about_questionnaires.html). A research nurse obtained measurements of height, weight, and fasting blood samples from each participant just prior to their colonoscopy examination.
Blood samples were immediately processed in a research laboratory and stored at −80° C. Serum RBP4 levels were measured by enzyme-linked immunosorbant assays (ELISA) (Alpco Diagnostics). Lab personnel were blind to the case and control status of each participant and RBP4 assays were performed twice, the average of which were used for the final value for each participant. Serum levels of insulin and glucose were available for 593 patients, and the homeostasis model assessment of insulin resistance (HOMA-IR) was calculated (glucose*insulin/405). Of the 33 patients missing HOMA-IR calculations, 17 were controls and 16 were cases, and there was no statistically significant difference of age, gender, and race between those with and without HOMA-IR data. All patients signed written consents and this study was approved by University Hospitals Case Medical Center Institutional Review Board.
We first performed univariate analyses of BMI, serum RBP4 and other covariates comparing the cases to the controls, using either ANOVA (for continuous variables) or a chi-square test (for discrete variables). We then applied unconditional logistic regression to estimate coefficients and 95% confidence intervals (CIs) for the association between log(RBP4) and colon adenomas. In our base model, we adjusted for age, race and gender. We then further adjusted for BMI, family history of colorectal cancer, smoking and NSAID use (full model). We also compared log(RBP4) results from multivariate logistic regression models with and without adjustment for HOMA (full model + HOMA). Odds ratio estimates for continuous scale were calculated for each 35µg/mL increment (approximately 1 standard deviation) of RBP4.
To examine potential modifying effect of BMI, we categorized each individual into “lower” or “higher” BMI based on the median value (27.8 kg/m2). RBP4 was then analyzed as categorical variable based on the tertile cut-off values of the entire study population. Categorical RBP4 were analyzed for both “lower” and “higher” BMI groups in all three models (base model, full model and full model + HOMA). Tests for trend were based on logistic regression coefficients for continuous scale. We tested for multiplicative interaction between RBP4 and BMI by including their cross-product term in the models. All p values were two-sided and computed using R i386 3.0.2.
Results
The cases had higher levels of RBP4 than the controls (mean = 58.5µg/mL ± 38.2 vs. 51.9µg/mL ± 32.5; p=0.03). Smoking, gender, and age differed significantly between the cases and the controls as well (Supplemental Table 1). In base model, log(RBP4) was statistically significantly associated with risk of colon adenoma (OR=3.10, CI = 1.15 – 8.66; p = 0.03). Further adjustment for BMI, NSAIDs, family history of colorectal cancer, diabetes mellitus (DM), and smoking (full model) had little impact on the results. Adding HOMA-IR to the full model yielded similar results. Because treatment of DM may reduce RBP4 levels, we next repeated the analyses excluding 90 patients with DM. The estimates were virtually identical to the analyses based on the entire sample, except for a slight decrease of β-slope in HOMA-IR adjusted model (Table 1).
Table 1.
Logistic Regression Analysis of Risk of Adenoma for Serum Levels of RBP4
| Including DM Pts.§ |
Excluding DM Pts. |
|||||||
|---|---|---|---|---|---|---|---|---|
| β ± SE | OR* | 95% C.I. | p | β ± SE | OR* | 95% C.I. | p | |
| Base model** | 0.32 ± 0.15 | 3.1 | 1.15 – 8.66 | 0.03 | 0.32 ± 0.16 | 3.13 | 0.01 – 9.49 | 0.04 |
| Full model† | 0.32 ± 0.15 | 3.1 | 1.11 – 8.97 | 0.03 | 0.32 ± 0.16 | 3.13 | 1.004 – 9.84 | 0.04 |
| Full model +HOMA‡ | 0.32 ± 0.16 | 3.1 | 1.04 – 8.97 | 0.04 | 0.29 ± 0.17 | 2.82 | 0.87 – 8.84 | 0.09 |
ORs for each 35 µg/mL (approximately 1 standard deviation) increment of RBP4.
Base model adjusts for age, race and gender.
Full model adjusts for age, race, gender, BMI (body mass index), non-steroidal anti-inflammatory drug use (6 months or longer), known family history and smoking (pack years).
33 missing values in HOMA-IR index.
diabetes treatment status is included in the three models as a covariate.
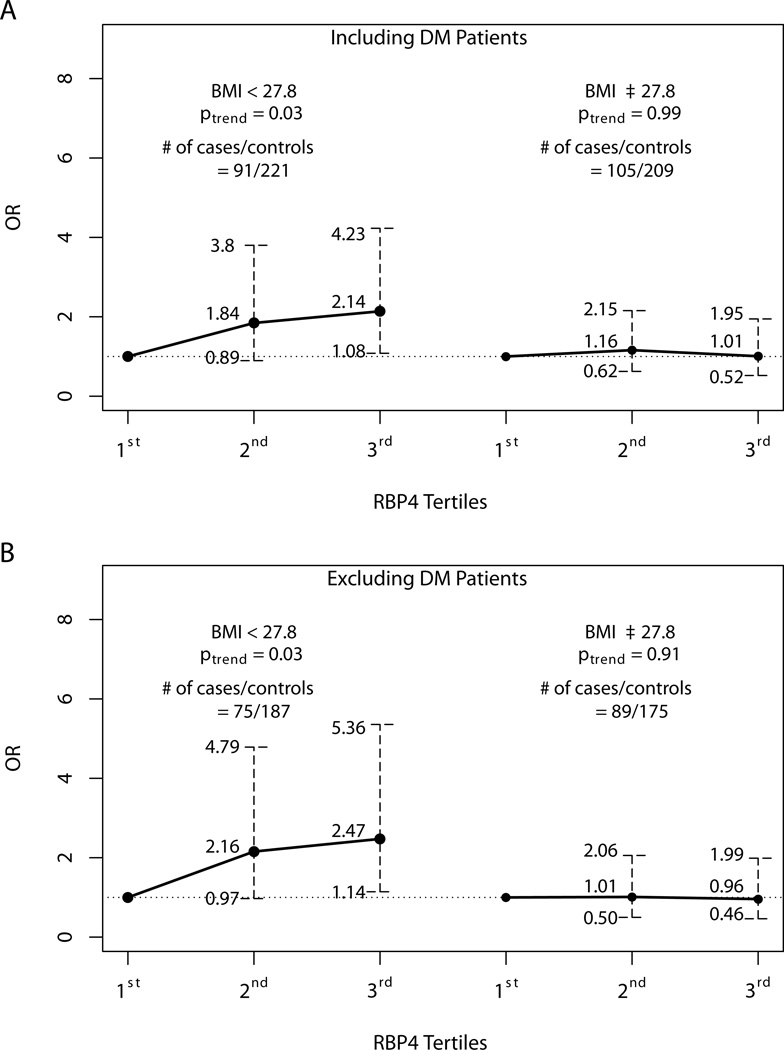
To explore potential effect modification by obesity, we performed analyses stratified by BMI [< 27.8 kg/m2 (median) vs. ≥ 27.8 kg/m2]. For those with lower BMI there is an approximately 2-fold increase of adenoma risk for the top tertile (OR=2.07, CI = 1.04–4.11) (ptrend = 0.04); in contrast, the corresponding OR estimate was 1.02 (CI = 0.53–1.97) for those with higher BMI (ptrend = 0.93). Excluding patients with DM revealed an even stronger association among those of lower BMI (Figure 1). Again, there is little evidence for association among those with higher BMI. Further adjustment of HOMA-IR showed similar results (data not shown). Tests for multiplicative interaction between RBP4 and BMI groups were not statistically significant (p > 0.10).
Figure 1.
Discussion
This is the first population-based study linking circulating levels RBP4 to risk of colon neoplasia. The mechanisms by which RBP4 may contribute to colon carcinogenesis are largely unknown. Our analysis was motivated by the discovery of RBP4 as a novel adipokine with a potentially causative role in insulin resistance (Yang Q, Graham TE, et al. 2005; Graham TE, Yang Q, et. al 2006). While our data indeed show a strong positive association, especially among patients with lower BMI, the observed association is independent of insulin resistance, suggesting that mechanisms other than insulin resistance may underlie the RBP4-colon neoplasia link. RBP4 is the principal transporter for retinol and a recent study has shown that the holo-RBP protein may act through its own receptor STRA6, which transduces JAK2/STAT3 signaling, to initiate carcinogenic transformations (Berry DC, Levi L et. al 2014). It is thus tempting to speculate that RBP4 may promote colon carcinogenesis through this pathway.
Our observation that the RBP4-adenoma association is limited to patients with lower BMI is intriguing. Adipose tissue is recognized as an active endocrine organ secreting a number of bioactive adipokines, which regulate physiological and pathological processes such as insulin sensitivity and resistance, inflammation, immunity and carcinogenesis (Kahn BB, Flier JS 2000; Van Kruijsdijk RCM, van der Wall E et. al 2009). We speculate that the wide-ranging effects of adipose tissue dysfunction in the state of obesity are so pervasive that the relative contribution of RBP4 is diminished.
There are limitations of our study. First, the cross-sectional nature of our study cannot readily dismiss reverse causality, i.e. the presence of adenoma may have raised the circulating levels of RBP4. Second, although we found striking difference in risk association between the ‘higher’ and the ‘lower’ BMI groups, our study with a moderate sample size has limited statistical power to detect multiplicative interaction between BMI and RBP4; as such, chance finding remains a potential explanation of the observed differential association between the two BMI groups. Finally, there were considerable differences in the smoking, gender, and age between the cases and controls. Although we have included these variables as covariates in our models, we recognize that statistical adjustment of smoking, gender, and age may not adequately control for confounding by these variables or other unmeasured factors.
In summary, we show that higher circulating levels RBP4 are associated, independent of insulin resistance and DM, with risk of colon adenoma, in particular among those with lower BMI (<27.8 kg/m2). Further investigation is merited to corroborate our findings and to unravel the underlying molecular mechanisms between RBP4 and colon adenomas.
Supplementary Material
Acknowledgments
Funding:
This work was supported by the National Cancer Institute grants (R01CA136726, U01CA181770), Case Center for Transdisciplinary Research on Energetics and Cancer (U54 CA 116867), and Case GI SPORE (P50CA50964).
Footnotes
Disclosure statement: The authors have no conflicts of interest to disclose.
References
- Berry DC, Levi L, Noy N. Holo-Retinol–Binding Protein and Its Receptor STRA6 Drive Oncogenic Transformation. Cancer Research. 2014;74:1–11. doi: 10.1158/0008-5472.CAN-14-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. The Journal of Nutrition. 2001;131:3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- Graham TE, Yang Q, Blüher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson P-A, Smith U, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. The New England Journal of Medicine. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Flier JS. Obesity and insulin resistance. The Journal of Clinical Investigation. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JP, Furr HC, Tanumihardjo SA. Retinol to Retinol-Binding Protein (RBP) is Low in Obese Adults due to Elevated apo-RBP. Experimental Biology and Medicine. 2008;233:1255–1261. doi: 10.3181/0803-RM-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz AP, Thompson CL, Chak A, Berger NA, Li L. Insulin resistance, central obesity, and risk of colorectal adenomas. Cancer. 2012;118:1774–1781. doi: 10.1002/cncr.26454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. Journal of the National Cancer Institute. 2002;94:972–980. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- Van Kruijsdijk RCM, van der Wall E, Visseren FLJ. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology. 2009;18:2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund and American Institute for Cancer Research. Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer. 2011 [Google Scholar]
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.