Abstract
Rationale: Survivors of critical illness report impaired health-related quality of life (HRQoL) after hospital discharge, but the degree to which these impairments are attributable to critical illness is unknown.
Objectives: We sought to examine changes in HRQoL associated with an intensive care unit (ICU) stay and the differential association of type of hospitalization (critical illness versus noncritical illness) on changes in HRQoL.
Methods: We identified 11,243 participants in the Ambulatory Care Quality Improvement Project (a multicenter randomized trial of Veterans conducted March 1997 to August 2000) completing at least two Medical Outcomes Study Short-Form 36 questionnaires over 2 years, and categorized patients by hospitalization status during the interval between measures. We used multiple linear regression with generalized estimating equations for analysis.
Measurements and Main Results: Our primary outcome was change in the Physical Component Summary score. Participants requiring hospitalization or ICU admission had significantly worse baseline HRQoL than those not hospitalized (P < 0.001). Compared with patients who were not hospitalized, follow-up Physical Component Summary scores were lower among non-ICU hospitalized patients and ICU patients (adjusted β-coefficient = −1.40 [95% confidence interval, −1.81, −0.99] and adjusted β-coefficient = −1.53 [95% confidence interval, −2.11, −0.95], respectively), with no difference between the two groups (P value = 0.80). Similar results were seen for the Mental Component Summary score and each of the Medical Outcomes Study Short-Form 36 subdomains.
Conclusions: Prehospital HRQoL is a significant determinant of HRQoL after hospitalization or ICU admission. Hospitalization is associated with increased risk of impairment in HRQoL after discharge, yet the overall magnitude of this reduction is small and similar between non-ICU hospitalized and critically ill patients.
Keywords: quality of life, critical illness, intensive care units, cohort studies, outcome assessment (health care)
Each year, millions of individuals are admitted to an intensive care unit (ICU), the majority of whom will survive their hospital stays (1). As short-term mortality from critical illness has declined, clinicians and scientists have become increasingly interested in its long-term consequences (2). Studies of survivors of critical illness demonstrate significant impairments in health-related quality of life (HRQoL) (3–8), functional status (7, 9, 10), cognition (10, 11), and mental health (12) compared with age-matched control subjects. Although these findings are consistent across cohorts of ICU survivors, important gaps in our knowledge remain (13). In particular, the extent critical illness or treatments provided in the critical care setting contribute to long-term outcomes is not clear. If reductions in HRQoL in ICU survivors are not actually caused by critical illness or critical care, future attempts to improve post-ICU outcomes focusing on critical care processes may be ineffective. Additionally, if all acute illness is the pertinent risk factor for reduced HRQoL and not critical illness per se, we risk profoundly underestimating the public health importance of impairment after hospitalization.
A major challenge to studying post-ICU morbidity is the need to identify patients’ pre-ICU health status. Prior studies have attempted to address this challenge by comparing ICU survivors to a healthy population (6–8, 14, 15), having patients provide retrospective preadmission HRQoL (15, 16), or asking caregivers to provide information on HRQoL on ICU presentation (14, 15). Each of these approaches has significant limitations. For example, proxies may underestimate, whereas survivors may later overestimate, prehospital admission HRQoL (17). Recent longitudinal studies measuring the trajectory of illness before hospital admission suggest that the morbidity observed in ICU survivor cohorts overestimates the attributable morbidity of critical illness (18). However, these studies have not included HRQoL as an outcome (10, 11).
Using a unique data source of prospectively collected longitudinal data from a randomized trial measuring HRQoL at regular intervals (19), we sought to determine the association of an ICU stay with declines in HRQoL. We also hoped to study the differential effect of hospitalization type (critical versus noncritical illness) on changes in HRQoL. We hypothesized ICU survivors would have a significant reduction in HRQoL and this decrement would be greater than that associated with either a hospital admission or that found among patients not hospitalized.
Some of the results were previously presented as an abstract at the American Thoracic Society International Conference in May 2011 (20).
Methods
Study Design and Population
We performed secondary analysis of data from the Ambulatory Care Quality Improvement Project (ACQUIP) (19), a multicenter randomized trial evaluating the effect of feedback to providers on patient health and satisfaction. The study was conducted from January 1997 to August 2000 at seven nationwide Veterans Affairs (VA) general internal medicine clinics. All patients visiting their primary care provider at least once in the year prior were eligible to participate. ACQUIP participants received a baseline Medical Outcomes Study Short-Form 36 (SF-36) HRQoL questionnaire (21, 22), with subsequent follow-up SF-36 questionnaires mailed to participants in the control arm at 12 and 24 months and those in the intervention arm at 3, 6, 12, 18, 24, and 30 months after enrollment. The intervention had no effect (19), and we included participants from both arms of the study. To be included in this analysis, participants had to have at least two SF-36 scores approximately 1 year apart, from the baseline or 12- or 24-month mailings (referred to here as Year 0, Year 1, and Year 2 questionnaires, respectively). To assess for differential loss to follow-up among the sickest patients, we compared characteristics, hospitalization status, and baseline HRQoL scores between responders and nonresponders to follow-up surveys after the first year of study.
Variables
Outcome
We chose change in the Physical Component Summary (PCS) score to be the primary outcome based on literature demonstrating an enduring impairment after critical illness (7). Our secondary outcomes included change in the Mental Component Summary (MCS) score and in each of the eight subdomains of the SF-36. Scores range from 0 to 100, with higher scores indicating better HRQoL (23). We defined the minimally important clinical difference as a decline of 5 points on the PCS and MCS and 10 points on each of the SF-36 subdomains (24).
Exposure
Our primary exposure was hospital or ICU admission during the 1-year interval between SF-36 measures. We identified hospitalizations using data from national administrative VA databases (25); data on hospitalizations occurring outside of the VA system were not available. We excluded hospitalizations occurring within 2 weeks before the follow-up SF-36 due to uncertainty as to whether or not the questionnaire was completed before or after hospitalization. We used a time-varying exposure variable to categorize patients’ hospitalization status using bed location codes. Patients were classified as experiencing no hospitalizations, at least one non-ICU hospitalization (and no ICU admissions), or at least one ICU hospitalization during each specified interval.
Potential confounders
We identified demographic information at study entry. We assessed the burden of comorbid illness in the year prior using the Deyo adaptation of the Charlson comorbidity index (26, 27), and identified self-reported diagnoses of depression. As the likelihood of admission to a VA facility differs based on distance from home (28), we calculated the distance participants lived from their local VA hospital. We identified participants who were comanaged with an outside provider.
Statistical Analysis
We used Chi-square tests, one-way analysis of variance, and paired Student t tests as appropriate to compare baseline characteristics and initial SF-36 scores by hospitalization status and mean SF-36 summary scores before and after hospital admission. Among patients who completed a baseline SF-36 (Year 0, or Year 1 if the Year 0 survey was not completed) and remained alive at the end of the subsequent year, we compared differences in baseline characteristics, hospitalization status, and most recent HRQoL scores between responders and nonresponders to the 12-month follow-up (Year 1 or Year 2, as appropriate) SF-36.
We used multiple linear regression with generalized estimating equations to estimate the association between hospitalization status and HRQoL while accounting for repeated measures (29). Our outcome was the SF-36 score obtained at 1-year follow-up. Each model was adjusted for the most recent prior SF-36 score, time since prior SF-36 score, and all of the potential confounders described above. In a restricted cohort excluding nonhospitalized patients, we made additional adjustment for length of stay and time between discharge and follow-up SF-36.
We hypothesized that any absence of a clinically significant decline in HRQoL after hospitalization could be partially due to a “floor effect,” or an inability of patients with already low HRQoL scores to decline further after hospitalization. We tested for effect modification of the association between hospitalization status and follow-up PCS by quartile of the most recent prior PCS score using an interaction term and performed analyses stratified by quartile of most recent PCS score. Finally, we used multiple logistic regression with generalized estimating equations to estimate the association between hospitalization status and a clinically significant decrease in HRQoL (29).
Sensitivity Analysis
We conducted a separate analysis in restricted cohort of patients with an ICU admission that also met criteria for critical illness using International Classification of Diseases (ICD)-9 diagnostic codes similar to that used by Ehlenbach and colleagues (11)
The University of Washington Human Subjects Division and all other participating sites approved the ACQUIP study protocol. The Institutional Review Boards of the VA Puget Sound approved the analyses presented here (VA MIRB #00109).
Results
Responders versus Nonresponders
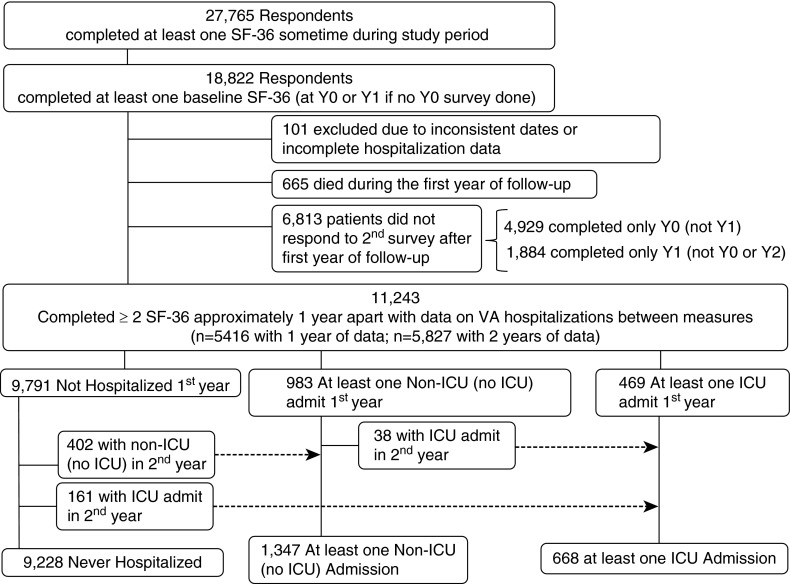
We identified 18,822 patients completing a baseline SF-36 (Figure 1). After excluding patients with inconsistent dates and incomplete hospitalization information, 18,721 patients remained. Of these, 665 patients died during the first year. Of the remaining 18,056 patients, 11,243 (62%) returned a 12-month follow-up SF-36. Among survivors of a hospitalization, response rates were no different among ICU patients compared with those with a non-ICU admission (60 vs. 58%, P value 0.36) (Table 1).
Figure 1.
Identification of study cohort. ICU = intensive care unit; SF-36 = Medical Outcomes Study Short-Form 36; VA = Veterans Affairs hospital.
Table 1.
Comparison of rates of death and differential response rates based on hospitalization status during the first year of follow-up
| Hospitalization Status* |
|||
|---|---|---|---|
| Not Hospitalized N (%) | Hospitalized N (%) | ICU Hospitalized N (%) | |
| All patients completing baseline survey | N = 15,889 | N = 1,872 | N = 960 |
| Died first year | 316 (2) | 173 (9) | 176 (18) |
| Survived first year | N = 15,573 | N = 1,699 | N = 784 |
| Nonresponder | 5,782 (37) | 716 (42) | 315 (40) |
| Responded to follow-up SF-36 | 9,791 (63) | 983 (58) | 469 (60) |
Definition of abbreviations: ICU = intensive care unit; SF-36 = Medical Outcomes Study Short-Form 36; Y = year.
For responders, hospitalization status represents admissions occurring between baseline survey (Y0 or Y1 if no Y0 completed) and when follow-up survey received (Y1 or Y2, respectively); among nonresponders, hospitalization status represents admissions occurring within 365 days after the baseline survey; among those dying during the first year of follow-up, hospitalization status represents admissions occurring between baseline survey and death.
We found small but statistically significant differences among responders and nonresponders to the follow-up SF-36 (see Table E1 in the online supplement). Nonresponders were younger, with fewer comorbid illnesses and increased rates of depression, and lived further away from their VA site of care. Importantly, there were no differences in the proportion of patients admitted to the ICU, although nonresponders experienced more hospital stays. There were small statistically, although not clinically, significant differences between groups regarding most recent HRQoL scores. In a restricted cohort of 2,483 survivors of hospitalization during the first year of follow-up, small differences in baseline HRQoL scores persisted between responders and nonresponders (Table E2).
Characteristics of the Cohort
Of the 11,243 patients with at least two SF-36 measures, 9,228 (82%) were never hospitalized, 1,347 (12%) experienced at least one non-ICU hospitalization, and 668 (6%) experienced at least one ICU hospitalization during the study period (Figure 1). Patients not hospitalized during the study were younger, more educated, and reported a higher annual income than patients experiencing a hospitalization or ICU admission (Table 2). Nonhospitalized patients also had fewer comorbid illness, had fewer admissions in the year before study entry, and were more likely to also receive care outside of the VA. Among hospitalized patients, there was no difference in mean time between discharge and next SF-36 between patients with (178.8 ± 100.3 d) or without an ICU stay (172.9 ± 100.0 d) (P = 0.20).
Table 2.
Baseline characteristics of patients by hospitalization status (anytime during study)
| Characteristics |
Not Hospitalized |
At Least 1 Non-ICU Hospitalization |
At Least 1 ICU Admission |
P Value |
|---|---|---|---|---|
| (N = 9,228) | (N = 1,347) | (N = 668) | ||
| Age at entry, mean (SD), yr | 64.8 (10.8) | 66.0 (10.7) | 66.8 (9.4) | <0.001 |
| White, N (%) | 7,059 (77) | 1,110 (82) | 547 (82) | <0.001 |
| Male, N (%) | 8,929 (97) | 1,297 (96) | 649 (97) | 0.538 |
| Annual income, N (%) | <0.001 | |||
| <$ 20,000 | 5,381 (58) | 836 (62) | 429 (64) | |
| $20,000–$40,000 | 2,417 (26) | 348 (26) | 152 (23) | |
| >$40,000 | 742 (8) | 62 (5) | 41 (6) | |
| Education, N (%) | <0.001 | |||
| <12th grade | 2,534 (27) | 428 (32) | 233 (35) | |
| High school grad ± some college | 4,999 (54) | 722 (54) | 355 (53) | |
| College grad ± grad work | 1,481 (16) | 165 (12) | 67 (10) | |
| Marital status, N (%) | 0.146 | |||
| Single/never married | 696 (8) | 103 (8) | 36 (5) | |
| Married | 5,748 (62) | 806 (60) | 410 (61) | |
| Separated/divorced/widowed | 2,625 (28) | 412 (31) | 212 (32) | |
| Outside care, N (%) | 3,509 (38) | 358 (27) | 162 (24) | <0.001 |
| Self-reported depression, N (%) | 2,006 (22) | 375 (28) | 171 (26) | <0.001 |
| Deyo-Charlson Comorbidity score, mean (SD) | 0.9 (1.4) | 1.3 (1.6) | 1.6 (1.8) | <0.001 |
| Distance to VA, mean (SD), miles | 46.3 (117.7) | 43.5 (108.9) | 36.8 (74.1) | 0.099 |
| Hospitalization in year prior, N (%) | <0.001 | |||
| Non-ICU admission | 733 (8) | 293 (22) | 119 (18) | |
| ICU admission | 296 (3) | 96 (7) | 96 (14) |
Definition of abbreviations: ICU = intensive care unit; VA = Veterans Affairs hospital.
Totals may not sum to 100% due to missing values.
Baseline HRQoL was poor among this cohort of Veterans, especially in physical domains, with a mean initial PCS score of 34.1 (SD, 11.7). Patients hospitalized with or without an ICU stay had significantly worse baseline HRQoL than patients not hospitalized at any point during the 2-year study period. However, there were no differences in baseline HRQoL scores between patients hospitalized with or without an ICU admission (Table 3).
Table 3.
Initial health-related quality of life scores by hospitalization status (anytime during study)
| SF-36 Score |
Not Hospitalized |
At Least 1 Non-ICU Hospitalization |
At Least 1 ICU Admission |
P Value* |
P Value† |
|---|---|---|---|---|---|
| (N = 9,228) | (N = 1,347) | (N = 668) | |||
| Summary Measures | |||||
| Physical Component Summary | 35.0 (11.8) | 30.5 (10.6) | 29.7 (10.5) | <0.001 | 0.146 |
| Mental Component Summary | 47.3 (12.8) | 44.7 (13.6) | 45.6 (13.0) | <0.001 | 0.156 |
| Domains | |||||
| Physical Functioning | 51.7 (29.5) | 39.8 (28.3) | 38.0 (27.1) | <0.001 | 0.176 |
| Role-Physical | 36.4 (41.7) | 23.7 (35.8) | 22.2 (35.2) | <0.001 | 0.404 |
| Bodily Pain | 50.5 (26.3) | 43.0 (25.3) | 43.0 (25.4) | <0.001 | 0.979 |
| General Health | 48.2 (24.0) | 40.4 (22.8) | 39.5 (22.2) | <0.001 | 0.391 |
| Vitality | 44.6 (24.5) | 36.9 (24.2) | 36.8 (23.5) | <0.001 | 0.946 |
| Social Functioning | 64.4 (29.9) | 53.8 (30.7) | 55.5 (30.0) | <0.001 | 0.235 |
| Role-Emotional | 58.9 (43.9) | 50.0 (44.3) | 50.2 (44.8) | <0.001 | 0.904 |
| Mental Health | 68.3 (22.7) | 63.4 (24.8) | 64.8 (23.6) | <0.001 | 0.217 |
Definition of abbreviations: ICU = intensive care unit; SF-36 = Medical Outcomes Study Short-Form 36.
Data are presented as mean (SD). Although all patients in this study had completed at least two SF-36 scores, the number of patients with sufficient data to complete each SF-36 subscale and Summary Measures differed slightly. Data were complete for more than 97% of each domain, for each of the three hospitalization categories.
P value for comparison across all three categories.
P value for comparison of non-ICU hospitalized and ICU hospitalized only.
Change in HRQoL by Hospitalization Status
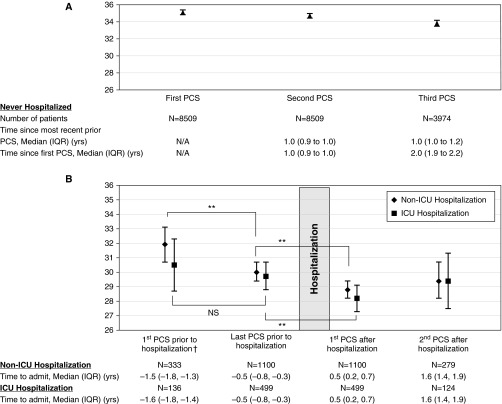
The mean 1-year change in PCS for nonhospitalized patients was −0.59 (SD, 7.22). Among patients experiencing their first hospitalization, the unadjusted mean PCS score was significantly lower after hospital discharge for patients with either an ICU or non-ICU hospitalization (Figure 2). Among those patients with two PCS scores before their first hospital admission, mean PCS scores also declined between the initial SF-36 and that just before admission, although this change only met statistical significance among non-ICU hospitalized patients.
Figure 2.
Trajectory of Physical Component Summary (PCS) among patients during the course of the study. Unadjusted mean PCS scores and 95% confidence intervals are shown. †Hospitalization refers to first hospital stay; patients also hospitalized in year preceding or year after hospitalization were excluded. **Indicates P value < 0.01 (paired Student t test). (A) Trajectory of PCS among patients not hospitalized during the course of the study. (B) Trajectory of PCS among patients hospitalized during the course of the study. ICU = intensive care unit; IQR = interquartile range; N/A = not applicable; NS = nonsignificant.
In adjusted analyses, follow-up PCS scores remained lower among subjects experiencing a non-ICU hospitalization (adjusted β-coefficient, −1.40; 95% confidence interval [CI], −1.81 to −0.99) or ICU hospitalization (adjusted β-coefficient, −1.53; 95% CI, −2.11 to −0.95) compared with nonhospitalized participants (Table 4). In an analysis restricted to hospitalized patients, and further adjusted for hospital length of stay and time between discharge to follow-up PCS score, there was essentially no difference in PCS scores between the non-ICU and ICU hospitalized patients (adjusted β-coefficient, −0.09; 95% CI, −0.80 to +0.62) (Table 5).
Table 4.
Difference in health-related quality of life scores (physical component summary and physical subdomains) by hospitalization status: comparison of intensive care unit and non–intensive care unit hospitalized patients to those not hospitalized during interval
| Follow-up SF-36 Scores | Unadjusted β-Coefficient (95% CI) | P Value | Adjusted β-Coefficient* (95% CI) | P Value |
|---|---|---|---|---|
| Physical component summary | ||||
| Not hospitalized | — | — | — | — |
| After non-ICU admission | −2.57 (−3.05, −2.10) | <0.001 | −1.40 (−1.81, −0.99) | <0.001 |
| After ICU admission | −3.07 (−3.75, −2.39) | <0.001 | −1.53 (−2.11, −0.95) | <0.001 |
| Physical subdomains | ||||
| Physical Functioning | ||||
| Not hospitalized | — | — | — | — |
| After non-ICU admission | −7.25 (−8.44, −6.07) | <0.001 | −3.89 (−4.93, −2.85) | <0.001 |
| After ICU admission | −7.59 (−9.28, −5.91) | <0.001 | −4.42 (−5.83, −3.01) | <0.001 |
| Role-Physical | ||||
| Not hospitalized | — | — | — | — |
| After non-ICU admission | −12.31 (−13.99, −10.63) | <0.001 | −7.01 (−8.60, −5.43) | <0.001 |
| After ICU admission | −13.85 (−16.22, −11.48) | <0.001 | −9.24 (−11.59, −6.90) | <0.001 |
| Bodily Pain | ||||
| Not hospitalized | — | — | — | — |
| After non-ICU admission | −5.66 (−6.82, −4.50) | <0.001 | −3.11 (−4.14, −2.09) | <0.001 |
| After ICU admission | −6.31 (−8.12, −4.49) | <0.001 | −3.38 (−4.95, −1.80) | <0.001 |
| General Health | ||||
| Not hospitalized | — | — | — | — |
| After non-ICU admission | −4.20 (−5.10, −3.29) | <0.001 | −2.46 (−3.24, −1.68) | <0.001 |
| After ICU admission | −4.32 (−5.62, −3.01) | <0.001 | −2.23 (−3.25, −1.21) | <0.001 |
Definition of abbreviations: CI = confidence interval; ICU = intensive care unit; SF-36 = Medical Outcomes Study Short-Form 36.
Linear regression with generalized estimating equations for repeated observations, using exchangeable correlation matrix and robust variance.
Adjusted for previous score, time since previous score, demographics (age, race, income, education, marital status), Deyo-Charlson comorbidity score, depression, distance to Veterans Affairs hospital, and receipt of outside care.
Table 5.
Difference in health-related quality of life scores (physical component summary and physical subdomains) by hospitalization status: comparison of intensive care unit to non–intensive care unit hospitalized patients
| Follow-up SF-36 Scores | Unadjusted β-Coefficient (95% CI) | P Value | Adjusted β-Coefficient* (95% CI) | P Value |
|---|---|---|---|---|
| Physical component summary | ||||
| After non-ICU admission | — | — | — | — |
| After ICU admission | −0.50 (−1.40, +0.39) | 0.271 | −0.09 (−0.80, +0.62) | 0.803 |
| Physical subdomains | ||||
| Physical Functioning | ||||
| After non-ICU admission | — | — | — | — |
| After ICU admission | −0.82 (−3.15, +1.51) | 0.491 | −0.29 (−2.07, +1.49) | 0.748 |
| Role-Physical | ||||
| After non-ICU admission | — | — | — | — |
| After ICU admission | −2.33 (−5.08, +0.43) | 0.098 | −1.84 (−4.46, +0.78) | 0.169 |
| Bodily Pain | ||||
| After non-ICU admission | — | — | — | — |
| After ICU admission | −0.39 (−2.69, +1.91) | 0.739 | −0.33 (−2.21, +1.55) | 0.733 |
| General Health | ||||
| After non-ICU admission | — | — | — | — |
| After ICU admission | −0.38 (−2.29, +1.53) | 0.698 | +0.21 (−1.08, +1.49) | 0.754 |
Definition of abbreviations: CI = confidence interval; ICU = intensive care unit; SF-36 = Medical Outcomes Study Short-Form 36.
Linear regression with generalized estimating equations for repeated observations, using exchangeable correlation matrix and robust variance.
Adjusted for hospital length of stay, time from discharge to follow-up health-related quality-of-life score, previous score, time since previous score, demographics (age, race, income, education, marital status), Deyo-Charlson comorbidity score, depression, distance to Veterans Affairs hospital, and receipt of outside care.
In adjusted analyses, we also found hospitalization to be associated with small decrements in HRQoL as measured by subdomains of the PCS (Table 4). The magnitude of this reduction was not significantly different between participants experiencing a non-ICU hospitalization or ICU admission (Table 5). We found similar results in analyses including MCS and its subdomains as outcomes (Table E3).
We found that both non-ICU hospitalized and ICU hospitalized patients had a significantly higher odds of a clinically meaningful decline in adjusted HRQoL than nonhospitalized patients (Table 6; Table E4), but there was no difference between non-ICU hospitalized and ICU hospitalized patients for PCS and the majority of the subdomains (Table 7). Patients experiencing an ICU hospitalization did have a significantly higher odds of a 5 or more point decline in the MCS (adjusted odds ratio, 1.29 [95% CI, 1.03, 1.62]) and a 10 or more point decline in the vitality subscale than those with a non-ICU hospitalization (adjusted odds ratio, 1.37 [95% CI, 1.10, 1.70]) (Table E4).
Table 6.
Odds of a clinically significant decline in health-related quality-of-life scores (physical component summary and physical subdomains) by hospitalization status: comparison of intensive care unit and non–intensive care unit hospitalized patients to those not hospitalized during interval
| Follow-up SF-36 Scores | Unadjusted OR (95% CI) | P Value | Adjusted OR* (95% CI) | P Value |
|---|---|---|---|---|
| Physical component summary† | ||||
| Not hospitalized | — | — | — | — |
| After non-ICU admission | 1.25 (1.10, 1.42) | <0.001 | 1.56 (1.36, 1.79) | <0.001 |
| After ICU admission | 1.26 (1.05, 1.51) | 0.011 | 1.61 (1.33, 1.95) | <0.001 |
| Physical subdomains | ||||
| Physical Functioning‡ | ||||
| Not hospitalized | — | — | — | — |
| After non-ICU admission | 1.21 (1.08, 1.36) | 0.001 | 1.38 (1.22, 1.56) | <0.001 |
| After ICU admission | 1.45 (1.24, 1.70) | <0.001 | 1.70 (1.44, 2.02) | <0.001 |
| Role-Physical‡ | ||||
| Not hospitalized | — | — | — | — |
| After non-ICU admission | 0.98 (0.86, 1.12) | 0.790 | 1.43 (1.24, 1.66) | <0.001 |
| After ICU admission | 1.11 (0.92, 1.34) | 0.280 | 1.70 (1.40, 2.07) | <0.001 |
| Bodily Pain‡ | ||||
| Not hospitalized | — | — | — | — |
| After non-ICU admission | 1.17 (1.05, 1.31) | 0.004 | 1.39 (1.24, 1.57) | <0.001 |
| After ICU admission | 1.18 (1.01, 1.38) | 0.034 | 1.39 (1.17, 1.65) | <0.001 |
| General Health‡ | ||||
| Not hospitalized | — | — | — | — |
| After non-ICU admission | 1.34 (1.20, 1.50) | <0.001 | 1.51 (1.34, 1.71) | <0.001 |
| After ICU admission | 1.29 (1.09, 1.51) | 0.002 | 1.44 (1.21, 1.73) | <0.001 |
Definition of abbreviations: CI = confidence interval; ICU = intensive care unit; OR = odds ratio; SF-36 = Medical Outcomes Study Short-Form 36.
Logistic regression with generalized estimating equations for repeated observations, using exchangeable correlation matrix and robust variance.
Adjusted for previous score, time since previous score, demographics (age, race, income, education, marital status), Deyo-Charlson comorbidity score, depression, distance to Veterans Affairs hospital, and receipt of outside care.
Clinically meaningful decline = 5 or more points.
Clinically meaningful decline = 10 or more points.
Table 7.
Odds of a clinically significant decline in health-related quality-of-life scores (physical component summary and physical subdomains) by hospitalization status: comparison of intensive care unit to non–intensive care unit hospitalized patients
| Follow-up SF-36 Scores | Unadjusted OR (95% CI) | P Value | Adjusted OR* (95% CI) | P Value |
|---|---|---|---|---|
| Physical component summary† | ||||
| After non-ICU admission | — | — | — | — |
| After ICU admission | 1.02 (0.82, 1.26) | 0.864 | 1.01 (0.79, 1.30) | 0.910 |
| Physical subdomains | ||||
| Physical Functioning‡ | ||||
| After non-ICU admission | — | — | — | — |
| After ICU admission | 1.20 (1.00, 1.45) | 0.056 | 1.24 (1.00, 1.55) | 0.054 |
| Role-Physical‡ | ||||
| After non-ICU admission | — | — | — | — |
| After ICU admission | 1.12 (0.90, 1.39) | 0.326 | 1.26 (0.93, 1.71) | 0.142 |
| Bodily Pain‡ | ||||
| After non-ICU admission | — | — | — | — |
| After ICU admission | 1.00 (0.83, 1.20) | 0.972 | 0.98 (0.80, 1.22) | 0.886 |
| General Health‡ | ||||
| After non-ICU admission | — | — | — | — |
| After ICU admission | 0.94 (0.77, 1.14) | 0.517 | 0.94 (0.76, 1.17) | 0.585 |
Definition of abbreviations: CI = confidence interval; ICU = intensive care unit; OR = odds ratio; SF-36 = Medical Outcomes Study Short-Form 36.
Logistic regression with generalized estimating equations for repeated observations, using exchangeable correlation matrix and robust variance.
Adjusted for hospital length of stay, time from discharge to follow-up health-related quality-of-life score, previous score, time since previous score, demographics (age, race, income, education, marital status), Deyo-Charlson comorbidity score, depression, distance to Veterans Affairs hospital, and receipt of outside care.
Clinically meaningful decline = 5 or more points.
Clinically meaningful decline = 10 or more points.
Examining the Influence of Most Recent PCS on the Decline in PCS after Discharge
We found that the magnitude of reduction in PCS score associated with hospitalization was greatest among participants with the highest most recent scores (P value for interaction term, <0.001) (Figure 3). Among patients with PCS scores in the highest quartile of the cohort (median PCS, 50.2; range, 43.2–68.3), hospitalization (β = −3.09 [95% CI, −4.49, −1.69]), and ICU admission (β = −5.00 [95% CI, −7.43, −2.57]) were associated with statistically significant (and among ICU hospitalized patients, clinically significant) decrements in PCS score compared with those not hospitalized. There were still no differences between hospitalized and ICU hospitalized groups (P = 0.17).
Figure 3.
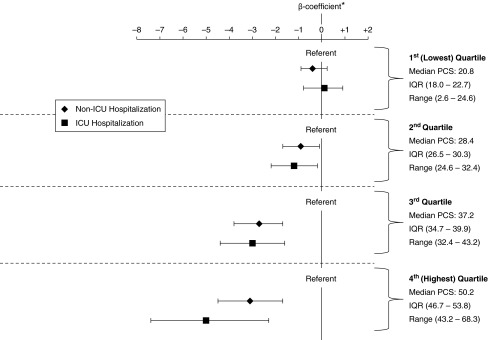
Differences in Physical Component Summary (PCS) score, stratified by quartiles of most recent prior PCS scores. *P value for interaction term of stratified most recent prior PCS score and hospitalization status in adjusted model < 0.001. Shown are β-coefficients and 95% confidence intervals for models stratified by quartile of most recent prior PCS score, adjusted for a priori confounders; ICU = intensive care unit; IQR = interquartile range.
Sensitivity Analysis
Among patients with a bed location code for the ICU, 29 patients were identified as having critical illness using ICD-9 diagnostic codes used by Ehlenbach and colleagues (11). We again found that non-ICU hospitalized and ICU hospitalized patients had greater decrements in their follow-up HRQoL scores than their nonhospitalized counterparts (Tables E5 and E6). In this subsample, HRQoL declined to a greater extent among patients with critical illness compared with those hospitalized without critical illness for several of the outcomes. In the final adjusted model, these differences were attenuated, and our results were similar to our primary analysis for most outcomes. Unlike our primary analysis, this subset of critically ill patients did have clinically significant declines in some of the HRQoL subdomains (Role-Physical, Bodily Pain, General Health, Social Functioning, and Role-Emotional) when compared with nonhospitalized patients; the magnitude of these reductions differed from those experiencing a non-ICU hospitalization only for the general health subdomain (adjusted β-coefficient comparing ICU to non-ICU hospitalized, −6.38 [95% CI, −11.00, −1.76]).
Discussion
In this large longitudinal cohort study with prospectively collected assessments of HRQoL, we found baseline HRQoL to be lower among patients with a subsequent hospitalization or ICU admission than those not requiring hospitalization. Patients who were hospitalized and received ICU-level care had larger 1-year decrements in HRQoL compared with patients with no hospitalizations, even after adjusting for prehospital SF-36 values. However, decrements in HRQoL after a hospitalization were similar for patients with or without an ICU stay, suggesting that for many patients any acute illness could be associated with worse physical and emotional functioning regardless of their receipt of critical care services.
We contend that our findings have two important implications. First, for the majority of patients, outcomes after critical illness may not differ substantially from outcomes after hospitalization for other acute conditions that do not result in ICU admission. Second, to adequately study differences in patient-centered outcomes after hospital admission, it is imperative that premorbid objective assessments of these outcomes are available.
Our findings need to be considered in the context of several limitations of our study design, including possible bias due to informative censoring among the sickest patients, heterogeneity of the acuity of illness among ICU patients, a possible lack of power to rule out differences between the most critically ill patients and their non-ICU hospitalized counterparts due to sample size, and the possibility of a floor effect due to very poor baseline quality of life among study participants. We acknowledge the importance of these challenges, and as a result we will address them first before discussing the implications of our findings in more detail.
First, as are all studies that collect longitudinal assessments after critical illness, our study is subject to bias due to informative censoring. Patients who are the sickest, and may have had the largest decrements in HRQoL, may not have been able to respond to the mailed questionnaires. At the same time, because only VA hospitalizations were captured, it is possible that the sickest patients were hospitalized at outside ICUs, were not included among our critically ill patients, and did not respond to follow-up surveys. As a result of these limitations, our results may overestimate the HRQoL among survivors of an ICU hospitalization, biasing us to not find a difference between critically ill and noncritically ill patients when one truly exists. Although we acknowledge these limitations, we are reassured by the fact that among patients surviving the first year of follow-up, there was no difference in nonresponse rates among patients with a non-ICU or ICU hospitalization. Furthermore, nonresponders in general tended to be younger, with less comorbid illness, indicating the magnitude of these biases is likely small.
Another limitation is that we lack a measure of illness severity for hospitalized or critically ill patients. It is possible that nonpatient factors influenced ICU triage decisions (30, 31), biasing us toward finding no difference between these groups. We attempted to address this limitation using a highly specific set of ICD-9 codes to define what we believe to be the sickest patients among our ICU cohort (11). Although the results of this sensitivity analysis did show clinically significant differences in some of the HRQoL domains when compared with nonhospitalized patients, these differences were not consistent across all domains of the SF-36 and did not involve our primary outcome, the PCS score. The small number of patients who met the Ehlenbach criteria limits our ability to rule out any difference between these very sick patients and noncritically ill hospitalized patients, although we did find a small difference in the General Health subscale of the SF-36 between these two groups.
Finally, the overall poor health status of our population at baseline may have induced a “floor effect,” limiting our ability to detect a significant decline in HRQoL. Indeed, the baseline PCS and MCS scores in our cohort are lower than post–critical illness scores reported in large cohorts of severely critically ill patients (7) and similar to that among inpatients hospitalized for an acute coronary event (32). Our findings are consistent with prior studies demonstrating HRQoL to be lower among U.S. Veterans than non-Veteran populations and may result from high rates of comorbidity and mental illnesses among the Veteran population (33). Despite these challenges, we contend that our results are compelling enough to have important implications for future research.
Outcomes after Critical Illness May Not Differ from Those after Non-ICU Hospitalization
We found no difference in the decline in HRQoL among hospitalized patients who did or did not require ICU care. Our findings are in contrast to one recent study of elderly Medicare beneficiaries showing survivors of a hospitalization involving mechanical ventilation had increased disability when compared with those without mechanical ventilation (9). However, the differences between disability scores among patients with and without mechanical ventilation in that study were relatively small (difference of 3.4 on a scale of 0–100). It may be that acute illnesses requiring hospitalization lead to long-term declines in overall health status regardless of the presence of critical illness. This hypothesis is supported by studies demonstrating that hospitalization, even for illnesses that are easily treatable and considered uncomplicated, is associated with significant disability, particularly among the elderly (34–36). There are a number of shared possible risk factors between hospitalized and critically ill patients that have the potential to negatively affect HRQoL, such as delirium, decreased mobility, depression, and malnutrition. The cumulative effect of such factors on HRQoL is not known and could be the focus of future investigations. Such studies have the potential to lead to a better understanding of which patient populations to target in future intervention studies designed to improve long-term HRQoL. Our results suggest that these interventions may be important for all hospitalized patients, not just those as identified as critically ill.
Premorbid Measures of HRQoL Are Needed to Assess Posthospitalization HRQoL
Several prior studies have shown HRQoL to be lower among survivors of critical illness compared with the general population, yet these studies suffer from several important limitations (3–6, 8, 14, 16). Many did not collect information about prehospitalization HRQoL, and, in those that did, baseline HRQoL was assessed using retrospective report among survivors or was estimated by surrogates at the time of admission (14–16). As a result, it is difficult to determine if the reduced HRQoL after critical illness described in prior studies was due to low baseline HRQoL or reflected changes due to hospitalization for critical illness (3). Although a handful of studies suggest that prehospitalization HRQoL is likely lower among patients admitted with critical illness (15, 16), ours is the first to confirm this finding using prospective assessments. Our results emphasize the importance of accurate assessments of prehospital functional status in determining the possible effect of acute or critical illness on posthospital quality of life. This is particularly true among our cohort of patients who reported very low HRQoL even before hospital admission. Evaluation of only post-ICU health status in such a cohort could lead to erroneous conclusions about the effect of hospitalization and critical illness on HRQoL. Our findings complement recent work that calls for prehospital assessment of patients’ functional status when examining the effect of critical illness on posthospital rates of geriatric conditions, such as injurious falls and chronic pain, as well as recent literature showing increased risk of hospitalization for pneumonia among patients already demonstrating evidence of cognitive decline (18, 37). Our finding of a significant interaction between baseline HRQoL and subsequent decline in PCS scores after hospitalization means that this premorbid assessment needs to be carefully considered when planning future studies and interventions targeting HRQoL among hospitalized patients.
In contrast to several other studies, we found the magnitude of the average change in HRQoL associated with critical illness to be relatively small. There are several explanations for this finding. Studies showing greater decrements in HRQoL may not have adequately measured and adjusted for prehospital HRQoL, or the overall poor health status of our population may have induced a “floor effect” limiting our ability to detect a significant decline in HRQoL, as suggested above. In addition, many prior studies of HRQoL have concentrated on specific populations of patients, such as those with severe adult respiratory distress syndrome or multiorgan dysfunction (6, 15). This prior work may not generalize to a more heterogeneous and possibly less severely ill group of ICU patients such as ours. However, given the variability of severity of illness that has been demonstrated among ICU patients in previous studies (38), our population may be more similar to many ICUs than the settings and populations in which long-term outcomes after critical illness has largely been studied to date. Finally, HRQoL after critical illness has been shown to improve over time (14). The mean time between discharge and follow-up SF-36 score in our cohort was approximately 6 months, and although we adjusted for time between discharge and follow-up assessment in our sensitivity analysis, we may have missed early negative effects on HRQoL due to critical illness. Nevertheless, we would argue that long-term, rather than reversible short-term, derangements are perhaps the most important outcomes for patients, their families, and their clinicians.
In addition to those discussed above, our study should be interpreted in the context of other more minor limitations. First, our Veteran population consists mostly of older, white men and therefore may not generalize to other populations. Second, the data used for this analysis are more than a decade old, during which time substantial improvements in survival have been achieved for a number of patients with critical illness, including those receiving mechanical ventilation (39). It is possible that patients surviving a more severe critical illness would have greater decrements in their HRQoL than those observed here. We believe that the strength of this study lies in its prospectively collected assessments of HRQoL; to our knowledge, it is the first of its kind, and given the challenges of measuring premorbid HRQoL it is unlikely that another study like it will be done in the near future.
In conclusion, prehospital HRQoL is a powerful determinant of HRQoL after hospitalization or ICU admission. Although hospitalization is associated with increased risk of clinically significant decrements in HRQoL after discharge, we found the overall magnitude of this reduction is small and similar between non-ICU hospitalized and critically ill patients. Patients with higher baseline HRQoL may be at greatest risk of subsequent decline in HRQoL resulting from the hospital stay and may be an important population to target in future intervention studies. These studies should include careful assessment of premorbid HRQoL, and all hospitalized patients, not just those who are identified as critically ill, may benefit from these interventions.
Footnotes
L.C.F. is supported by Mentored Clinical Research Career Development Award grant K23HL111116 from the National Institutes of Health, National Heart, Lung, and Blood Institute and by the Department of Veterans Affairs, Health Services Research and Development (VA HSR&D). D.H.A. and V.S.F. are supported by the VA HSR&D. C.R.C. is supported by Mentored Clinical Scientist Research Career Development Award grant K08 HS020672 from the Agency for Healthcare Research and Quality. W.J.E. is supported by a Beeson Career Development Award in aging, sponsored by The National Institute on Aging, The Atlantic Philanthropies, The John A. Hartford Foundation, the Starr Foundation, the National Institute on Neurological Disorders and Strokes, and an anonymous donor.
The views expressed here are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the National Institutes of Health.
Author Contributions: L.C.F., C.R.C., G.D.R., C.L.H., and V.S.F. contributed to the conception and design of the study and planned the data analysis. V.S.F. was responsible for acquisition of the data; L.C.F. performed all statistical analysis. All of the authors contributed to the interpretation of the data. L.C.F. wrote the initial draft of the manuscript; all of the authors contributed to revising the manuscript critically for important intellectual content and provided final approval of the version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303:849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 2.Needham DM, Kamdar BB, Stevenson JE. Rehabilitation of mind and body after intensive care unit discharge: a step closer to recovery. Crit Care Med. 2012;40:1340–1341. doi: 10.1097/CCM.0b013e31823b8df7. [DOI] [PubMed] [Google Scholar]
- 3.Dowdy DW, Eid MP, Sedrakyan A, Mendez-Tellez PA, Pronovost PJ, Herridge MS, Needham DM. Quality of life in adult survivors of critical illness: a systematic review of the literature. Intensive Care Med. 2005;31:611–620. doi: 10.1007/s00134-005-2592-6. [DOI] [PubMed] [Google Scholar]
- 4.Heyland DK, Hopman W, Coo H, Tranmer J, McColl MA. Long-term health-related quality of life in survivors of sepsis. Short Form 36: a valid and reliable measure of health-related quality of life. Crit Care Med. 2000;28:3599–3605. doi: 10.1097/00003246-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Hofhuis JG, Spronk PE, van Stel HF, Schrijvers AJ, Rommes JH, Bakker J. The impact of severe sepsis on health-related quality of life: a long-term follow-up study. Anesth Analg. 2008;107:1957–1964. doi: 10.1213/ane.0b013e318187bbd8. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF., Jr Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 7.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 8.Combes A, Costa MA, Trouillet JL, Baudot J, Mokhtari M, Gibert C, Chastre J. Morbidity, mortality, and quality-of-life outcomes of patients requiring >or=14 days of mechanical ventilation. Crit Care Med. 2003;31:1373–1381. doi: 10.1097/01.CCM.0000065188.87029.C3. [DOI] [PubMed] [Google Scholar]
- 9.Barnato AE, Albert SM, Angus DC, Lave JR, Degenholtz HB. Disability among elderly survivors of mechanical ventilation. Am J Respir Crit Care Med. 2011;183:1037–1042. doi: 10.1164/rccm.201002-0301OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJ, Carson SS, Curtis JR, Larson EB. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davydow DS, Hough CL, Langa KM, Iwashyna TJ. Symptoms of depression in survivors of severe sepsis: a prospective cohort study of older Americans. Am J Geriatr Psychiatry. 2013;21:887–897. doi: 10.1016/j.jagp.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubenfeld GD. Does the hospital make you older faster? Am J Respir Crit Care Med. 2012;185:796–798. doi: 10.1164/rccm.201202-0267ED. [DOI] [PubMed] [Google Scholar]
- 14.Hofhuis JG, Spronk PE, van Stel HF, Schrijvers GJ, Rommes JH, Bakker J. The impact of critical illness on perceived health-related quality of life during ICU treatment, hospital stay, and after hospital discharge: a long-term follow-up study. Chest. 2008;133:377–385. doi: 10.1378/chest.07-1217. [DOI] [PubMed] [Google Scholar]
- 15.Wehler M, Geise A, Hadzionerovic D, Aljukic E, Reulbach U, Hahn EG, Strauss R. Health-related quality of life of patients with multiple organ dysfunction: individual changes and comparison with normative population. Crit Care Med. 2003;31:1094–1101. doi: 10.1097/01.CCM.0000059642.97686.8B. [DOI] [PubMed] [Google Scholar]
- 16.Graf J, Koch M, Dujardin R, Kersten A, Janssens U. Health-related quality of life before, 1 month after, and 9 months after intensive care in medical cardiovascular and pulmonary patients. Crit Care Med. 2003;31:2163–2169. doi: 10.1097/01.CCM.0000079607.87009.3A. [DOI] [PubMed] [Google Scholar]
- 17.Scales DC, Tansey CM, Matte A, Herridge MS. Difference in reported pre-morbid health-related quality of life between ARDS survivors and their substitute decision makers. Intensive Care Med. 2006;32:1826–1831. doi: 10.1007/s00134-006-0333-0. [DOI] [PubMed] [Google Scholar]
- 18.Iwashyna TJ, Netzer G, Langa KM, Cigolle C. Spurious inferences about long-term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841. doi: 10.1164/rccm.201109-1660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fihn SD, McDonell MB, Diehr P, Anderson SM, Bradley KA, Au DH, Spertus JA, Burman M, Reiber GE, Kiefe CI, et al. Effects of sustained audit/feedback on self-reported health status of primary care patients. Am J Med. 2004;116:241–248. doi: 10.1016/j.amjmed.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Cecere LM, Cooke CR, Hough CL, Rubenfeld GD, Ehlenbach WJ, Au DH, Fan VS. Intensive care admission is no worse for long-term quality of life than hospitalization [abstract] Am J Respir Crit Care Med. 2011;183:A4125. [Google Scholar]
- 21.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 22.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Ware J, Kosinkski M, Gandek B.SF-36 health survey manual and interpretation guide Lincoln, RI: Quality Metric2005 [Google Scholar]
- 24.Wyrwich KW, Nelson HS, Tierney WM, Babu AN, Kroenke K, Wolinsky FD. Clinically important differences in health-related quality of life for patients with asthma: an expert consensus panel report. Ann Allergy Asthma Immunol. 2003;91:148–153. doi: 10.1016/S1081-1206(10)62169-2. [DOI] [PubMed] [Google Scholar]
- 25.Maynard C, Chapko MK. Data resources in the Department of Veterans Affairs. Diabetes Care. 2004;27:B22–B26. doi: 10.2337/diacare.27.suppl_2.b22. [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 28.Burgess JF, Jr, DeFiore DA. The effect of distance to VA facilities on the choice and level of utilization of VA outpatient services. Soc Sci Med. 1994;39:95–104. doi: 10.1016/0277-9536(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 29.Fitzmaurice GM, Laird NM, Ware JH.Applied longitudinal analysis Hoboken, NJ: Wiley-Interscience2004 [Google Scholar]
- 30.Seymour CW, Iwashyna TJ, Ehlenbach WJ, Wunsch H, Cooke CR. Hospital-level variation in the use of intensive care. Health Serv Res. 2012;47:2060–2080. doi: 10.1111/j.1475-6773.2012.01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen LM, Render M, Sales A, Kennedy EH, Wiitala W, Hofer TP. Intensive care unit admitting patterns in the Veterans Affairs health care system. Arch Intern Med. 2012;172:1220–1226. doi: 10.1001/archinternmed.2012.2606. [DOI] [PubMed] [Google Scholar]
- 32.Emery CF, Frid DJ, Engebretson TO, Alonzo AA, Fish A, Ferketich AK, Reynolds NR, Dujardin JP, Homan JE, Stern SL. Gender differences in quality of life among cardiac patients. Psychosom Med. 2004;66:190–197. doi: 10.1097/01.psy.0000116775.98593.f4. [DOI] [PubMed] [Google Scholar]
- 33.Kazis LE, Ren XS, Lee A, Skinner K, Rogers W, Clark J, Miller DR. Health status in VA patients: results from the Veterans Health Study. Am J Med Qual. 1999;14:28–38. doi: 10.1177/106286069901400105. [DOI] [PubMed] [Google Scholar]
- 34.Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA. 2011;306:1782–1793. doi: 10.1001/jama.2011.1556. [DOI] [PubMed] [Google Scholar]
- 35.Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304:1919–1928. doi: 10.1001/jama.2010.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davydow DS, Hough CL, Levine DA, Langa KM, Iwashyna TJ. Functional disability, cognitive impairment, and depression after hospitalization for pneumonia. Am J Med. 2013;126:615–624.e5. doi: 10.1016/j.amjmed.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah FA, Pike F, Alvarez K, Angus D, Newman AB, Lopez O, Tate J, Kapur V, Wilsdon A, Krishnan JA, et al. Bidirectional relationship between cognitive function and pneumonia. Am J Respir Crit Care Med. 2013;188:586–592. doi: 10.1164/rccm.201212-2154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O’Brien CR, Rubenfeld GD. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006;355:41–50. doi: 10.1056/NEJMsa053993. [DOI] [PubMed] [Google Scholar]
- 39.Esteban A, Frutos-Vivar F, Muriel A, Ferguson ND, Peñuelas O, Abraira V, Raymondos K, Rios F, Nin N, Apezteguía C, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med. 2013;188:220–230. doi: 10.1164/rccm.201212-2169OC. [DOI] [PubMed] [Google Scholar]