ABSTRACT
Gestational protein restriction results in intrauterine growth restriction and hypertension in adult female growth-restricted rats. Enhanced vascular responsiveness to angiotensin II is observed, and blockade of the renin-angiotensin system abolishes hypertension in adult growth-restricted rats, suggesting that the renin-angiotensin system contributes to intrauterine growth restriction-induced hypertension. Moreover, growth-restricted adult rats have higher plasma testosterone levels, and antiandrogen treatment abolishes hypertension, indicating an important role for testosterone. We hypothesized that androgens may play a pivotal role in the enhanced responsiveness to Ang II and hypertension. Female offspring of pregnant rats fed 20% protein (control) or 6% protein diet (protein restricted), at 6 mo of age, were studied. Plasma testosterone and mean arterial pressure in protein-restricted offspring were significantly higher compared to controls. Flutamide treatment (10 mg/kg/day subcutaneously for 10 days) reduced mean arterial pressure in protein-restricted offspring but was without significant effect in controls. Vascular Agtr1/Agtr2 ratio was significantly higher in protein-restricted offspring, an effect that was reversed by flutamide. Flutamide treatment did not have any effect on Agtr1/Agtr2 ratio in controls. Enhanced contractile response to angiotensin II in mesenteric arteries was observed in protein-restricted offspring compared with control. Flutamide treatment reversed the enhanced contractile response to angiotensin II in protein-restricted offspring without significant effect in controls. Vascular reactivity to phenylephrine was similar between the control and protein-restricted offspring with and without flutamide treatment, suggesting that enhanced contractile response and flutamide's reversal effect is specific to angiotensin II. These results suggest that prenatally protein-restricted rats exhibit an enhanced responsiveness to angiotensin II that is testosterone-dependent.
Keywords: Agtr1, angiotensin II, blood pressure, flutamide, mesenteric arteries, pregnancy, protein restriction, testosterone, vascular function
INTRODUCTION
Suboptimal influences during early life leads to increased risk for cardiovascular disease during adult life [1–9]. Using a model of protein restriction during rat pregnancy, our laboratory has begun to elucidate the mechanisms linking intrauterine growth restriction and adult life blood pressure [3, 5–7]. We previously reported that female growth-restricted offspring from protein-restricted dams exhibit a marked increase in arterial pressure in adulthood that is associated with an increase in expression of mesenteric arterial angiotensin (Ang II) receptor, Agtr1a [7], suggesting that inappropriate activation of the systemic renin-angiotensin system (RAS) is observed in hypertensive adult female protein-restricted offspring. Moreover, blockade of the RAS abolishes hypertension in adult prenatal protein-restricted offspring [7], suggesting that the RAS contributes to the etiology of hypertension in female protein-restricted offspring; yet, the exact mechanism by which the RAS contributes to hypertension in this model is not clear.
We and others have shown that prenatal protein restriction leads to development of hypertension associated with increase in plasma testosterone levels in the adult females [6, 10]. In these hypertensive females, ovariectomy exacerbated blood pressure to adult male levels, but estradiol replacement only reversed that part of blood pressure increase that was induced by ovariectomy [6], whereas, antiandrogen treatment completely normalized blood pressure to control levels [3]. Flutamide treatment abolishing hypertension in adult female protein-restricted rats with no significant effect on blood pressure in adult female control rats suggest that hypertension in adult female protein-restricted rats is testosterone dependent [3]. The mechanism by which testosterone modulates blood pressure in adult female protein-restricted rats is not yet elucidated. Furthermore, whether modulation of the RAS by testosterone plays an important role in mediating hypertension induced by prenatal protein restriction in female protein-restricted offspring is also not known.
A role for modulation of the RAS by testosterone is observed in genetic models of hypertension. Testosterone interacts with the RAS, upregulating the classical constrictor pathway via upregulation of angiotensinogen gene expression, renin activity, and Agtr1 expression [11–13]. Testosterone is implicated to play a critical role in mediating hypertension in the spontaneously hypertensive rat (SHR) [14–16], and modulation of the RAS by testosterone exacerbates hypertension in male SHR [17]. Upregulation of vascular angiotensin receptor expression by testosterone in the SHR may serve as one mechanism by which modulation of the RAS by testosterone contributes to the development of hypertension in the SHR [18]. Androgens also increase Agtr1a receptor mRNA abundance in the abdominal but not thoracic aortas of male mice [19]. However, expression of the renal Agtr1 is not altered by castration in the New Zealand genetically hypertensive rat [20], yet the enhanced pressor response to acute Ang II observed in male New Zealand genetically hypertensive rats is abolished by castration, indicating that increased sensitivity to Ang II is testosterone dependent in this genetic model of hypertension [20]. Furthermore, RAS blockade in male and female SHRs abolishes the sex difference in the arterial pressure observed [17]. Taken together, these data demonstrate that testosterone interacts with the RAS, increasing activation of the classical pathway resulting in Agtr1 activation by Ang II.
A critical role for the RAS is indicated in models of developmental programming of hypertension [7, 21–23], and we showed that exaggerated vasomotor responses to Ang II [7] may serve as a potential mechanism. Therefore, the aim of this study was to determine whether the enhanced responsiveness to Ang II and hypertension observed in adult female protein-restricted rats is testosterone dependent.
MATERIALS AND METHODS
Animals
All the experimental procedures were in accordance with National Institutes of Health guidelines, and our protocol was approved by the Animal Care and Use Committee at the University of Texas Medical Branch. Rats were housed in a temperature-controlled room (23°C) with a 12L:12D cycle with food and water available ad libitum. Timed pregnant Sprague-Dawley rats were purchased from Harlan. The rats were allocated to ad libitum isocaloric diet containing either 20% (control, n = 8) or 6% (protein-restricted group, n = 9) casein, as in our previous studies [3, 5, 6]. After the delivery of pups, dams were returned to regular chow. Pups were weaned at 3 wk of age to regular chow, and females were housed separately and examined at 6 mo of age. Blood samples were collected between 0900 and 1000 from control and protein-restricted offspring on the day of estrus, as determined by vaginal cytology. Plasma was separated by centrifugation and stored at −20°C until the time of measurement. A subset of female offspring at 6 mo of age were treated with vehicle or androgen receptor antagonist flutamide (10 mg·kg−1·day−1 subcutaneously) [3] for 10 days. Following flutamide treatment, changes in arterial pressure were determined. After blood pressure measurement, the animals were sacrificed, a portion of the mesenteric arteries were separated for vascular reactivity studies, and the remaining were quickly frozen for RNA isolation and determination of expression of Ang II receptors (Agtr1 and Agtr2). Unless specified otherwise, one animal per litter was used for the different studies.
Experimental Procedures
Plasma testosterone levels.
Testosterone levels in the samples were measured using an enzyme imuunoassay radioimmunoassay kit (Enzo Life Sciences) according to the manufacturer's instructions. The minimum detectable concentration of testosterone was 6 pg/ml. The intra- and interassay coefficients of variation were lower than 5%.
Mean arterial pressure.
Mean arterial pressure in conscious free-moving female offspring of control and protein-restricted dams was determined at 6 mo of age using indwelling carotid arterial catheters as described in our previous publication [6]. Briefly, rats under anesthesia (ketamine, 45 mg/kg; xylazine, 5 mg/kg) (Burns Veterinary Supply) were surgically instrumented with flexible catheters (polyethylene 50 tubing) in the left carotid artery. The catheters were tunneled to the nape of the neck and exteriorized. After a 24 h recovery period, when the animals are fully conscious and in free-moving state, arterial catheter was connected to a pressure transducer and arterial blood pressure was obtained using a data acquisition system (DBP001 direct BP system and Workbench for Windows software, both from Kent Scientific). Following a 30 min stabilization period, the arterial pressure was monitored continuously for 30 min and averaged to determine the baseline values.
Quantitative real-time PCR.
Immediately following the measurements of baseline blood pressure, the whole mesenteric arteries were collected and instantly frozen in liquid nitrogen and later processed for total RNA extraction using TRIzol (Invitrogen). All the RNA isolates were made DNA-free by treatment with DNAse and further purified with RNeasy cleanup kit (Qiagen Inc.). Total RNA concentration and integrity were determined using an ND-1000 Nanodrop spectrophotometer (Thermo Fisher Scientific) and RNA gel. One microgram of total RNA was reverse transcribed using a modified Maloney murine leukemia virus-derived reverse transcript (New England BioLabs Inc.) and a blend of oligo (dT) and random hexamer primers (Invitrogen). The reaction was carried out at 28°C for 15 min and 42°C for 50 min, then stopped by heating at 94°C for 5 min followed by 4°C before storage at −20°C until further analysis. One microliter of the diluted cDNA corresponding to 100 ng RNA was amplified by real-time PCR using FAM (Invitrogen) as the fluorophore in a CFX96 real-time thermal cycler (Bio-Rad). PCR conditions were 2 min at 50°C for one cycle; 10 min at 95°C, 15 sec at 95°C, and 1 min at 60°C for 40 cycles; and a final dissociation step (0.05 sec at 65°C and 0.5 sec at 95°C). The efficiency of these assays was 100%. Results were calculated using the 2−ΔΔCT method and expressed in fold increase and/or decrease of the gene of interest in protein-restricted versus control rats. All the reactions were performed in duplicate, and 18S was used as an internal control. The following TaqMan assays were done in 10 μl for real-time PCR at a final concentration of 250 nM TaqMan probe and 900nM of each primer; Assays-on-Demand for Agtr1a (Rn01435427_m1), Agtr1b (Rn02132799_s1), and Agtr2 (Rn00560677_s1) were obtained from Applied Biosystems.
Ex vivo vascular reactivity studies.
Freshly excised third-order mesenteric arteries from 6-mo-old female offspring were placed in ice-cold modified Krebs bicarbonate solution (KBS) of the following composition: 118 mM NaCl, 4.7 mM KCl, 25 mM NaHCO3, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, and 11 mM dextrose. The mesenteric arteries were cleaned of adherent connective tissue and cut into rings of 2 mm length. Two to four rings from one rat were used for one experiment, and the variable n presented with each figure represents the number of animals studied. We have previously shown that protein-restricted offspring have endothelial dysfunction [5]; hence, studies were done in endothelium-denuded arterial rings to avoid confounding and to assess the Ang II receptor-mediated effects on the vascular smooth muscle. Endothelium was denuded by gently rubbing with tungsten wires. Two 25-μm tungsten wires were threaded through the lumen, and the rings were mounted in an isometric wire myograph system (model 610M wire myography; Danish Myo Technology). The rings were bathed in 6 ml KBS, gassed with 95% oxygen and 5% carbon dioxide, maintained at a temperature of 37°C, and allowed to equilibrate for 30 min before normalization to an internal diameter of 0.9 of L13.3kPa (resting transluminal pressure of 100 mmHg, in vivo) by using a standardized procedure [24] and normalization software package (Myodata; Danish Myo Technology). This corresponds to a transmural pressure of ∼90 mmHg. Following normalization, rings were repeatedly exposed to 80 mM KCl to test their viability and to determine a standard contractile response for each of them. The rings were contracted with a α1-adrenergic receptor agonist, 3 μM phenylephrine (Sigma), and when responses were stable, endothelium-denudation was confirmed by absence of relaxation to 10 μM acetylcholine (Sigma). Rings were then allowed to recover for 60 min, after which cumulative concentration-response curves were generated with Ang II (10−13 to 10−8 M) (Sigma) and phenylephrine (10−9 to 10−5 M).
Statistical Analysis
For the comparison of arterial pressure and gene expression, analysis was performed using two way ANOVA (diet and flutamide treatment as factors), with adjustments for multiple comparisons. Cumulative concentration-response curves were analyzed by computer fitting to a four-parameter sigmoid curve using the Prism 5 program (GraphPad) to evaluate the half-maximal effective concentration (pD2 = −log half-maximal concentration) and the maximum asymptote of the curve (Emax). All the values are expressed as means ± SEM, and a P < 0.05 was considered significant.
RESULTS
Plasma Testosterone Levels
Plasma testosterone levels significantly increased by 2.4-fold in adult female protein-restricted offspring (135 ± 19.93 pg/ml, n = 7) compared to controls (59 ± 8.96 pg/ml, n = 5, P < 0.05).
Mean Arterial Pressure
Mean arterial pressure measured in conscious free-moving rats via indwelling carotid catheter was significantly higher in protein-restricted females (116 ± 3.6 mmHg, n = 8) compared to controls (98 ± 2.4 mmHg, n = 8, P < 0.05; Fig. 1). Flutamide treatment significantly decreased the blood pressure in protein-restricted females (106 ± 1.4 mmHg, n = 8, P < 0.05) and was without significant effect in controls (105 ± 2.9 mmHg, n = 8; Fig. 1).
FIG. 1.
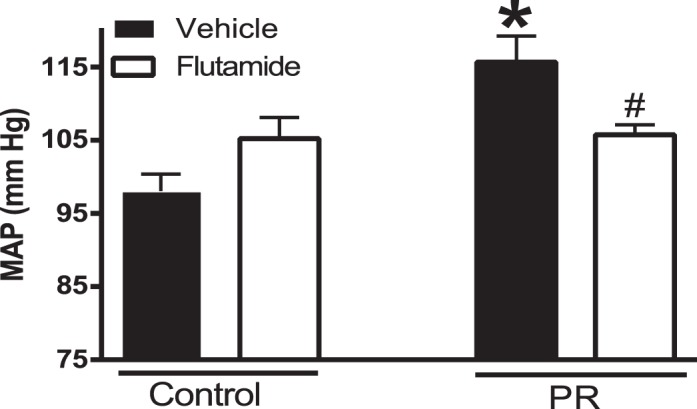
Changes in blood pressure in control and prenatal protein-restricted females. Mean arterial pressure (MAP) was measured through carotid arterial catheters in conscious free-moving control (n = 8) and prenatal protein-restricted females (n = 8) at 6 mo of age after treatment with vehicle or flutamide (10 mg·kg−1·day−1 subcutaneously) for 10 days. Data points represent the mean ± SEM. *P < 0.05 versus vehicle-treated control; #P < 0.05 versus vehicle-treated protein-restricted group.
Expression of Agtr1 and Agtr2 Receptors in the Mesenteric Artery
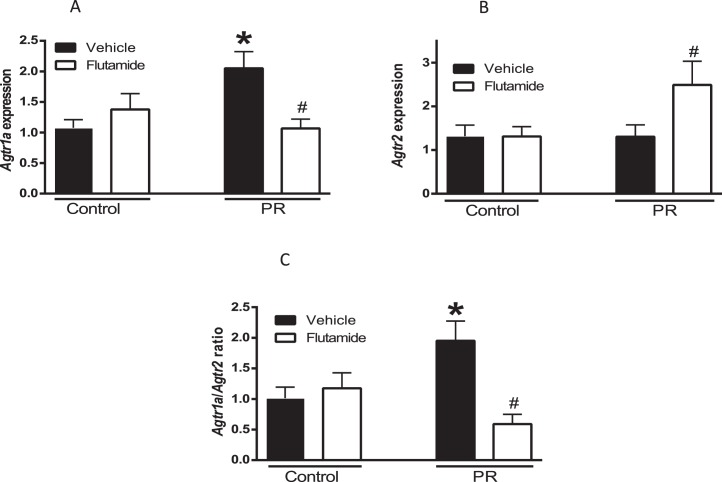
At the mRNA level, rodents possess two Agtr1 receptor isoforms, designated Agtr1a and Agtr1b. Agtr1b was undetectable in the rat mesenteric arteries, which is similar to the finding in previous studies [7, 25]. The mesenteric vascular expression of Agtr1a mRNA was 2-fold higher in protein-restricted rats compared to control (n = 6 in each group, P < 0.05; Fig. 2A). Agtr2 mRNA was similar between control and protein-restricted females (n = 6 in each group; Fig. 3B). The vascular Agtr1a/Agtr2 ratio was significantly higher by 2.0-fold in protein-restricted females compared with control females (n = 6; Fig. 2C).
FIG. 2.
Changes in mesenteric vascular angiotensin receptors in female control and protein-restricted offspring. Real-time PCR was used to assess vascular Agtr1a (A) and Agtr2 (B) mRNA expression at 6 mo of age. Quantitation of vascular Ang II receptors was normalized relative to 18S levels. The ratio of Agtr1a/Agtr2 (C) is presented (n = 6 in each group). *P < 0.05 versus vehicle-treated control; #P < 0.05 versus vehicle-treated protein-restricted group.
FIG. 3.
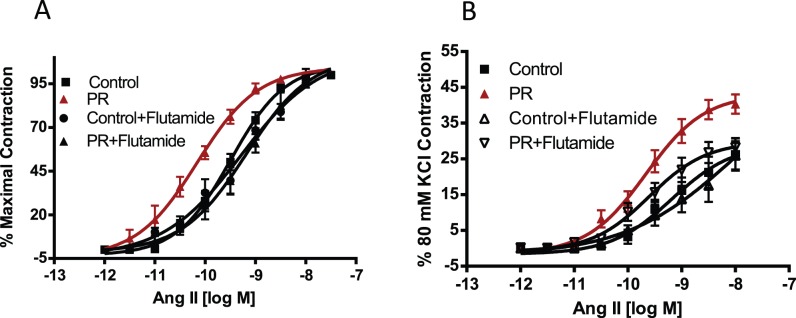
Ang II-induced contraction in mesenteric arterial rings of control and protein-restricted offspring with and without flutamide. Endothelium-denuded mesenteric arterial rings were incubated in Krebs buffer and then stimulated with increasing concentrations of Ang II. A) Ang II contraction was measured and presented as percentage of maximal contraction. B) Ang II-induced contractions were also calculated as percentage of 80 mM KCl contractions (n = 14–16 mesenteric arterial rings from seven to eight rats of each group).
Flutamide significantly decreased Agtr1a (n = 6, P < 0.05; Fig. 2A) and increased Agtr2 (n = 6, P < 0.05; Fig. 2B) mRNA expression in the mesenteric arteries of protein-restricted females compared to vehicle-treated protein-restricted females (n = 6 in each group, P < 0.05; Fig. 2A). Flutamide did not affect Agtr1a and Agtr2 mRNA expression in the controls (n = 6 in each group; Fig. 2, A and B). Thus, flutamide significantly decreased mesenteric arterial Agtr1a/Agtr2 ratio in protein-restricted females and was without significant effect in controls (n =6, P < 0.05; Fig. 2C).
Ex Vivo Vasomotor Responses
Ang II induced a dose-dependent increase in contractile responses in mesenteric arterial rings. However, the Ang II-induced contractile responses were exaggerated with a leftward shift in the dose-response curves as well as an increase in maximal responses in the protein-restricted (pD2 = 10.16 ± 0.10, Emax = 40.35 ± 2.73, n = 7) compared to control rats (pD2 = 9.49 ± 0.08, Emax = 26.2 ± 5.52, n = 8, P < 0.05; Fig. 3).
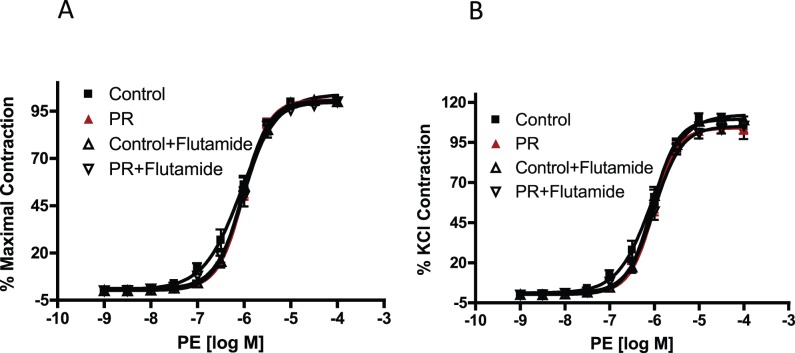
Flutamide treatment reversed the enhanced contractile response to angiotensin II in protein-restricted females (pD2 = 9.18 ± 0.14, Emax = 28.21 ± 2.62, n = 6) with no significant effect in controls (pD2 = 9.29 ± 0.14, Emax = 25.95 ± 3.97, n = 6; Fig. 3). Contractile responses to phenylephrine (n = 5 in each group; Fig. 4) were not increased in protein-restricted females compared to controls. Flutamide treatment did not affect contractile responses to phenylephrine in both protein-restricted and control females (n = 5 in each group; Fig. 4).
FIG. 4.
Phenylephrine (PE)-induced contraction in mesenteric arterial rings of control and protein-restricted offspring with and without flutamide. Endothelium-denuded mesenteric arterial rings were incubated in Krebs buffer and then stimulated with increasing concentrations of PE. A) Phenylephrine contraction was measured and presented as percentage of maximal contraction. B) Phenylephrine -induced contractions were also calculated as percentage of 80 mM KCl contraction (n = 10–12 mesenteric arterial rings from five rats in each group).
DISCUSSION
This study tested the hypothesis whether testosterone contributes to the enhanced responsiveness to Ang II and hypertension observed in adult female protein-restricted rats. The main findings indicate that not only increased blood pressure, but the enhanced mesenteric vasomotor response mediated by Ang II in female protein-restricted rats is abolished by flutamide. The increased Agtr1a expression and Agtr1a/Agtr2 ratio in the mesenteric arteries of protein-restricted females was also reversed by flutamide. Importantly, the vascular contractile responses to another vasoconstrictor, phenylephrine, was not altered by flutamide, suggesting that flutamide's reversal of contractile effect is specific to Ang II. Therefore, we suggest that testosterone may regulate increases in vascular Agtr1 and Ang II stimulated responses to mediate increase in blood pressure in the female offspring that are exposed to prenatal protein restriction.
It is now well-established that a variety of insults, when experienced in the prenatal period, can have long-term influences on the health of the individual. Consistent with previous reports [6, 10], prenatal protein-restricted adult females have increased plasma testosterone levels. This increased testosterone levels in protein-restricted females is of both ovarian and extraovarian origin and is associated with increased blood pressure [3, 5–7] similar to that observed in this study. As reported previously by our group [3] and now confirmed by direct carotid artery catheter, blood pressure was significantly reversed by antiandrogen flutamide, suggesting that hypertension in adult protein-restricted females is androgen receptor dependent.
RAS, a regulatory system important in the long-term control of blood pressure, is altered in the offspring of protein-restricted dams, and is shown to contribute to the etiology of programmed hypertension. Consistently, we and others have shown that blocking of Ang II formation with angiotensin converting enzyme blocker [26] or inhibition of Ang II action at Ang II type-1 receptor (AT1R) with antagonist [7, 27] prevents elevation of blood pressure in the offspring of protein-restricted dams. We not only confirm that prenatal protein restriction leads to exaggerated mesenteric vascular responses to Ang II in the adult females [7], but we also provide novel data that treatment with a selective androgen receptor antagonist, flutamide, abolished the exaggerated mesenteric vascular responses to Ang II. This finding suggests that the enhanced responsiveness to Ang II in female protein-restricted rats is testosterone dependent and, importantly, indicates that involvement of the RAS in prenatal protein restriction-induced hypertension may necessitate modulation by testosterone. Similar responses of testosterone-dependent enhancement in responsiveness to Ang II is observed in adult growth-restricted male rats born to pregnant rats with reduced uterine perfusion [28].
The finding that increased expression of Agtr1a in the mesenteric arteries was reversed by flutamide suggests that testosterone may mediate the enhanced sensitivity to Ang II in female protein-restricted rats through upregulation of Agtr1a-mediated mechanisms. There are evidences that Agtr1 expression is androgen dependent. In rat epididymis, castration reduced Agtr1 receptor protein that was restored when rats were treated with testosterone [29]. Moreover, androgen was reported to increase Ang II receptors in bovine adrenal glomerulosa cells [30]. In addition, incubation of human prostate cancer cells with androgens increased Agtr1 receptor expression [31], supporting androgens regulation of Agtr1. However, the mechanism by which androgens regulate Agtr1 expression has been relatively unexplored. Possible mechanisms of testosterone-dependent upregulation of Agtr1a receptor mRNA expression include modulation of receptor transcription through androgen response elements. Preliminary analysis of Agtr1 promoter reveals the presence of partial androgen response elements [30]. Androgens may also activate Agtr1 transcription from alternative response elements such as the glucocorticoid response element [32, 33] by enhancing assembly of general transcription factors, including TATA-box-binding protein [34], stimulating activator protein-1, nuclear Factor-KappaB [35, 36], or increasing mRNA stability [37]. In addition to stimulating receptor mediated effects, testosterone may activate Agtr1 postreceptor mechanisms [38]. Testosterone is shown to potentiate renal vascular responses to Ang II partially through upregulation of the Rho kinase-signaling pathway [39]. Whether testosterone-modulated control of Rho kinase or other downstream-signaling pathways contributes to the enhanced responsiveness to acute Ang II in the female protein-restricted rats is unknown. Thus, testosterone-dependent hypertension mediated via modulation of the RAS may involve both receptor and postreceptor mechanisms.
Actions of the Agtr2 are less clear but seem to counterbalance some of the actions of the Agtr1 leading to vasodilation [40]. The reason for this specific increase in Agtr2 expression in flutamide-treated protein-restricted females is unclear and is an area of future investigation. Studies indicate that the Agtr1a/Agtr2 ratio plays a crucial role in the development of the hypertensive phenotype [18], and the vascular Agtr1/Agtr2 ratio relates to the magnitude of blood pressure elevation observed in SHR rats [18]. Generation of nitric oxide has been implicated in mediating the effects related to Agtr2 actions [41] In the present study, we demonstrated that flutamide significantly reversed the increased mesenteric arterial ratio of Agtr1/Agtr2 in female offspring relating to the magnitude of blood pressure decrease.
Evidence indicates that androgens can contribute to blood pressure control in women because young women with conditions such as polycystic ovarian syndrome, women after menopause, and African-American women have higher plasma testosterone levels, and the frequency of hypertension is greater in these populations. In this study, higher testosterone levels during adult life significantly increases blood pressure. We have shown that increased Agtr1 expression plays a role in the hypertensive activity of androgen. Regulation of Agtr1 expression by androgens via the androgen receptor has significant functional consequences because we determined that androgens exert a significant increase in Ang II-mediated vasoconstriction. The exact mechanism(s) responsible for testosterone modulation of the RAS in this model of hypertension is not clear but the finding that flutamide treatment attenuated mesenteric arterial sensitivity to Ang II with associated decrease in Agtr1a expression in female protein-restricted rats suggest that testosterone potentiates sensitivity to Ang II via its receptor-mediated mechanisms. Further studies are needed to clarify the complex pathways that mediate hypertension and Ang II sensitivity in adult female protein-restricted offspring. Importantly, experimental studies investigating the role of sex hormones in mediating hypertension programmed in response to fetal insult may provide insight into the critical mechanisms linking sex hormones and factors key to the long-term control of blood pressure.
Footnotes
Support from the National Institute of Health (NIH) through grants HL119869 awarded to K.S. and HL102866 and HL58144 awarded to C.Y. is greatly appreciated. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- Brawley L, Itoh S, Torrens C, Barker A, Bertram C, Poston L, Hanson M. Dietary protein restriction in pregnancy induces hypertension and vascular defects in rat male offspring. Pediatr Res. 2003;54:83–90. doi: 10.1203/01.PDR.0000065731.00639.02. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Olson DM, Symonds ME. Maternal nutrient restriction alters renal development and blood pressure regulation of the offspring. Proc Nutr Soc. 2006;65:116–124. doi: 10.1079/pns2005484. [DOI] [PubMed] [Google Scholar]
- Gangula PR, Reed L, Yallampalli C. Antihypertensive effects of flutamide in rats that are exposed to a low-protein diet in utero. Am J Obstet Gynecol. 2005;192:952–960. doi: 10.1016/j.ajog.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Mesquita FF, Gontijo JA, Boer PA. Expression of renin-angiotensin system signalling compounds in maternal protein-restricted rats: effect on renal sodium excretion and blood pressure. Nephrol Dial Transplant. 2010;25:380–388. doi: 10.1093/ndt/gfp505. [DOI] [PubMed] [Google Scholar]
- Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C. Protein restriction during pregnancy induces hypertension and impairs endothelium-dependent vascular function in adult female offspring. J Vasc Res. 2008;46:229–239. doi: 10.1159/000166390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C. Protein restriction during pregnancy induces hypertension in adult female rat offspring—influence of oestradiol. Br J Nutr. 2012;107:665–673. doi: 10.1017/S0007114511003448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathishkumar K, Balakrishnan M, Chinnathambi V, Gao H, Yallampalli C. Temporal alterations in vascular angiotensin receptors and vasomotor responses in offspring of protein-restricted rat dams. Am J Obstet Gynecol. 2012;206:507–510. doi: 10.1016/j.ajog.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrens C, Brawley L, Barker AC, Itoh S, Poston L, Hanson MA. Maternal protein restriction in the rat impairs resistance artery but not conduit artery function in pregnant offspring. J Physiol. 2003;547:77–84. doi: 10.1113/jphysiol.2002.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Guzman C, Cabrera R, Cardenas M, Larrea F, Nathanielsz PW, Zambrano E. Protein restriction during fetal and neonatal development in the rat alters reproductive function and accelerates reproductive ageing in female progeny. J Physiol. 2006;572:97–108. doi: 10.1113/jphysiol.2005.103903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Naftilan AJ, Oparil S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension. 1992;19:456–463. doi: 10.1161/01.hyp.19.5.456. [DOI] [PubMed] [Google Scholar]
- Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ. Androgen regulation of rat renal angiotensinogen messenger RNA expression. J Clin Invest. 1989;83:1941–1945. doi: 10.1172/JCI114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz FH, Roper EF. Testosterone effect on renin system in rats. Proc Soc Exp Biol Med. 1977;155:330–333. doi: 10.3181/00379727-155-39800. [DOI] [PubMed] [Google Scholar]
- Reckelhoff JF, Zhang H, Srivastava K, Granger JP. Gender differences in hypertension in spontaneously hypertensive rats: role of androgens and androgen receptor. Hypertension. 1999;34:920–923. doi: 10.1161/01.hyp.34.4.920. [DOI] [PubMed] [Google Scholar]
- Jenkins C, Salisbury R, Ely D. Castration lowers and testosterone restores blood pressure in several rat strains on high sodium diets. Clin Exp Hypertens. 1994;16:611–625. doi: 10.3109/10641969409067965. [DOI] [PubMed] [Google Scholar]
- Martin DS, Biltoft S, Redetzke R, Vogel E. Castration reduces blood pressure and autonomic venous tone in male spontaneously hypertensive rats. J Hypertens. 2005;23:2229–2236. doi: 10.1097/01.hjh.0000191903.19230.79. [DOI] [PubMed] [Google Scholar]
- Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension. 2000;35:480–483. doi: 10.1161/01.hyp.35.1.480. [DOI] [PubMed] [Google Scholar]
- Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res. 2004;62:587–593. doi: 10.1016/j.cardiores.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Henriques T, Zhang X, Yiannikouris FB, Daugherty A, Cassis LA. Androgen increases AT1a receptor expression in abdominal aortas to promote angiotensin II-induced AAAs in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:1251–1256. doi: 10.1161/ATVBAHA.107.160382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Kost CK, Jr, Martin DS. Androgens augment renal vascular responses to ANG II in New Zealand genetically hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1608–R1615. doi: 10.1152/ajpregu.00364.2005. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Jackson AA. Captopril normalises systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem Physiol A Physiol. 1995;110:223–228. doi: 10.1016/0300-9629(94)00177-u. [DOI] [PubMed] [Google Scholar]
- Sherman RC, Langley-Evans SC. Early administration of angiotensin-converting enzyme inhibitor captopril, prevents the development of hypertension programmed by intrauterine exposure to a maternal low-protein diet in the rat. Clin Sci (Lond) 1998;94:373–381. doi: 10.1042/cs0940373. [DOI] [PubMed] [Google Scholar]
- Ceravolo GS, Franco MC, Carneiro-Ramos MS, Barreto-Chaves ML, Tostes RC, Nigro D, Fortes ZB, Carvalho MH. Enalapril and losartan restored blood pressure and vascular reactivity in intrauterine undernourished rats. Life Sci. 2007;80:782–787. doi: 10.1016/j.lfs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Spiers A, Padmanabhan N. A guide to wire myography. Methods Mol Med. 2005;108:91–104. doi: 10.1385/1-59259-850-1:091. [DOI] [PubMed] [Google Scholar]
- Lee S, Ji H, Wu Z, Zheng W, Hassan A, Sandberg K. Translational regulation of ANG II type 1 receptors in proliferating vascular smooth muscle cells. Am J Physiol Regul Integr Comp Physiol. 2006;290:R50–R56. doi: 10.1152/ajpregu.00448.2005. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Jackson AA. Captopril normalises systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem Physiol A Physiol. 1995;110:223–228. doi: 10.1016/0300-9629(94)00177-u. [DOI] [PubMed] [Google Scholar]
- Sherman RC, Langley-Evans SC. Antihypertensive treatment in early postnatal life modulates prenatal dietary influences upon blood pressure in the rat. Clin Sci (Lond) 2000;98:269–275. [PubMed] [Google Scholar]
- Ojeda NB, Royals TP, Black JT, Dasinger JH, Johnson JM, Alexander BT. Enhanced sensitivity to acute angiotensin II is testosterone dependent in adult male growth-restricted offspring. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1421–R1427. doi: 10.1152/ajpregu.00096.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung PS, Wong TP, Chung YW, Chan HC. Androgen dependent expression of AT1 receptor and its regulation of anion secretion in rat epididymis. Cell Biol Int. 2002;26:117–122. doi: 10.1006/cbir.2001.0830. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Goodfriend TL. Androgen modulation of adrenal angiotensin receptors. Science. 1984;224:1009–1011. doi: 10.1126/science.6326265. [DOI] [PubMed] [Google Scholar]
- Uemura H, Hasumi H, Ishiguro H, Teranishi J, Miyoshi Y, Kubota Y. Renin-angiotensin system is an important factor in hormone refractory prostate cancer. Prostate. 2006;66:822–830. doi: 10.1002/pros.20407. [DOI] [PubMed] [Google Scholar]
- Adler AJ, Danielsen M, Robins DM. Androgen-specific gene activation via a consensus glucocorticoid response element is determined by interaction with nonreceptor factors. Proc Natl Acad Sci U S A. 1992;89:11660–11663. doi: 10.1073/pnas.89.24.11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdarina IG, King PJ, Clark AJ. Characterization of the angiotensin (AT1b) receptor promoter and its regulation by glucocorticoids. J Mol Endocrinol. 2009;43:73–80. doi: 10.1677/JME-09-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwan IJ, Gustafsson J. Interaction of the human androgen receptor transactivation function with the general transcription factor TFIIF. Proc Natl Acad Sci U S A. 1997;94:8485–8490. doi: 10.1073/pnas.94.16.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo DF, Inagami T. The genomic organization of the rat angiotensin II receptor AT1B. Biochim Biophys Acta. 1994;1218:91–94. doi: 10.1016/0167-4781(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Lee DK, Chang C. Molecular communication between androgen receptor and general transcription machinery. J Steroid Biochem Mol Biol. 2003;84:41–49. doi: 10.1016/s0960-0760(03)00005-0. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Strehlow K, Wassmann S, Baumer AT, Albory K, Sauer H, Bohm M. Differential effects of estrogen and progesterone on AT(1) receptor gene expression in vascular smooth muscle cells. Circulation. 2000;102:1828–1833. doi: 10.1161/01.cir.102.15.1828. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- Song J, Kost CK, Jr, Martin DS. Androgens potentiate renal vascular responses to angiotensin II via amplification of the Rho kinase signaling pathway. Cardiovasc Res. 2006;72:456–463. doi: 10.1016/j.cardiores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Hagihara GN, Lobato NS, Filgueira FP, Akamine EH, Aragao DS, Casarini DE, Carvalho MH, Fortes ZB. Upregulation of ERK1/2-eNOS via AT2 receptors decreases the contractile response to angiotensin II in resistance mesenteric arteries from obese rats. PLoS One. 2014;9:e106029. doi: 10.1371/journal.pone.0106029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan RE, Davis EA, Widdop RE. Functional role of angiotensin II AT2 receptor in modulation of AT1 receptor-mediated contraction in rat uterine artery: involvement of bradykinin and nitric oxide. Br J Pharmacol. 2003;140:987–995. doi: 10.1038/sj.bjp.0705484. [DOI] [PMC free article] [PubMed] [Google Scholar]