ABSTRACT
Chorioamnionitis, an infection/inflammation of the fetomaternal membranes, is frequently associated with preterm delivery. The mechanisms of inflammation in chorioamnionitis are poorly understood. We hypothesized that neutrophils recruited to the decidua would be the major producers of proinflammatory cytokines. We injected intra-amniotic (IA) interleukin 1beta (IL-1beta) at ∼80% gestation in rhesus macaque monkeys, Macaca mulatta, delivered the fetuses surgically 24 h or 72 h after IA injections, and investigated the role of immune cells in the chorion-amnion decidua. IA IL-1beta induced a robust infiltration of neutrophils and significant increases of proinflammatory cytokines in the chorioamnion decidua at 24 h after exposure, with a subsequent decrease at 72 h. Neutrophils in the decidua were the major source of tumor necrosis factor alpha (TNFalpha) and IL-8. Interestingly, IA IL-1beta also induced a significant increase in anti-inflammatory indoleamine 2,3-dioxygenase (IDO) expression in the decidua neutrophils. The frequency of regulatory T cells (Tregs) and FOXP3 mRNA expression in the decidua did not change after IA IL-1beta injection. Collectively, our data demonstrate that in this model of sterile chorioamnionitis, the decidua neutrophils cause the inflammation in the gestational tissues but may also act as regulators to dampen the inflammation. These results help to understand the contribution of neutrophils to the pathogenesis of chorioamnionitis-induced preterm labor.
Keywords: chorioamnionitis; decidua; IDO; indoleamine 2,3-dioxygenase; inflammation; neutrophils
INTRODUCTION
Preterm birth is a major public health problem, which accounted for 11.5% of all births in the United States in 2012 [1]. Furthermore, prematurity is responsible for 75% of perinatal mortality [2] with approximately 1 million deaths/year world wide [3]. Chorioamnionitis, diagnosed by infection or inflammation in the fetal membranes or the amniotic fluid, is associated with >40% of preterm labor [2, 4–6]. Apart from maternal morbidity, chorioamnionitis increases the risk for brain white matter injury [7, 8], necrotizing enterocolitis [9], and chronic lung disease in preterm infants. However, the mechanisms of chorioamnionitis-induced preterm labor and fetal inflammation are poorly defined.
While chorioamnionitis is most frequently an ascending infection, elegant clinical and experimental studies in macaques have demonstrated that microbial invasion of amniotic cavity is required to elicit the full-blown syndrome of preterm labor and fetal organ injury [10–13]. On the other hand, focal deciduitis with increased amniotic fluid (AF) cytokines without microbial invasion is sufficient to cause fetal lung inflammation/injury but not preterm labor [11–13]. First, we used a model of direct intra-amniotic injection (IA) of a proinflammatory agonist exposure. Second, the non-human primate model of chorioamnionitis is particularly relevant for the study of mechanisms of infection associated with preterm labor because the reproductive biology and immunology is very similar to that of humans [14, 15]. Our experimental approach to model chorioamnionitis is also supported by intra-amniotic injections of proinflammatory agonists in rodents, rabbit, and sheep [16, 17].
Although human studies are informative [18, 19], the lack of appropriate preterm controls and confounding by commonly administered medications such as betamethasone are problematic. In a series of studies by Adams et al. [20], Grigsby et al. [21], and Novy et al. [22], IA lipopolysaccharides (LPS), IL-1β, or live Ureaplasma parvum increased AF levels of several proinflammatory cytokines and induced preterm labor in rhesus macaque, Macaca mulatta. Similarly, we recently reported that preterm rhesus macaque exposed to IA IL-1β developed chorioamnionitis and fetal inflammation characterized by a skewed balance between T regulatory cells (Tregs) and IL-17+ cells in fetal lymphoid tissue [23]. However, the source of cytokines and mechanisms of preterm labor were not defined in those studies.
Macrophages and T cells infiltrate the choriodecidua, the tissue at the maternal-fetal interface, prior to the onset of labor in humans and experimental animals [18, 24, 25]. Extraplacental fetal membranes can secrete proinflammatory mediators in vitro in response to a proinflammatory stimulus or when harvested from laboring women [26, 27]. However, very little is known about the immunology of the decidua leukocytes and expression of cytokines/chemokines by fetal membranes in vivo during chorioamnionitis.
To understand the pathogenesis of inflammation-induced prematurity, we used injection of IA IL-1β as our model, because previous studies demonstrated that this single cytokine induced a cascade of inflammation leading to preterm labor [28–32]. To understand the early events preceding preterm labor, rhesus macaques at ∼80% gestation were given IA IL-1ß, and fetuses were delivered surgically 24 h or 72 h later. Given the predominance of neutrophil infiltration in the decidua during acute chorioamnionitis [33], we hypothesized that neutrophils recruited by IA IL-1β to the decidua would be a major source of cytokine expression. We report both the proinflammatory and counter regulatory factors induced in the decidua after IA IL-1β.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of California Davis. Experiments were conducted with the Society for the Study of Reproduction's specific guidelines and standards. Normally cycling adult female rhesus macaques (Macaca mulatta [n = 23]) were time mated. Animals were randomized to receive either 10 μg of human recombinant IL-1β (Peprotech, Rockyhill, NJ) in 1 ml of saline or 1 ml of saline by ultrasonography-guided IA injection. Hysterotomies were performed 24 or 72 h post-IA injection with delivery of the fetus at 130 ± 2 day gestational age (term = 165 ± 10 days) (Table 1) as previously described [23]. The time points of 24 h or 72 h after IA IL-1ß were chosen to capture the kinetics of early amniotic fluid/chorioamnion-decidua inflammation prior to overt preterm labor, reported to occur at approximately 3 days after IA IL-1β [30]. The animals were delivered at 80% term gestation to model preterm delivery in the third trimester. There were no deaths or overt preterm labor in the animals.
TABLE 1.
Description of the animals included in the study.
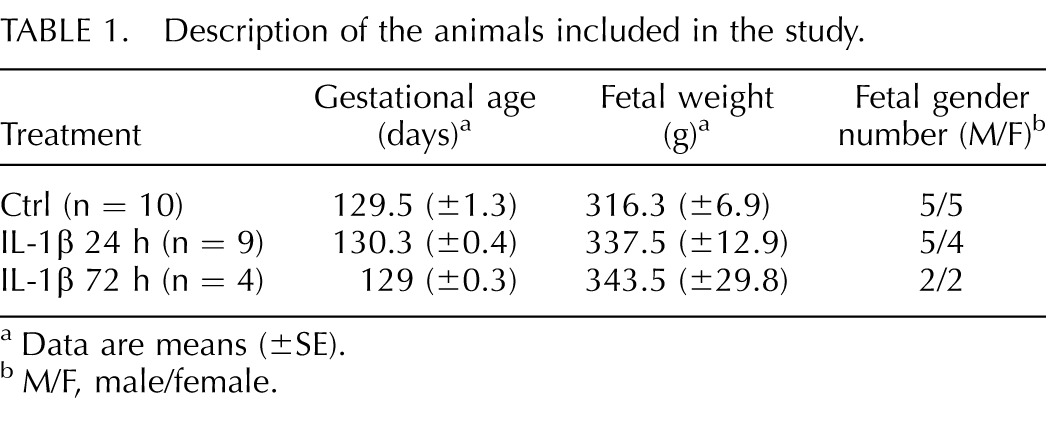
Data are means (±SE).
M/F, male/female.
Decidua Cell Suspensions
Purified decidua cell suspensions were prepared as previously described with some modifications [34]. Briefly, the membranes were dissected from each placenta. Decidua parietalis was scraped from the underlying chorion, washed, and digested at 37°C with 125 mg/100 ml dispase II (Life Technologies, Grand Island, NY) plus 50 mg/100 ml collagenase A (Roche, Indianapolis, IN) in Dulbecco modified Eagle medium-F12 (DMEM-F12) medium with antibiotics. After 30 min, 0.4 mg/100 ml DNase I (Roche) was added to the cell suspension for an additional 30 min at 37°C on a shaking platform. Cell suspensions were filtered through 70-μm cell strainers twice, washed in PBS, and counted. Red blood cell lysis was performed using an ammonium chloride-potassium carbonate-ethylenediaminetetraacetic acid solution. Viability was >90% by trypan blue exclusion test.
Flow Cytometry
For multiparameter flow cytometry (LSR Fortessa; BD Biosciences, San Diego, CA), a cocktail of conjugated antibodies validated for the rhesus macaque was used (http://www.nhpreagents.org/NHP/clonelist.aspx?ID=15). For most of the immunophenotyping studies, we used freshly isolated purified decidua cell suspensions. The following monoclonal antibodies were used: anti-CD3 (SP34-2), anti-CD16 (3G8), anti-CD56 (NCAM16.2), anti-CD45 (D058-1283), anti-Ki67 (B56), anti-CD4 (L200), anti-CD25 (M-A251), anti-tumor necrosis factor α (TNFα; clone Mab11; BD Biosciences); anti-FOXP3 (clone PCH101; eBioscience, San Diego, CA); anti-HLA-DR (L243; Biolegend, San Diego, CA); anti-CD14 (TUK14; Life Technologies); anti-NKp46 (BAB281; Beckam Coulter, Brea, CA); anti-NKG2a (REA110; Myltenyi Biotech, San Diego, CA); anti-CD88 (P12/1; AbD Serotec, Hercules, CA). In preliminary experiments, we noted that CD4 and CD8 expression levels were down-regulated by the protease digestion procedures, consistent with other reports [35–37]. Therefore, to evaluate the T-cell subset phenotype, cells were cultured overnight at 37°C, in 5% CO2 in DMEM:F12 containing 10% fetal bovine serum, 100 IU/ml penicillin, 100 IU/ml streptomycin, and 2 mmol/L glutamine (viability >85% by trypan blue exclusion test). To evaluate baseline intracellular cytokine production induced by in vivo exposures, decidua parietalis cells were used for flow cytometry either immediately after isolation or after an overnight culture and 1 μl/ml monensin (eBioscience) plus 10 μg/ml brefeldin A (Sigma-Aldrich, St. Louis, MO) for 5 h prior to staining (both procedures yielded similar results). Cells were treated with 20 μg/mL human immunoglobulin G (IgG) to block Fc receptors, stained for surface markers for 30 min at 4°C in PBS, washed, and fixed in 1% paraformaldehyde. Intracellular staining for TNFα was performed by cytokine staining (Cytofix/Cytoperm; BD Bioscience) according to the manufacturer's instructions. All antibodies were titrated for optimal detection of positive populations and mean fluorescence intensity. A total of 500 000 events were recorded for each sample. Doublets were excluded on the basis of forward scatter properties, and dead cells were excluded using LIVE/DEAD Fixable Aqua dead cell stain (Life Technologies). Unstained and isotype controls were used to determine positive staining for each marker. We used a negative biological population for FOXP3 (CD3−CD4− cells) as reference to set up the cut-off for FOXP3+ cells in CD3+CD4+ T cells [38]. Data were analyzed using FlowJo version 9.5.2 software (TreeStar Inc., Ashland, OR).
Immunohistochemistry
Immunohistochemistry was performed as previously described [23]. Briefly, the sections were incubated with either anti-human CD68 (product KP1; 1:100 dilution; Dakocytomation, Glostrup, Denmark), vimentin (product C-20; 1:50 dilution; Santa Cruz Biotechnology, Dallas, TX), pan-cytokeratin (product AE1/AE3; 1:500 dilution; Invitrogen), IL-8/CXCL8 (product G265-8; 1:25 dilution; BD Biosciences), neutrophil elastase (product ab68672; 1:100 dilution; Abcam, Cambridge, MA), IDO1 (product 10.1; 1:25 dilution; Millipore, Billerica, MA) in 4% normal goat serum at 4°C overnight, followed by biotin-labeled secondary antibody. Immunostaining was visualized using Vectastain ABC peroxidase Elite kit (Vector Labs, Burlingame, CA) to amplify antigen-antibody complexes. Additionally, immunofluorescence staining was performed using the previously mentioned antibodies in 10% normal horse serum/0.2% Tween-20 at 4°C overnight. Staining was visualized using fluorescently labeled secondary antibodies (AF488 or AF594; 1:200 dilution; Invitrogen) for 1 h at room temperature. Nuclear counterstain was achieved using Vector Shield Hard-Set mounting medium with 4′,6-diamidino-2-phenylindole (DAPI; Vector Labs).
RNA Isolation, cDNA Generation, and Quantitative RT-PCR
Total RNA was extracted from snap-frozen chorioamnion-decidua, and the amnion was peeled from choriodecidua (in a subset of animals) and a full-thickness section of the uterus after homogenizing in TRIzol (Invitrogen). RNA concentration and quality were measured by Nanodrop spectrophotometer (Thermo-Scientific). Reverse transcription of the RNA was performed using Verso cDNA synthesis kit (Thermo-Scientific), following the manufacturer's protocol. Quantitative RT-PCR was carried out in a StepOnePlus real-time PCR system (Life Technologies) following standard cycling conditions. Quantitative RT-PCR assays were performed with rhesus-specific TaqMan gene expression primers (Life Technologies). Eukaryotic 18S rRNA (Life Technologies) was endogenous control for normalization of the target RNAs, and a sample from an IA saline animal was used as the calibrator. The cytokine values after IA IL-1β were expressed relative to the average value of the IA saline control group.
Cytokine Measurement
The chorioamnion tissue was homogenized in 50 mM Tris-HCl buffer (pH7.5), 1 mM EGTA, and 1 mM EDTA containing protease inhibitor cocktail and 1M phenylmethylsulfonyl fluoride (Sigma-Aldrich). Homogenates were centrifuged, supernatants were collected, and the protein concentration was determined by BCA protein assay reagent kit (Pierce, Rockford, IL). Cytokine/chemokine concentrations were determined by Luminex using non-human primate multiplex kits (Millipore) according to the manufacturer's protocol. Concentrations were calculated from standard curves using recombinant proteins. For the AF, the cytokine values were expressed as picograms per milliliter, and for the chorioamnion-decidua or amnion tissue homogenates, the values were expressed as picograms per nanograms of total protein concentration.
Statistical Analyses
Prism version 5.0b software (GraphPad, La Jolla, CA) was used to analyze data. Values were expressed as means ± SE. Kruskal-Wallis tests were used to determine whether the three groups were significantly different. Intra-group comparisons were analyzed using the paired t-tests. Intergroup comparison was evaluated by using Mann-Whitney U tests. Results were considered significant for P values of ≤0.05. However, due to the limited number of samples per group, we also report trends (P value between 0.05 and 0.15). Where not reported, P is >0.15.
RESULTS
We confirmed that IA IL-1β caused acute chorioamnionitis characterized by a large neutrophil infiltration of the fetal membranes (Supplemental Fig. S1; all Supplemental Data are available online at www.biolreprod.org), consistent with our previous results [23].
Intra-Amniotic IL-1β Increased Cytokine Expression in Amniotic Fluid and Chorioamnion-Decidua Tissue
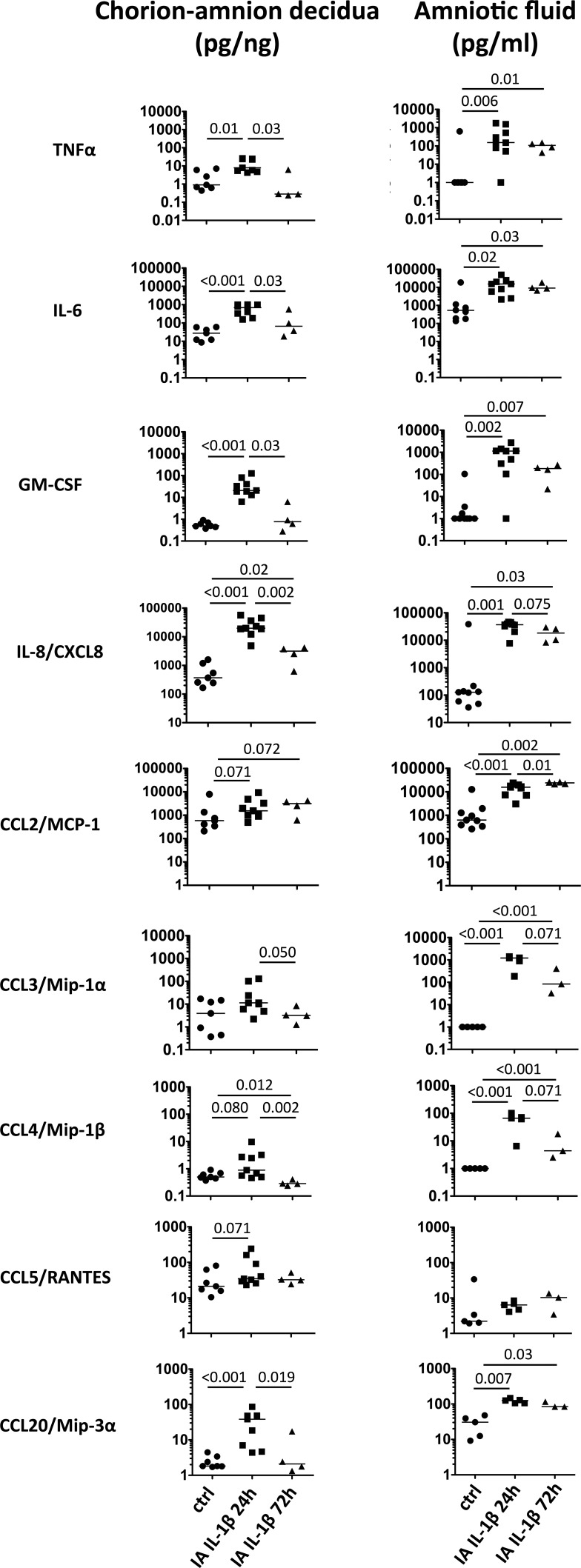
Amniotic fluid cytokines can be used as an adjunct to characterize the degree of intrauterine inflammation [39, 40]. To assess cytokine profile induced by IA IL-1β in the gestational tissues, we measured the levels of pro- and anti-inflammatory cytokines in chorioamnion-decidua tissue homogenate and AF. The choice of cytokines/chemokines were based on querying a broad target of factors reported in the preterm labor reports, representative genes for the different T-cell subset pathways [41, 42], and the availability of rhesus-specific antibodies for multiplex ELISA. IA IL-1β increased several proinflammatory cytokines and chemokines (TNFα, IL-6, GM-CSF, IL-8/CXCL8, CCL2/MCP-1, CCL3/Mip-1α, CCL4/Mip-1β, CCL5/RANTES, and CCL20/Mip-3α) in parallel with the chorioamnion-decidua and AF (Fig. 1). Values were high at 24 h and decreased to control levels at 72 h for most cytokines, except for IL-8 and CCL2 in chorioamnion-decidua. In contrast, AF levels remained higher than those of control at 72 h. IA IL-1β exposure did not change the levels of IL-10 in chorioamnion-decidua or in the AF (data not shown). IL-12p40, IL-17A, and interferon γ (IFN-γ) levels were undetectable in both chorioamnion-decidua and AF (not shown).
FIG. 1.
IA IL-1β increased inflammatory cytokines in chorioamnion-decidua and AF. Dot plots show cytokine levels in chorioamnion-decidua tissue homogenates and AF from controls (ctrl); IA IL-1β animals exposed for 24 h and 72 h. Horizontal bars correspond to medians, and P values are shown (n = 4–9 animals/group).
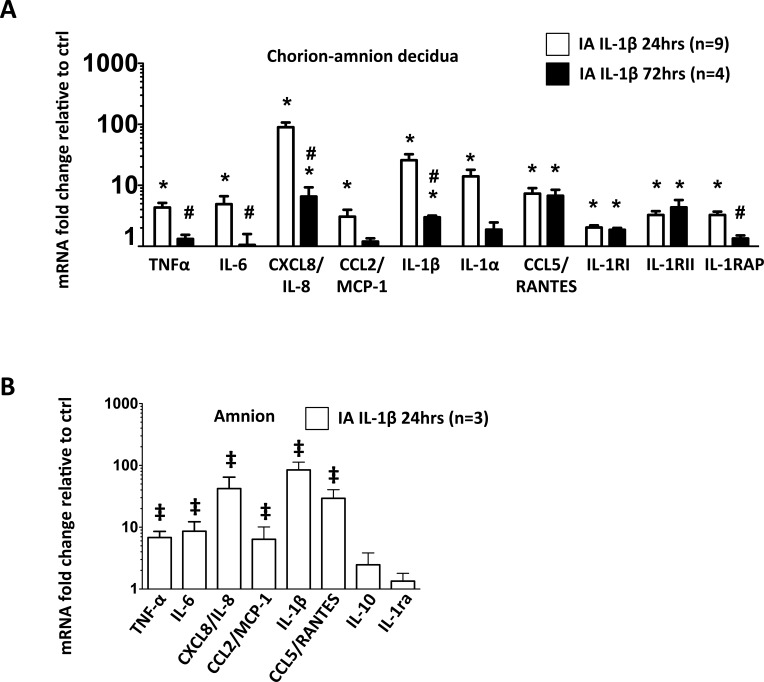
Expression of cytokine mRNA from intact chorioamnion-decidua tissue confirmed the protein levels (Fig. 2A). Moreover, our data also showed a significant increase in IL-1 receptors type I (IL-1RI), type II (IL-1RII), and IL-1 receptor accessory protein (IL-1RAP) mRNA levels in chorioamnion-decidua of IA IL-1β-injected animals compared to those in control animals (Fig. 2A).
FIG. 2.
IA IL-1β increased proinflammatory cytokine mRNAs in chorioamnion-decidua and amnion tissue. mRNAs were measured by quantitative RT-PCRs and normalized to the expression of eukaryotic 18S rRNA in chorion-amnion decidua (A), and amnion dissected free of the chorion and decidua parietalis (B). Values for each gene in experimental animals were expressed as fold change relative to a pooled average of control animals in the chorioamnion-decidua tissue (A) and amnion tissue (B). *P < 0.05 compared to controls; #P < 0.05 compared to IA IL-1β 24-h group; ‡P ≤ 0.1 compared to controls.
To understand the role of the amnion, the tissue in contact with AF, in mediating inflammation, we measured mRNAs for different cytokines in the amnion separated from the chorion and the decidua in a subset of animals. mRNA expression of proinflammatory cytokines increased by 24 h after IA IL-1β injection (Fig. 2B). Of note, compared to proinflammatory cytokines, the anti-inflammatory cytokines IL-10 and IL-1 receptor antagonist (IL-1ra) did not increase after IA IL-1β exposure (P = 0.2 vs. control, Fig. 2B). Similar increases in cytokine proteins were also detected in the amnion tissue (not shown). No significant apoptosis, as indicated by TUNEL staining and activated caspase-3 immunostaining, was evident in the fetal membranes of either control or experimental animals (data not shown). Together, these data showed that the amnion is a source of proinflammatory cytokine production.
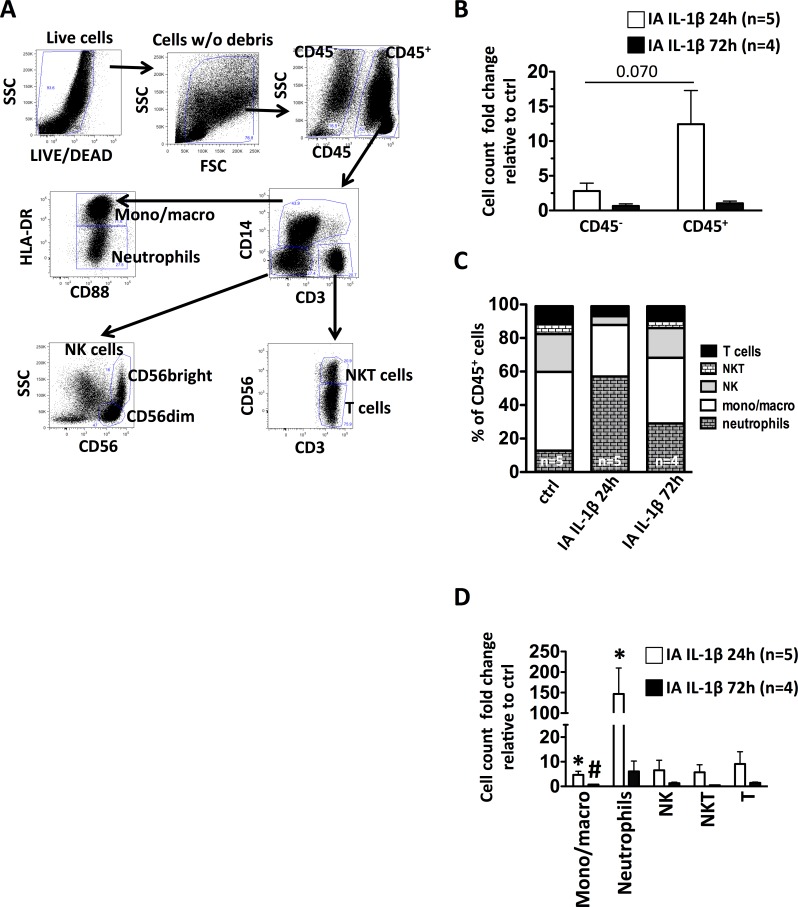
Intra-Amniotic IL-1β Induced a Neutrophil-Dominant Leukocyte Infiltration in Decidua Parietalis
We analyzed different decidua cell populations by multiparameter flow cytometry (Fig. 3A). IA IL-1β caused an increase in CD45+ cell number (Fig. 3B) as well as a change in cell population frequency in the decidua parietalis (Fig. 3C). Compared to the controls, the number of CD45− cells were approximately 2-fold increased, whereas CD45+ cells were ∼15-fold increased at 24 h after IA IL-1β, with a subsequent decrease to near control levels at 72 h (Fig. 3B). Within the CD45+ cell population, the relative frequency of monocyte/macrophages (defined as CD3−CD14highCD88+HLADR+ cells) did not change between the controls and the IA IL-1β-exposed animals (Fig. 3C). However, their numbers were ∼4.5-fold increased at 24 h, with continued modest increases at 72 h after IA IL-1β because the overall leukocyte population increased (Fig. 3D). Neutrophils (defined as CD3−CD14lowHLADRlowCD88+) were ∼100-fold increased at 24 h after IA IL-1β exposure and remained increased at 72 h (Fig. 3D). The relative frequency of CD14−CD3+CD56+ NKT cells and CD14−CD56−CD3+ T cells did not change after IA IL-1β, whereas the relative frequency of CD3−CD14−CD56+ NK cells (CD56dim and CD56bright) decreased at 24 h (Fig. 3C). However, the overall numbers of NKT, T cells, and NK cells increased, albeit nonsignificantly, 24 h after IA IL-1β (Fig. 3D).
FIG. 3.
CD45+ cell recruitment in decidua parietalis after IA IL-1β exposure. Decidua parietalis cell suspensions were analyzed by multiparameter flow cytometry. A) Representative gating strategy was used to characterize the different leukocyte subpopulations in decidua parietalis: live cells were first identified by the absence of LIVE/DEAD stain and forward-/side-scatter expression, excluding cell debris. Then, leukocytes were gated as CD45+ cells. Inside the CD45+ cells, the leukocyte subpopulations were gated as monocytes/macrophages (CD3−CD14highCD88+HLA-DR+); neutrophils (CD3−CD14lowHLADRlowCD88+); NK cells (CD3−CD14−CD56+); T cells (CD14−CD3+CD56−); and NKT cells (CD14−CD3+CD56+). B) IA IL-1β exposure increased the number of decidua CD45+ leukocytes at 24 h. (C) Relative frequency of leukocyte subpopulations. (D) IA IL-1β exposure increased significantly the number of decidua neutrophils and monocytes/macrophages. *P < 0.05 compared to controls; #P < 0.05 compared to the 24-h IA-IL-1β group. SSC, side scatter; FSC, forward scatter.
In the first trimester of gestation, rhesus macaque decidua CD56+ NK cells are the most abundant leukocyte population, with functional differences in subsets with different levels of CD56 expression (CD56dim vs. CD56bright) [43]. At 80% term gestation, CD56dim NK frequency was higher than CD56bright NK cells in all groups and achieved significance in the 24-h IL-1β-treated animals (control: 56 ± 7% vs. 39 ± 7%; IL-1β at 24 h: 77 ± 5% vs. 17 ± 3%, P = 0.031; IL-1β at 72 h: 62 ± 13% vs. 28 ± 13%). Compared to CD56dim NK cells, CD56bright NK cells expressed higher levels of CD16 and NKp46 (Table 2). However, the expression of NKG2a (CD159a), a negative regulator of NK cell-mediated target-cell lysis, did not change between CD56dim and CD56bright NK cells (Table 2).
TABLE 2.
Activation markers in NK cell subsets from decidua parietalis.a
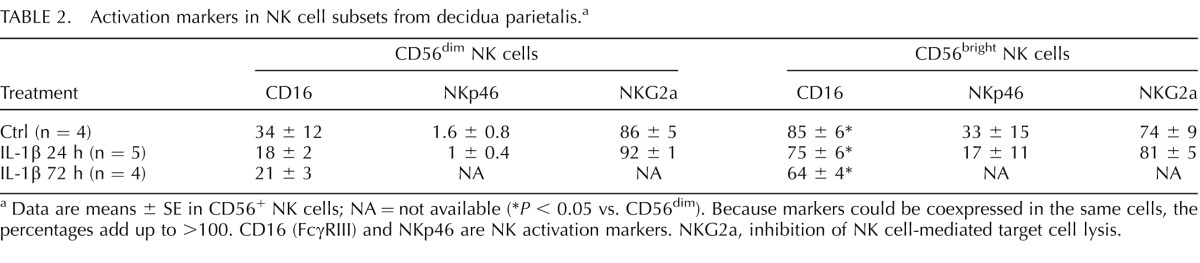
Data are means ± SE in CD56+ NK cells; NA = not available (*P < 0.05 vs. CD56dim). Because markers could be coexpressed in the same cells, the percentages add up to >100. CD16 (FcγRIII) and NKp46 are NK activation markers. NKG2a, inhibition of NK cell-mediated target cell lysis.
Lymphocyte numbers in the decidua were low. Most CD3+ T cells in the decidua were CD3+CD4− T cells, suggesting that they were either CD8+ cells or possibly subsets of γδ+ T cells or CD4−CD8−αβ+ T cells [44], but IA IL-1β did not change the frequency of these T cells (72 ± 3.4% vs. 63 ± 6.9% of CD3+ cells in control and at 24 h IL-1β, respectively, P = 0.7).
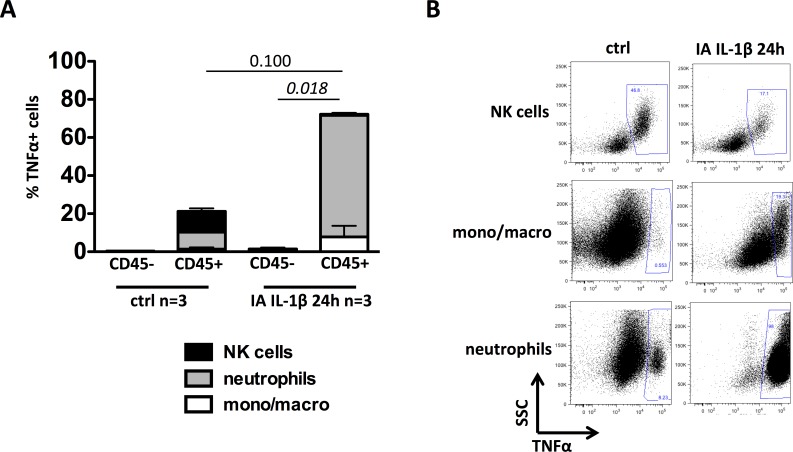
Intra-Amniotic IL-1β Increased Decidua Neutrophil Production of TNFα
We measured intracellular expression of TNFα in decidua cells by flow cytometry ex vivo without additional in vitro stimulation. TNFα+ cells were almost exclusively in CD45+ cells compared to CD45− cells (Fig. 4A). Moreover, in IA IL-1β-treated animals, the frequency of TNFα+CD45+ cells tended to be higher than in control animals (P = 0.100) (Fig. 4A). We then examined the relative contribution of different leukocytes to TNFα production. In controls, the NK cells and neutrophils were the major producers of TNFα (Fig. 4, A and B). However, after 24 h, IA IL-1β, the neutrophils and monocyte/macrophages were the predominant TNFα+ cells, whereas the frequency of TNFα+ NK cells greatly decreased (Fig. 4, A and B). NKT and T cells did not express TNFα in controls or after IA IL-1β exposure (not shown).
FIG. 4.
IA IL-1β exposure increased the frequency of TNFα+ neutrophils in decidua parietalis. A) Frequency of TNFα-producing cells in both CD45+ and CD45− cell populations and their relative distribution: monocytes (mono)/macrophages (macro) (white), neutrophils (gray), and NK cells (black). B) Representative dot plots show TNFα production by NK cells, neutrophils, and mono/macro cells in both controls and animals at 24 h after exposure to IA IL-1β. SSC, side scatter.
Intra-Amniotic IL-1β Increased Decidua Neutrophil Production of IL-8
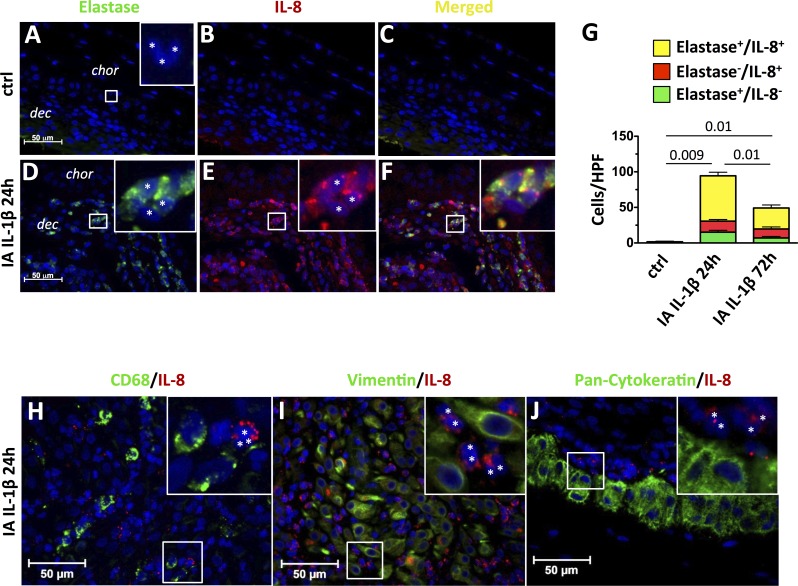
Because IL-8 mRNA was the chemokine most up-regulated by IA IL-1β, we localized the cellular source of IL-8 production in the chorioamnion-decidua tissue by using immunohistology colocalization. Control animals had a few polymorphonuclear neutrophils (Fig. 5A), confirming our results by flow cytometry (Fig. 3C). These cells were neutrophil elastase negative consistent with non-activated neutrophils (Inset in Fig. 5A). Compared to controls, the expression of both neutrophil elastase and IL-8 increased 24 h after IA IL-1β (compare Figs. 5, A and B, to 5, D and E). In the merged field, most neutrophil elastase+ cells were also IL-8 positive (Fig. 5F, inset). Neutrophil elastase+ cells were polymorphonuclear cells, confirming that they were neutrophils (Fig. 5D, inset). At the choriodecidual junction, neutrophil elastase+/IL-8+ cells at 24 h and 72 h after IA IL-1β accounted for approximately 75% of all IL-8+ cells (Fig. 5G). Of note, we did not find significant colocalization of IL-8 and CD68, IL-8 and vimentin, or IL-8 and pan-cytokeratin, indicating that macrophages, decidua stroma cells, and trophoblast cells were not important sources of decidua IL-8 (Figs. 5, H, I, and J, respectively).
FIG. 5.
Neutrophils but not macrophages, decidua stromal cells, or fetal trophoblast cells produce IL-8 after IA IL-1β exposure. Chorioamnion-decidua sections from paraffin-embedded blocks were used for immunocolocalization studies and stained with neutrophil elastase (green) plus IL-8 (red) plus DAPI (blue). Representative sections are shown from control animals (A–C) and from animals exposed to IA IL-1β (D–F) at 24 h. In controls animals, cells with polymorphonuclear neutrophil morphology did not express neutrophil elastase (A) or IL-8 (B) or both (C). In contrast, IA IL-1β injection increased the proportion of polymorphonuclear cells (*inset) that expressed neutrophil elastase (D) or IL-8 (E). F) Merged images show colocalization of IL-8 and neutrophil elastase in the same cells. A, D, E, and F insets) Higher magnification pictures. G) Cell count/high powered field (HPF, ×40) of cytoplasmic neutrophil elastase+/IL-8−, elastase−/IL-8+, or neutrophil elastase+/IL-8+ (double-positive) cells in the chorion-decidua junction. P values for neutrophil elastase+/IL8+ neutrophils between the three groups of animals are shown. H–J) Representative sections from IA IL-1β 24-h animals of IL-8 colocalization and either macrophages (CD68 [green; H]), stromal cells (vimentin [green; I]), or trophoblast cells (pancytokeratin [green; J]). CD68+, vimentin+, or pancytokeratin+ cells do not colocalize with IL-8. In all panels, IL-8 expression is in red and DAPI is in blue. H, I, and J insets) Higher magnification picture, and white asterisks indicate IL-8+ polymorphonuclear cells. Original magnification ×4 (insets in A, D–F) and ×3 (insets in H–J).
Decidua Neutrophils Express IDO in Response to IA IL-1β
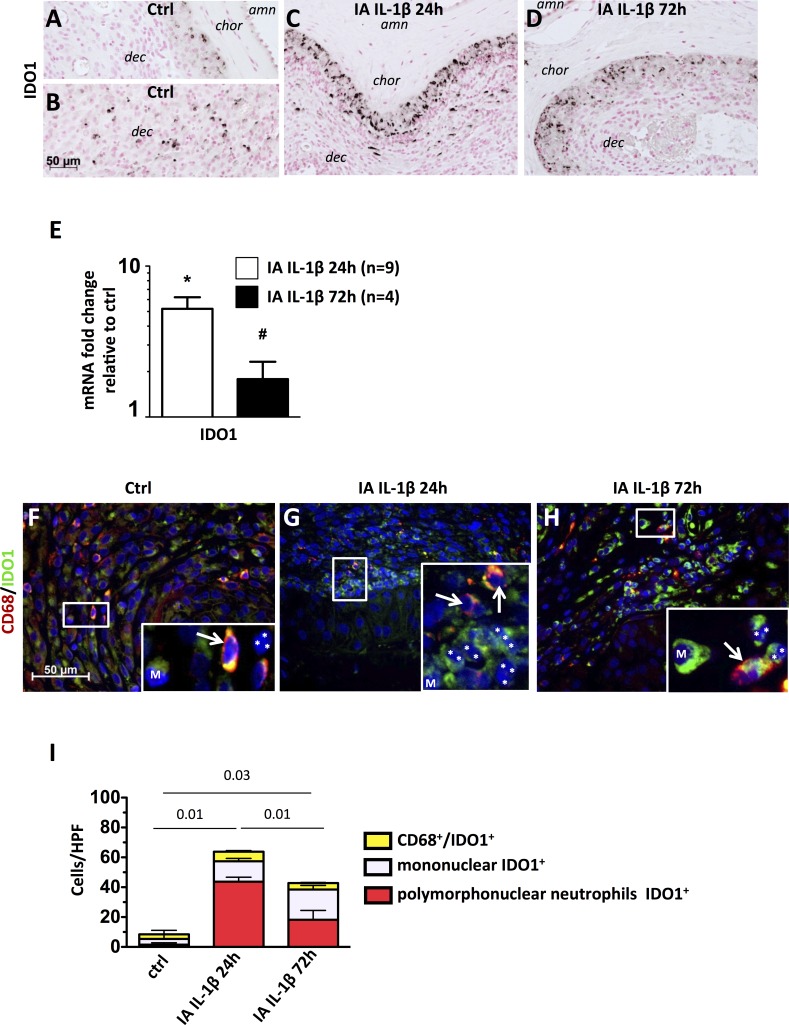
In order to understand counterbalancing mechanisms, we measured Tregs frequency and expression of indoleamine, IDO, IL-10, and IL-1ra. Very few IDO1+ cells were found at the chorion-decidua junction in control animals (Fig. 6A), but some were present in the deep decidua (Fig. 6B). IDO1+ cells at the chorion-decidua junction increased greatly after IL-1β exposure at 24 h (Fig. 6C) and to a lesser extent at 72 h (Fig. 6D). In parallel, compared to controls, mRNA expression for the IDO1 were ∼5-fold increased at 24 h after IA IL-1β exposure (P < 0.001) and remained ∼2-fold increased after 72 h (P = 0.016) (Fig. 6E). IDO1-producing cells were largely polymorphonuclear neutrophils and not CD68+ cells (Fig. 6, F–H, note lobes [asterisks] of the neutrophil nucleus in panel insets). Quantitation of the cell types producing IDO1 demonstrated that in the controls, macrophages and other decidua cells produced IDO1, whereas the neutrophils accounted for 40% of IDO1+ cells at 24 h and for 20% of IDO1+ cells at 72 h after IA IL-1β (Fig. 6I).
FIG. 6.
IA IL-1β exposure increased IDO1 production by neutrophils. A–D) Representative photomicrographs show immunohistology of IDO1-expressing cells in chorioamnion-decidua of control animals (A, B), those exposed to IA IL-1β after 24 h (C), and those exposed to IA IL-1β after 72 h. D) amn = amnion; chor = chorion; dec = decidua. E) Target mRNAs in chorioamnion-decidua were measured by quantitative RT-PCR and normalized to the expression of eukaryotic 18S rRNA. Values in experimental animals were expressed as fold change relative to that of a pooled average in the control animals (n = 5). *P < 0.05 compared to controls; #P < 0.05 compared to the group exposed to IA IL-1β at 24 h. IA IL-1β exposure increased IDO1. F–H) Representative photomicrographs from the merged frames from CD68 (green) plus IDO1 immunocolocalization. F) Higher magnification (insets ×40) views of samples from control animals show that only mononuclear cells (M) or CD68+ cells (white arrows) express IDO1, whereas IDO1+ cells are the predominant polymorphonuclear cells (nuclear lobes are shown by white asterisks) in IA IL-1β 24 h (G) and IA IL-1β 72 h (H) animals. I) Graph shows the cell count/HPF (×40) of IDO1+ cells in the choriodecidua junction: control animals show few CD68+IDO1+. In contrast, in IL-1β-treated animals, most polymorphonuclear neutrophils (nuclei indicated by white asterisks) are IDO1+. P values compare polymorphonuclear neutrophils IDO+ counts among the three groups of animals are shown. Original magnification ×3 (insets in F–H).
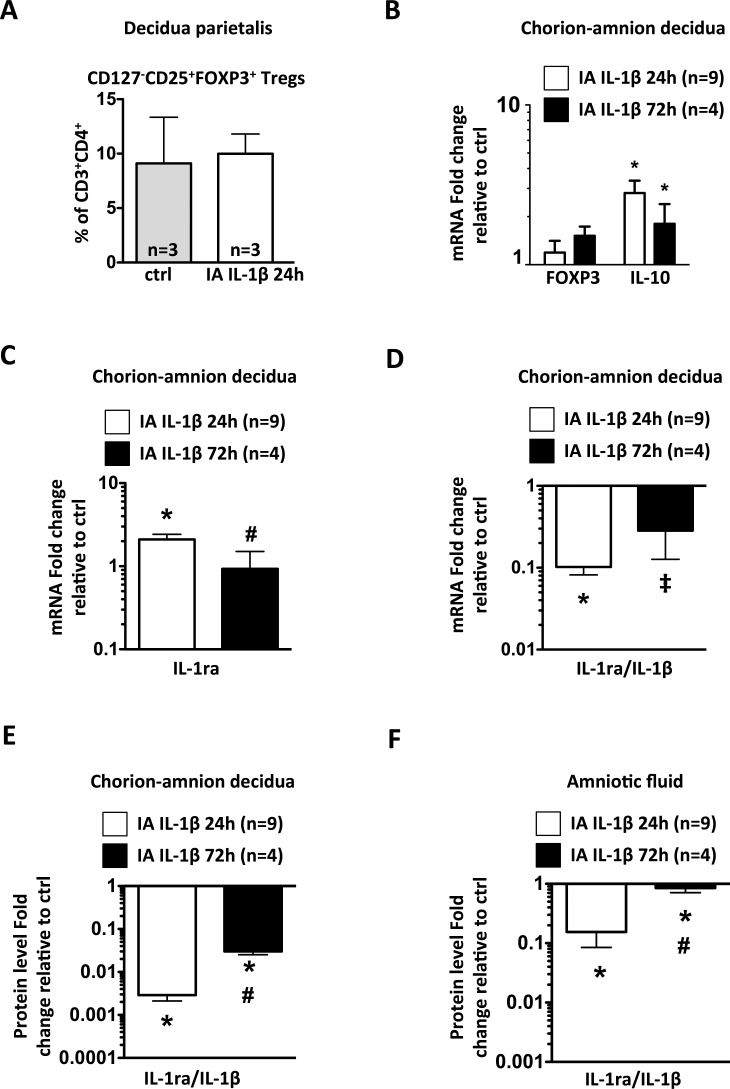
The frequency of Tregs (defined as CD3+CD4+CD127−CD25+FOXP3+ cells) in decidua parietalis and FOXP3 mRNA expression in chorioamnion-decidua did not change with IA IL-1β exposure (Fig. 7, A and B). Compared with controls, anti-inflammatory cytokine IL-10 mRNA increased significantly in the chorioamnion-decidua 24 h after IA IL-1β exposure (P = 0.001) and decreased at 72 h, although it remained marginally significantly higher than in controls (P = 0.053) (Fig. 7B). In the chorioamnion-decidua tissue, expression of the anti-inflammatory IL-1ra mRNA increased modestly (Fig. 7C) compared to large increases in IL-1β mRNA (Fig. 2A), resulting in a decreased IL-1ra:IL-1β ratio (Fig. 7D). The net result would be predicted to be an unbalanced IL-1β activity. These results were confirmed at the protein level (Fig. 7E). Similar changes in the IL-1ra/IL-1β axis were also observed in the AF (Fig. 7F).
FIG. 7.
IA IL-1β exposure modestly increased IL-10 mRNA expression and decreased the IL-1RA:IL-1β ratio in chorioamnion-decidua. A) Frequency of CD3+CD4+CD127−CD25+FOXP3+ Tregs in decidua parietalis was measured by flow cytometry. B–D) mRNAs were measured by quantitative RT-PCRs in chorioamnion-decidua and normalized to the expression of eukaryotic 18S rRNA. Values in experimental animals were expressed as fold change relative to a pooled average in the control animals (n = 5). B) IA IL-1β exposure significantly increased IL-10 mRNA but not FOXP3 mRNA. C) A modest increase of IL-1ra mRNA was observed after IA IL-1β 24-h exposure. D) Graph shows a significant decrease of IL-1ra/IL-1β mRNA ratio after IA IL-1β exposure. Graphs show a significant decrease of IL-1ra/IL-1β protein level ratio after IA IL-1β exposure in chorion-amnion decidua (E) and amniotic fluid (F). *P < 0.05 and ‡P = 0.072 compared to controls; #P < 0.05 compared to IA IL-1β 24-h group.
IA IL-1β-Induced Uterine Connexin-43
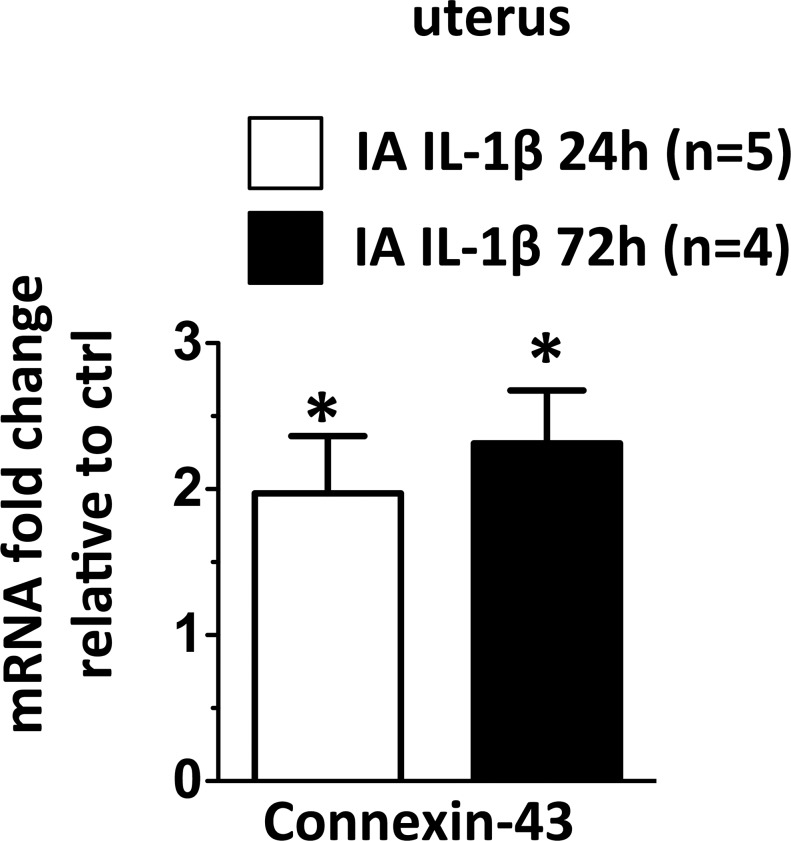
Our study was designed to understand the immunologic changes in gestational tissues preceding overt preterm labor. We therefore measured expression of gap junction protein connexion-43 (Cx-43) mRNA in the uterus, an established marker of early labor [45–47]. Cx-43 mRNA expression increased at 24 h (P = 0.05) and remained elevated at 72 h (P = 0.03) following IA IL-1β (Fig. 8).
FIG. 8.
IA IL-1β exposure increased Cx-43 expression in the uterus. Graph shows Cx-43 mRNA and the values in experimental animals were expressed as fold change relative to those of pooled average in the control animals (n = 5). *P < 0.05 compared to controls.
DISCUSSION
Leukocyte infiltration of several gestational tissues with elaboration of proinflammatory mediators occurs around the time of parturition [48–50], with most of the leukocyte infiltration occurring in the decidua, the tissue at the maternal-fetal interface [18]. Macrophages and T cells have been implicated in the pathogenesis of labor, because macrophages also infiltrate the decidua prior to preterm or term labor in mice, rats, and humans [18, 24, 25]. However, during acute chorioamnionitis, neutrophils are the predominant leukocytes infiltrating the decidua [33]. Although acute chorioamnionitis is an important cause of preterm labor in humans [51], very little information is available about the role of the immune cells in the setting of chorioamnionitis. In this study, we report the novel observation that in a clinically relevant model of acute sterile chorioamnionitis in the rhesus macaque, neutrophils rapidly and robustly infiltrated the decidua, were activated, and were the predominant source of expression of proinflammatory cytokines/chemokines and the regulatory enzyme indoleamine, IDO. The finding is significant because of the large surface area of decidua parietalis and its close proximity to the myometrium. Although decidua stroma cells can produce cytokines in vitro [34, 52], we demonstrate that the decidua stroma cells or other CD45− resident decidua cells are not important sources of TNFα and IL-8 during acute sterile chorioamnionitis.
Although experimental models using intra-amniotic injections of proinflammatory agonists have been reported extensively [17, 20, 29–32], there are some concerns about how accurately they might reflect human chorioamnionitis. In particular, the question is whether the lower genital organisms ascend into the upper genital tract, cause a diffuse choriodeciduitis, and then invade the amniotic cavity, resulting in an “outside-in” signaling from the decidua to the amniotic fluid. In this regard, based on careful testing of the amniotic fluid, fetal membrane histology and in situ hybridization for the bacterial 18S RNA in human samples, Kim et al. [10] concluded that the lower genital organisms initially disseminate into the amniotic cavity from a focal and not generalized choriodeciduitis, resulting in signaling to the decidua from amniotic cavity in an “inside-out” fashion. Direct infusion of live microorganisms into the choriodecidua of non-human primates resulted in an inconsistent generalized chorioamnionitis [11, 12]. However, preterm labor occurred with detection of microorganisms in the amniotic fluid, extensive chorioamnionitis, and high levels of cytokines in the amniotic fluid, underscoring the importance of inflammation in the amniotic cavity. Recent studies in a large number of women presenting with preterm labor demonstrated that the most cases with high levels of IL-6 in the amniotic fluid have no detectable microorganisms in the amniotic fluid by culture and sensitive molecular microbiological methods [53, 54]. Furthermore, the correlation with preterm labor was with severe amniotic fluid inflammation with or without amniotic fluid infection. Although these clinical and experimental studies do not settle the question of how lower genital microorganisms induce inflammation in the amniotic cavity, they lend support to the validity of using direct injection of proinflammatory agonists in the amniotic fluid to model chorioamnionitis.
The mechanisms of leukocyte infiltration in the decidua during chorioamnionitis are not known. Although we did not determine their origins, the decidual leukocytes are reported to be largely from maternal circulation, whereas the leukocytes in AF are largely of fetal origin [55, 56]. Furthermore, neutrophil infiltration in the fetal membranes during acute chorioamnionitis preferentially occurs at the choriodecidual junction [33], with relative sparing of the amnion, the tissue in closest proximity to the AF. We demonstrate that in response to a proinflammatory signal in the amniotic cavity, the amnion produces several proinflammatory cytokines/chemokines in vivo (e.g., IL-8, IL-1β, MCP-1, RANTES), which are known chemoattractants for neutrophils and monocytes. These cytokines produced by the amnion likely diffuse into the amniotic fluid. Of note, among the anti-inflammatory cytokines tested, the expression of IL-10 and IL-1ra did not increase in the amnion. Our results demonstrate a potential novel function of the amniotic epithelium to orchestrate decidua leukocyte influx in response to a proinflammatory signal in the AF.
The leukocyte pool size was 10–15-fold increased in the decidua 24 h after IA IL-1β. This estimate is likely valid because the entire decidua was sampled rather than a small fraction used in histologic analyses [18]. The recruited cells were largely neutrophils (∼60%) and monocyte/macrophages (∼25%). Our results are consistent with a predominantly neutrophilic influx in the decidua of women with preterm labor due to infection compared to that in women delivering at term with no labor [19]. These neutrophils were activated because they expressed neutrophil elastase, and they produced TNFα and IL-8. In fact, most of the TNFα and IL-8 expression in response to IA IL-1β was from the neutrophils and to a lesser extent from the monocytes/macrophages. Most of the neutrophils were located in the choriodecidual junction. Overall our results are consistent with the hypothesis that during chorioamnionitis, the initial amniotic epithelium inflammatory signal is amplified by decidua leukocytes leading to preterm labor.
Neutrophils are often viewed as effector cells of innate immunity with a limited capacity for regulatory activity [57]. Recently, Loughman et al. [58] reported that Escherichia coli induced the expression of inhibitory tryptophan-catabolizing enzyme IDO in human blood polymorphonuclear leukocytes. Consistent with this in vitro observation, our in vivo results demonstrated a potential regulatory role for decidua neutrophils during chorioamnionitis, because they also expressed IDO1. Although IDO1 expression was reported in the endometrium, the decidua, and the glandular epithelium of the uterus [59, 60], to our knowledge, expression in decidua neutrophils during chorioamnionitis has not been reported. The results are significant because inhibition of IDO1 in pregnant mice resulted in T-cell-mediated rejection of the fetus with paternally mismatched antigens [61, 62]. We did not measure IDO enzymatic activity. However, placenta appears to be an important organ for catabolizing tryptophan via the kynurenine pathway (requiring IDO enzyme activity) because plasma kynurenine levels were higher in fetal lambs than in their mothers in response to maternal tryptophan loading [63]. The functions of IDO1 expressing neutrophils in the decidua remain to be elucidated, although roles for neutrophils in controlling inflammation [64] and modulating uterus repair and remodeling have been postulated [25, 65]. Our data for decidua neutrophil infiltration, expression of proinflammatory cytokines associated with labor, and upregulation of the gap junction protein Cx-43 in the uterus suggest a role for neutrophils in mediating preterm labor. However, a recent study in mice demonstrated that neutrophil depletion using anti-Gr-1 and anti-Ly-6G antibodies inhibited chorioamnion-decidua production of IL-1β and TNFα but not preterm labor induced by intrauterine LPS [65]. Of note, a relatively large LPS dose (∼800 μg/kg) is needed to induce preterm labor in mouse compared to that in rhesus macaque (∼1 μg/kg) [20]. The anatomic site of injection of LPS was the uterine lumen and not the amniotic cavity in the mouse [65]. However, progression of inflammation in the amniotic cavity is essential for chorioamnionitis-induced preterm labor and fetal inflammation in rhesus [11], sheep [66], and humans [10]. The role for neutrophils in preterm labor has not been reported in the rhesus. Thus, experiments in different species will be needed to definitively determine the role of neutrophils in initiating chorioamnionitis-induced preterm delivery.
Proinflammatory cytokines and chemokines that were up-regulated after IA IL-1β included TNFα, IL-6, GM-CSF, IL-8/CXCL8, CCL2/MCP-1, CCL3/Mip-1α, CCL4/Mip-1β, CCL5/RANTES, and CCL20/Mip-3α. In contrast, IFNγ, IL-12p40, IL-17, IL-2, IL-15, IL-17A, IL-18, CXCL9/MIG, and CXCL10/IP-10 were not detectable. This profile is consistent with no significant accumulation or activation of T cells and is similar to that reported in humans with preterm labor due to chorioamnionitis [19]. Of note, IA IL-1β-induced expression of IL-1β, its receptors RI and RII and its accessory protein (RAP) in chorion-amnion decidua signifying a feed-forward loop. The relative pools of CD3+ T cells, CD4+ T cells, or the Treg subset did not change significantly in the decidua following IA IL-1β, consistent with reduced T-cell trafficking reported in pregnancy [67]. Although NK cells are one of the most abundant leukocytes in the decidua, their numbers increased only modestly after IA IL-1β. In contrast to data showing that the CD56bright NK cell are the most common NK cell subset in the first trimester rhesus decidua [43], we found a higher frequency of the CD56dim NK cells in the third trimester decidua. The frequency of the CD56dim NK cells increased further after IA IL-1ß. The functional significance of the reversed decidua NK cell subsets during first and third trimester needs to be more fully explored.
In summary, we demonstrated that in a preterm rhesus macaque model of chorioamnionitis, the amnion expressed chemotactic chemokines/cytokines, and activated neutrophils rapidly accumulated in the decidua with both pro- and anti-inflammatory responses occurring in the decidua. A better understanding of neutrophil activation and regulatory activities will be useful to design therapeutic strategies important to prevent preterm labor resulting from chorioamnionitis.
Supplementary Material
ACKNOWLEDGMENT
We thank the staff at the California National Primate Research Center for invaluable contributions, particularly Sarah Davis, for help with all aspects of animal management.
Footnotes
Supported by a California National Primate Research Center pilot grant to C.A.C., the Burroughs-Wellcome Fund Preterm Initiative to C.A.C., and U.S. National Institutes of Health grant R01 HD57869 to S.G.K.
These authors contributed equally to this work.
REFERENCES
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2012. Nat Vital Stat Rep. 2013;62:1–87. [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravett MG, Rubens CE; Global Alliance to Prevent Pematurity and Stillbirth Technical Team. A framework for strategic investments in research to reduce the global burden of preterm birth. Am J Obstet Gynecol. 2012;207:368–373. doi: 10.1016/j.ajog.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY, Jun JK. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189:919–924. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, Kim CJ, Erez O, Edwin S, Relman DA. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YW, Colford JM., Jr. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284:1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- Shatrov JG, Birch SC, Lam LT, Quinlivan JA, McIntyre S, Mendz GL. Chorioamnionitis and cerebral palsy: a meta-analysis. Obstet Gynecol. 2010;116:387–392. doi: 10.1097/AOG.0b013e3181e90046. [DOI] [PubMed] [Google Scholar]
- Been JV, Lievense S, Zimmermann LJ, Kramer BW, Wolfs TG. Chorioamnionitis as a risk factor for necrotizing enterocolitis: a systematic review and meta-analysis J Pediatr 2013. 162 236 242 e232. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Romero R, Gervasi MT, Kim JS, Yoo W, Lee DC, Mittal P, Erez O, Kusanovic JP, Hassan SS, Kim CJ. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Invest. 2009;89:924–936. doi: 10.1038/labinvest.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigsby PL, Novy MJ, Waldorf KM, Sadowsky DW, Gravett MG. Choriodecidual inflammation: a harbinger of the preterm labor syndrome. Reprod Sci. 2010;17:85–94. doi: 10.1177/1933719109348025. [DOI] [PubMed] [Google Scholar]
- Adams Waldorf KM, Gravett MG, McAdams RM, Paolella LJ, Gough GM, Carl DJ, Bansal A, Liggitt HD, Kapur RP, Reitz FB, Rubens CE. Choriodecidual group B streptococcal inoculation induces fetal lung injury without intra-amniotic infection and preterm labor in Macaca nemestrina. PLoS One. 2011;6:e28972. doi: 10.1371/journal.pone.0028972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams RM, Vanderhoeven J, Beyer RP, Bammler TK, Farin FM, Liggitt HD, Kapur RP, Gravett MG, Rubens CE. Adams Waldorf KM. Choriodecidual infection downregulates angiogenesis and morphogenesis pathways in fetal lungs from Macaca nemestrina. PLoS One. 2012;7:e46863. doi: 10.1371/journal.pone.0046863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravett MG, Haluska GJ, Cook MJ, Novy MJ. Fetal and maternal endocrine responses to experimental intrauterine infection in rhesus monkeys. Am J Obstet Gynecol. 1996;174:1725–1732. 1731–1723. doi: 10.1016/s0002-9378(96)70203-x. discussion. [DOI] [PubMed] [Google Scholar]
- Golos TG, Bondarenko GI, Dambaeva SV, Breburda EE, Durning M. On the role of placental Major Histocompatibility Complex and decidual leukocytes in implantation and pregnancy success using non-human primate models. Int J Dev Biol. 2010;54:431–443. doi: 10.1387/ijdb.082797tg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd I, Balakrishnan B, Kannan S. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol. 2012;67:287–294. doi: 10.1111/j.1600-0897.2012.01110.x. [DOI] [PubMed] [Google Scholar]
- Kramer BW. Chorioamnionitis—new ideas from experimental models. Neonatology. 2011;99:320–325. doi: 10.1159/000326620. [DOI] [PubMed] [Google Scholar]
- Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A, Lye SJ, Jones RL. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod. 2012;86:39. doi: 10.1095/biolreprod.111.095505. [DOI] [PubMed] [Google Scholar]
- Hamilton SA, Tower CL, Jones RL. Identification of chemokines associated with the recruitment of decidual leukocytes in human labour: potential novel targets for preterm labour. PLoS One. 2013;8:e56946. doi: 10.1371/journal.pone.0056946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams Waldorf KM, Persing D, Novy MJ, Sadowsky DW, Gravett MG. Pretreatment with toll-like receptor 4 antagonist inhibits lipopolysaccharide-induced preterm uterine contractility, cytokines, and prostaglandins in rhesus monkeys. Reprod Sci. 2008;15:121–127. doi: 10.1177/1933719107310992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigsby PL, Novy MJ, Sadowsky DW, Morgan TK, Long M, Acosta E, Duffy LB, Waites KB. Maternal azithromycin therapy for urea plasma intra-amniotic infection delays preterm delivery and reduces fetal lung injury in a primate model Am J Obstet Gynecol 2012. 207 475: e471 e414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, Cassell GH, Waites KB. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16:56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- Kallapur SG, Presicce P, Senthamaraikannan P, Alvarez M, Tarantal AF, Miller LM, Jobe AH, Chougnet CA. Intra-amniotic IL-1beta induces fetal inflammation in rhesus monkeys and alters the regulatory T cell/IL-17 balance. J Immunol. 2013;191:1102–1109. doi: 10.4049/jimmunol.1300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol. 2013;69:212–230. doi: 10.1111/aji.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shynlova O, Nedd-Roderique T, Li Y, Dorogin A, Nguyen T, Lye SJ. Infiltration of myeloid cells into decidua is a critical early event in the labour cascade and post-partum uterine remodelling. J Cell Mol Med. 2013;17:311–324. doi: 10.1111/jcmm.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, Vega-Sanchez R, Vadillo-Ortega F. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J Reprod Immunol. 2009;80:122–131. doi: 10.1016/j.jri.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Estrada-Gutierrez G, Gomez-Lopez N, Zaga-Clavellina V, Giono-Cerezo S, Espejel-Nunez A, Gonzalez-Jimenez MA. Espino y Sosa S, Olson DM, Vadillo-Ortega F. Interaction between pathogenic bacteria and intrauterine leukocytes triggers alternative molecular signaling cascades leading to labor in women. Infect Immun. 2010;78:4792–4799. doi: 10.1128/IAI.00522-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165:969–971. doi: 10.1016/0002-9378(91)90450-6. [DOI] [PubMed] [Google Scholar]
- Baggia S, Gravett MG, Witkin SS, Haluska GJ, Novy MJ. Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Investig. 1996;3:121–126. doi: 10.1177/107155769600300304. [DOI] [PubMed] [Google Scholar]
- Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195:1578–1589. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Sadowsky DW, Haluska GJ, Gravett MG, Witkin SS, Novy MJ. Indomethacin blocks interleukin 1beta-induced myometrial contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2000;183:173–180. doi: 10.1067/mob.2000.105968. [DOI] [PubMed] [Google Scholar]
- Sadowsky DW, Novy MJ, Witkin SS, Gravett MG. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2003;188:252–263. doi: 10.1067/mob.2003.70. [DOI] [PubMed] [Google Scholar]
- Redline RW. Inflammatory response in acute chorioamnionitis. Semin Fetal Neonatal Med. 2012;17:20–25. doi: 10.1016/j.siny.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Keelan JA, Wong PM, Bird PS, Mitchell MD. Innate inflammatory responses of human decidual cells to periodontopathic bacteria. Am J Obstet Gynecol. 2010;202:471.e1–471.e11. doi: 10.1016/j.ajog.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Mulder WM, Koenen H, van de Muysenberg AJ, Bloemena E, Wagstaff J, Scheper RJ. Reduced expression of distinct T-cell CD molecules by collagenase/DNase treatment. Cancer Immunol Immunother. 1994;38:253–258. doi: 10.1007/BF01533516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme N, Baeten D, De Vos M, Demetter P, Elewaut D, Mielants H, Verbruggen G, Cuvelier C, Veys EM, De Keyser F. Chemical agents and enzymes used for the extraction of gut lymphocytes influence flow cytometric detection of T cell surface markers. J Immunol Methods. 2000;236:27–35. doi: 10.1016/s0022-1759(99)00243-4. [DOI] [PubMed] [Google Scholar]
- Abuzakouk M, Feighery C, O'Farrelly C. Collagenase and dispase enzymes disrupt lymphocyte surface molecules. J Immunol Methods. 1996;194:211–216. doi: 10.1016/0022-1759(96)00038-5. [DOI] [PubMed] [Google Scholar]
- Presicce P, Moreno-Fernandez ME, Lages CS, Orsborn KI, Chougnet CA. Association of two clones allows for optimal detection of human FOXP3. Cytometry A. 2010;77:571–579. doi: 10.1002/cyto.a.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon B, Jun J, Romero R, Park K, Gomez R, Choi J, Kim I. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- Yoon B, Romero R, Park J, Chang J, Kim Y, Kim J, Kim K. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179:1254–1260. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cell Mol Immunol. 2014;11:571–581. doi: 10.1038/cmi.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambaeva SV, Durning M, Rozner AE, Golos TG. Immunophenotype and cytokine profiles of rhesus monkey CD56bright and CD56dim decidual natural killer cells. Biol Reprod. 2012;86:1–10. doi: 10.1095/biolreprod.111.094383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilburgs T, Strominger JL. CD8+ effector T cells at the fetal-maternal interface, balancing fetal tolerance and antiviral immunity. Am J Reprod Immunol. 2013;69:395–407. doi: 10.1111/aji.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lye SJ, Nicholson BJ, Mascarenhas M, MacKenzie L, Petrocelli T. Increased expression of connexin-43 in the rat myometrium during labor is associated with an increase in the plasma estrogen:progesterone ratio. Endocrinology. 1993;132:2380–2386. doi: 10.1210/endo.132.6.8389279. [DOI] [PubMed] [Google Scholar]
- Pierce BT, Calhoun BC, Adolphson KR, Lau AF, Pierce LM. Connexin 43 expression in normal versus dysfunctional labor. Am J Obstet Gynecol. 2002;186:504–511. doi: 10.1067/mob.2002.121108. [DOI] [PubMed] [Google Scholar]
- Tong D, Lu X, Wang HX, Plante I, Lui E, Laird DW, Bai D, Kidder GM. A dominant loss-of-function GJA1 (Cx43) mutant impairs parturition in the mouse. Biol Reprod. 2009;80:1099–1106. doi: 10.1095/biolreprod.108.071969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Nisula LT, Aalto ML, Kirkinen PP, Kosma VM, Heinonen ST. Myometrial inflammation in human delivery and its association with labor and infection. Am J Clin Pathol. 2003;120:217–224. doi: 10.1309/KC6K-DTX9-8LFY-B3J7. [DOI] [PubMed] [Google Scholar]
- Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9:41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79:50–57. doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Gravett MG, Rubens CE, Nunes TM. Global report on preterm birth and stillbirth (2 of 7): discovery science BMC Pregnancy Childbirth 2010. 10 (suppl 1): S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley D, Edwin S, Van WJ, Augustine N, Hill H, Mitchell M. Regulation of decidual cell chemokine production by group B streptococci and purified bacterial cell wall components. Am J Obstet Gynecol. 1997;177:666–672. doi: 10.1016/s0002-9378(97)70162-5. [DOI] [PubMed] [Google Scholar]
- Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Yeo L. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72:458–474. doi: 10.1111/aji.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, Rael J, Grove T, Morgan TK, Clewell W, Miller H, Luthy D, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210:125.e1–125.e15. doi: 10.1016/j.ajog.2013.11.032. [DOI] [PubMed] [Google Scholar]
- Namba F, Hasegawa T, Nakayama M, Hamanaka T, Yamashita T, Nakahira K, Kimoto A, Nozaki M, Nishihara M, Mimura K, Yamada M, Kitajima H, et al. Placental features of chorioamnionitis colonized with Ureaplasma species in preterm delivery. Pediatr Res. 2010;67:166–172. doi: 10.1203/PDR.0b013e3181c6e58e. [DOI] [PubMed] [Google Scholar]
- Macias AE, Wong SW, Sadowsky DW, Luetjens CM, Axthelm MK, Gravett MG, Haluska GJ, Novy MJ. Maternal or fetal origin of rhesus monkey (Macaca mulatta) amniotic fluid leukocytes can be identified by polymerase chain reaction using the zinc finger Y gene. Am J Primatol. 2001;55:159–170. doi: 10.1002/ajp.1049. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- Loughman JA, Hunstad DA. Induction of indoleamine 2,3-dioxygenase by uropathogenic bacteria attenuates innate responses to epithelial infection. J Infect Dis. 2012;205:1830–1839. doi: 10.1093/infdis/jis280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Rango U, Krusche CA, Beier HM, Classen-Linke I. Indoleamine-dioxygenase is expressed in human decidua at the time maternal tolerance is established. J Reprod Immunol. 2007;74:34–45. doi: 10.1016/j.jri.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Drenzek JG, Breburda EE, Burleigh DW, Bondarenko GI, Grendell RL, Golos TG. Expression of indoleamine 2,3-dioxygenase in the rhesus monkey and common marmoset. J Reprod Immunol. 2008;78:125–133. doi: 10.1016/j.jri.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Sivakumar J, Chandler P, Smith K, Molina H, Mao D, Munn DH. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol. 2001;2:64–68. doi: 10.1038/83183. [DOI] [PubMed] [Google Scholar]
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- Nicholls T, Nitsos I, Walker DW. Tryptophan metabolism in pregnant sheep: increased fetal kynurenine production in response to maternal tryptophan loading. Am J Obstet Gynecol. 1999;181:1452–1460. doi: 10.1016/s0002-9378(99)70391-1. [DOI] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi SF, Catalano RD, Wade J, Rossi AG, Norman JE. Decidual neutrophil infiltration is not required for preterm birth in a mouse model of infection-induced preterm labor J Immunol 2014. 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss TJ, Nitsos I, Newnham JP, Ikegami M, Jobe AH. Chorioamnionitis induced by subchorionic endotoxin infusion in sheep. Am J Obstet Gynecol. 2003;189:1771–1776. doi: 10.1016/s0002-9378(03)00810-x. [DOI] [PubMed] [Google Scholar]
- Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336:1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.