Abstract
Rationale: Sepsis therapeutics have a poor history of success in clinical trials, due in part to the heterogeneity of enrolled patients. Pharmacometabolomics could differentiate drug response phenotypes and permit a precision medicine approach to sepsis.
Objectives: To use existing serum samples from the phase 1 clinical trial of l-carnitine treatment for severe sepsis to metabolically phenotype l-carnitine responders and nonresponders.
Methods: Serum samples collected before (T0) and after completion of the infusion (T24, T48) from patients randomized to either l-carnitine (12 g) or placebo for the treatment of vasopressor-dependent septic shock were assayed by untargeted 1H-nuclear magnetic resonance metabolomics. The normalized, quantified metabolite data sets of l-carnitine- and placebo-treated patients at each time point were compared by analysis of variance with post-hoc testing for multiple comparisons. Pathway analysis was performed to statistically rank metabolic networks.
Measurements and Main Results: Thirty-eight metabolites were identified in all samples. Concentrations of 3-hydroxybutyrate, acetoacetate, and 3-hydroxyisovalerate were different at T0 and over time in l-carnitine-treated survivors versus nonsurvivors. Pathway analysis of pretreatment metabolites revealed that synthesis and degradation of ketone bodies had the greatest impact in differentiating l-carnitine treatment response. Analysis of all patients based on pretreatment 3-hydroxybutyrate concentration yielded distinct phenotypes. Using the T0 median 3-hydroxybutyrate level (153 μM), patients were categorized as either high or low ketone. l-Carnitine-treated low-ketone patients had greater use of carnitine as evidenced by lower post-treatment l-carnitine levels. The l-carnitine responders also had faster resolution of vasopressor requirement and a trend toward a greater improvement in mortality at 1 year (P = 0.038) compared with patients with higher 3-hydroxybutyrate.
Conclusions: The results of this preliminary study, which were not readily apparent from the parent clinical trial, show a unique metabolite profile of l-carnitine responders and introduce pharmacometabolomics as a viable strategy for informing l-carnitine responsiveness. The approach taken in this study represents a concrete example for the application of precision medicine to sepsis therapeutics that warrants further study.
Keywords: sepsis, 3-hydroxybutyric acid, ketone bodies, nuclear magnetic resonance, individualized medicine
Although advances in the delivery of care for patients with sepsis appear to have led to gradual improvements in sepsis survival, sepsis continues to be a significant hazard to human health with mortality from severe sepsis and septic shock greater than 20 and 40%, respectively (1–4). Importantly, novel pharmacologic therapies have had a checkered history in sepsis, with multiple promising therapeutics failing to demonstrate efficacy in phase 3 clinical trials (5). Although the reasons for such disappointments are complex, two factors that cloud the execution of sepsis clinical trials that might contribute to these results are an inability to prospectively identify a homogeneous group of patients who are most likely to benefit from a novel therapy and the absence of predictive parameters for the monitoring of drug delivery and response. These deficiencies provide the rationale for precision medicine (6, 7), which extends personalized medicine beyond the genome to include a broader systems, multilevel approach to the tailoring of therapeutics to individual patients. To date, efforts in personalized medicine in sepsis have been relatively incremental (8). Pharmacogenomics has yielded some success in identifying treatment response phenotypes (9) but pharmacometabolomics (10–12) may hold more promise. This is because the metabolome represents the end result of gene and protein function and activity (13–15) and therefore may provide a more sensitive readout of drug response phenotypes because most drugs impact components of metabolism (16, 17).
In this study, we applied pharmacometabolomics to existing serum samples that were collected as part of a study of the clinical and metabolic response of patients with vasopressor-dependent septic shock to a novel, metabolic therapy: levo (l)-carnitine (Carnitor injection, levocarnitine; Sigma-Tau Pharmaceuticals, Gaithersburg, MD) (14, 18). There is a growing body of evidence that suggests a series of metabolic changes are induced in the setting of sepsis (19–21). The most well recognized of these is hyperlactatemia, which has traditionally been attributed to tissue hypoperfusion. However, numerous studies have demonstrated that the lactate elevation in sepsis is multifactorial, and besides tissue hypoperfusion, it can result from stimulation of Na+/K+ ATPase or inhibition of various enzymes within the mitochondria (22–27). In addition to changes in lactate metabolism, other metabolic changes have been described in the setting of sepsis including alterations in pyruvate, glucose, alanine, and ketone bodies (Figure 1), demonstrating the central metabolic component of this disease process (21, 28). However, the degree of disruption in metabolic homeostasis varies dramatically from individual to individual, and it is plausible that responsiveness to therapy will also likewise vary. Identification of a group of patients most likely to benefit from a therapy is important not only for clinical practice, but also for future clinical trial design, and is one of the goals of precision medicine. Thus, we sought to metabolically phenotype early septic shock patients enrolled in a phase 1 randomized controlled clinical trial of l-carnitine treatment (18). To achieve this, we conducted nuclear magnetic resonance (NMR) metabolite profiling of existing serum samples that were collected before and after l-carnitine or placebo treatment. We used statistical and pathway analyses of the generated metabolomics data and the clinical phenotype data from the clinical trial to test the feasibility of a pharmacometabolomics approach to identifying a pretreatment metabolome of l-carnitine responders and nonresponders. Some of the results of this study have been previously reported in the form of an abstract (29).
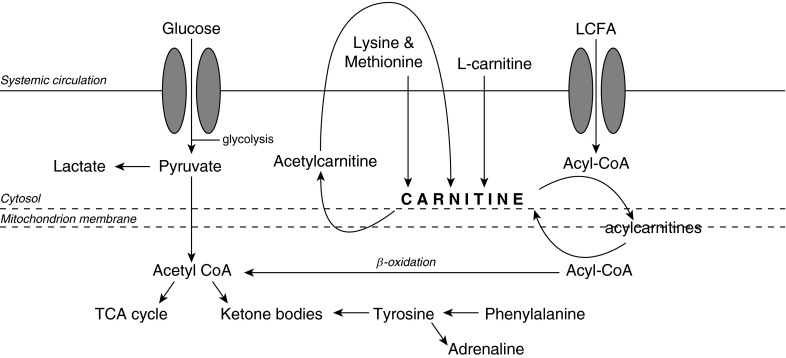
Figure 1.
Metabolic pathways of carnitine, showing the relationship of several of the differentiating metabolites of l-carnitine treatment response (47). Lysine and methionine are precursors of carnitine, which is required for the transport of long-chain fatty acids (LCFAs) into the mitochondria. Acetylcarnitine is formed by the acetylation of carnitine. Subsequently, it is hydrolyzed by plasma esterases to form carnitine. Ketone bodies can be produced via acetyl-coenzyme A (acetyl-CoA) and β-oxidation of LCFAs in the mitochondria. Phenylalanine is metabolized to tyrosine, which participates in the synthesis of adrenaline. TCA = tricarboxylic acid cycle.
Methods
Description of the Clinical Trial
The phase 1 study of l-carnitine infusion for the treatment of vasopressor-dependent shock has been previously published in full (18) and took place at a single tertiary care medical center and was approved by the Carolinas Medical Center Institutional Review Board in accordance with the ethical standards put forth in the Helsinki Declaration of 1975 (as revised in 1983). l-Carnitine was approved for investigational use in septic shock by the Food and Drug Administration (Investigational New Drug #107,086) and the study was registered on clinicaltrials.gov (NCT01193777) (18). Abbreviated inclusion criteria were as follows: suspicion of or confirmed infection; two or more systemic inflammatory response syndrome criteria; hypotension requiring vasopressors despite volume resuscitation; a cumulative vasopressor index of 3 or more (30) after 4 hours of administration; and a Sequential Organ Failure Assessment (SOFA) score of at least 5 (31). Abbreviated exclusion criteria were as follows: age less than 18 years; greater than 16 hours after septic shock recognition; a Do Not Resuscitate order; or known inborn error of metabolism. A total of 31 patients were enrolled, after which patients were randomized to either intravenous carnitine (12 g delivered as a 4-g bolus followed by an 8-g infusion over 12 h) or an equivalent volume of saline placebo. The demographic data of the patients randomized to each group have been previously published (18). All patients, clinicians, and investigators were blinded to study assignment until study completion. The study demonstrated no significant difference in adverse events between groups, and a significant reduction in mortality at 28 days (4 of 16 vs. 9 of 15; P = 0.048) with a trend toward improved 1-year survival (P = 0.057). To further study and confirm these findings, a multicenter phase 2 clinical trial is currently ongoing.
This metabolomics study used serum samples from the aforementioned clinical trial but was independent of the clinical trial. Blood samples were collected via an existing intravenous or arterial catheter before administration (T0) of l-carnitine or placebo, and 24 (±4) hours (T24) and 48 (±4) hours (T48) after enrollment. Blood was drawn into a serum separator tube (10 ml; Becton Dickinson, Franklin Lakes, NJ) and was allowed to clot at room temperature for at least 30 minutes, after which it was centrifuged (1,800 × g at 15°C for 10 min) to obtain serum, which was aliquoted (500 μl) and stored (–80°C) until the time of metabolomics assay.
Serum Sample Processing and 1H-NMR Metabolomics
Serum samples were sent frozen on dry ice to the University of Michigan (Ann Arbor, MI) by priority overnight express shipping. On delivery they were immediately transferred to a freezer (–80°C), where they were stored until the time of assay. At the time of assay, samples were thawed on ice and were subjected to 1:1 methanol (MeOH)–chloroform (CHCl3) extraction to precipitate macromolecules as previously described (32–34). The aqueous fraction of each extracted serum sample was assayed by one-dimensional (1-D) 1H-NMR using a Varian (now Agilent Inc., Santa Clara, CA) 11.74-T (500-MHz) spectrometer (additional details are provided in the online supplement).
The resulting NMR spectra were processed using the Processor module in Chenomx NMR Suite 7.6 (Chenomx, Inc., Edmonton, AB, Canada; chenomx.com), which permits phase, baseline, and shim correction (35, 36). Compounds were identified and quantified in the Profiler module of the software, which accounts for the pH of the sample and references to the known concentration of the internal standard, formate; metabolite concentrations were corrected for dilution secondary to the addition of formate. Metabolites were named using the Chenomx Compound Library containing 312 compounds. Spectral processing and compound identification and quantification were completed by a single user who was unaware of the treatment allocation of samples.
Data Analysis
The quantified metabolite data were log2 transformed and range scaled to achieve normalization. The mean normalized value of each metabolite at each time point for l-carnitine- and placebo-treated patients was compared by analysis of variance followed by Šidák’s multiple comparison test (37) (GraphPad Prism version 6 for the Mac; GraphPad Software, La Jolla, CA; www.graphpad.com). Descriptions of additional statistical testing as well as the analysis workflow scheme are provided in the online supplement and as shown in Figure E1 in the online supplement.
Results
Of the 31 enrolled patients, serum samples from all 16 in the l-carnitine arm and 14 of the 15 in the placebo arm underwent metabolomics analysis; one set of placebo-treated samples was inadvertently excluded from the shipment and were not further analyzed. Analysis of 1-D 1H-NMR spectra resulted in a total of 38 identified and quantified aqueous metabolites in each serum sample (Table E1). As expected, carnitine and acetylcarnitine levels at T24 were higher in patients treated with l-carnitine than placebo (Figures 2A and 2B). The concentrations of only three other metabolites differed between the two groups (Figures 2C–2E), and there were no differences in metabolite levels between the two groups at T0. Comparison of the metabolite profiles of l-carnitine-treated survivors and nonsurvivors revealed changes in a number of metabolites over time, including 3-hydroxybutyrate (3-OHB), acetoacetate, and 3-hydroxyisovalerate (Figures 3A–3F). Notably, carnitine and acetylcarnitine (Figures 3G and 3H) were higher in nonsurvivors, the elevation of which could not be completely explained by an association with renal function (i.e., serum creatinine; see Figure E2). There were several metabolites that were different between l-carnitine-treated survivors and nonsurvivors at T0 (Figures 3A–3D), and nonsurvivors had higher T0 acetylcarnitine (AC):carnitine (C) ratios (Figure 3I) than survivors. The pretreatment (T0) glucose (P = 0.993), lactate (P = 0.956), and total SOFA scores (31) (P = 0.111) were not different between l-carnitine survivors and nonsurvivors. Pathway analysis (Table E2) of all pretreatment metabolites of l-carnitine-treated patients indicated that the synthesis and degradation of ketone bodies was the most significant and impactful pathway that differentiated l-carnitine-treated survivors and nonsurvivors (Figure E3). A repeat of this analysis, using the pretreatment metabolites of all patients (regardless of treatment) categorized as either survivors or nonsurvivors, produced the same results.
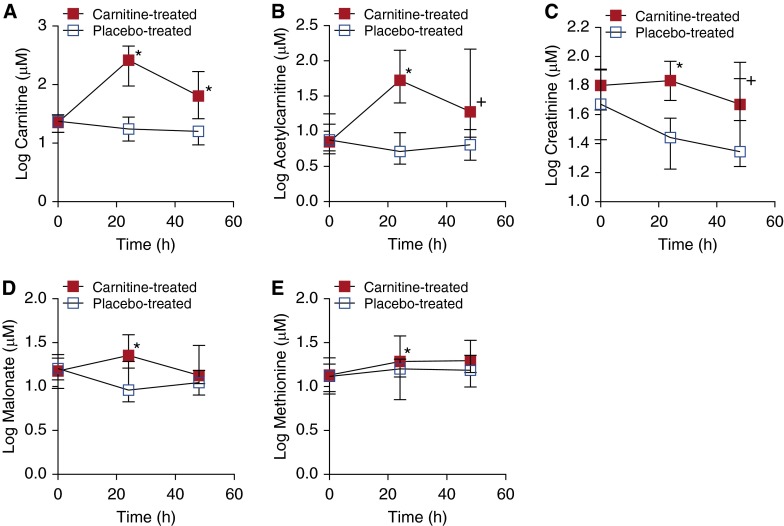
Figure 2.
Differences in aqueous serum metabolite concentrations between l-carnitine- and placebo-treated patients with septic shock. (A) Carnitine (*P < 0.001), (B) acetylcarnitine (*P < 0.001; +P = 0.001), and (C) creatinine (*P = 0.008; +P = 0.050) levels were different at 24 and 48 hours and (D) malonate (*P = 0.002) and (E) methionine (*P = 0.032) levels were different at 24 hours. There were no differences in metabolite concentrations between the two groups at T0 (before treatment). Data represent medians ± the interquartile range of 11–16 l-carnitine- and placebo-treated patients at each time point.
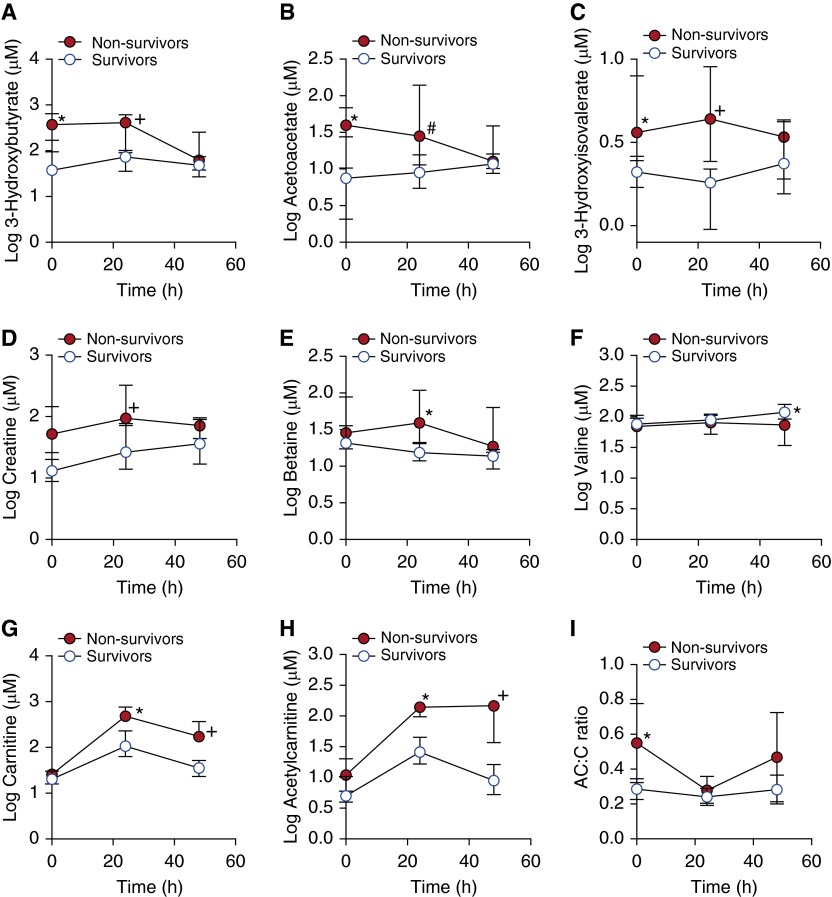
Figure 3.
Metabolite profiles were different in l-carnitine-treated survivors and nonsurvivors. The ketone bodies (A) 3-hydroxybutyrate and (B) acetoacetate were higher at T0 (before treatment) and T24 (24 [±4] h after treatment) in nonsurvivors (*P < 0.001, +P = 0.065, and #P = 0.019). The metabolites (C) 3-hydroxyisovalerate (*P < 0.001, +P = 0.005) and (D) creatine (+P = 0.0496) were also elevated in nonsurvivors at T24 compared with survivors. (E) Betaine (*P = 0.005) was higher at T24 and (F) valine was higher (*P = 0.031) at T48 (48 [±4] h after treatment) in survivors. Notably, both (G) carnitine (*P = 0.001, +P < 0.001) and (H) acetylcarnitine (*P = 0.004, +P = 0.001) were increased in nonsurvivors, suggesting differences in l-carnitine metabolism between the two groups. (I) The acetylcarnitine–carnitine (AC:C) ratio, an indicator of carnitine homeostasis, was higher at T0 (*P = 0.001) and more variable over time in nonsurvivors compared with survivors, suggesting a greater disturbance in carnitine metabolism. There were no differences in the T0 concentrations of betaine, valine, carnitine, or acetylcarnitine between survivors and nonsurvivors. Data represent medians ± the interquartile range of seven or eight patients per group.
Using the aforementioned finding that ketone bodies discriminated l-carnitine response, the data were analyzed further, by categorizing the entire data set (l-carnitine and placebo groups) on the basis of the median pretreatment concentration of 3-OHB (153 μM), the most abundant ketone body (representative 1H-NMR spectra are shown in Figure E4), to test its potential predictiveness, regardless of known outcome. Figures 4A and 4B show the pretreatment levels of 3-OHB and acetoacetate in carnitine- and placebo-treated patients categorized as either “high” ketone or “low” ketone. We also found that the AC:C ratio, which is representative of carnitine homeostasis (38), was higher in high-ketone patients compared with the low-ketone patients (Figure 4C). There were no differences in measured serum (clinical) creatinine or total bilirubin concentrations between the four groups at any of the time points (Figure E5). Despite the magnitude of difference in ketone body concentrations between high- and low-ketone patients at T0, there were no differences in patient demographics or clinical characteristics between the groups (Table 1). More specifically, no differences were observed in severity of illness as estimated by SOFA score (31) (Figure 4D), point-of-care lactate levels (Figure 4E), or glucose levels (Figure 4F).
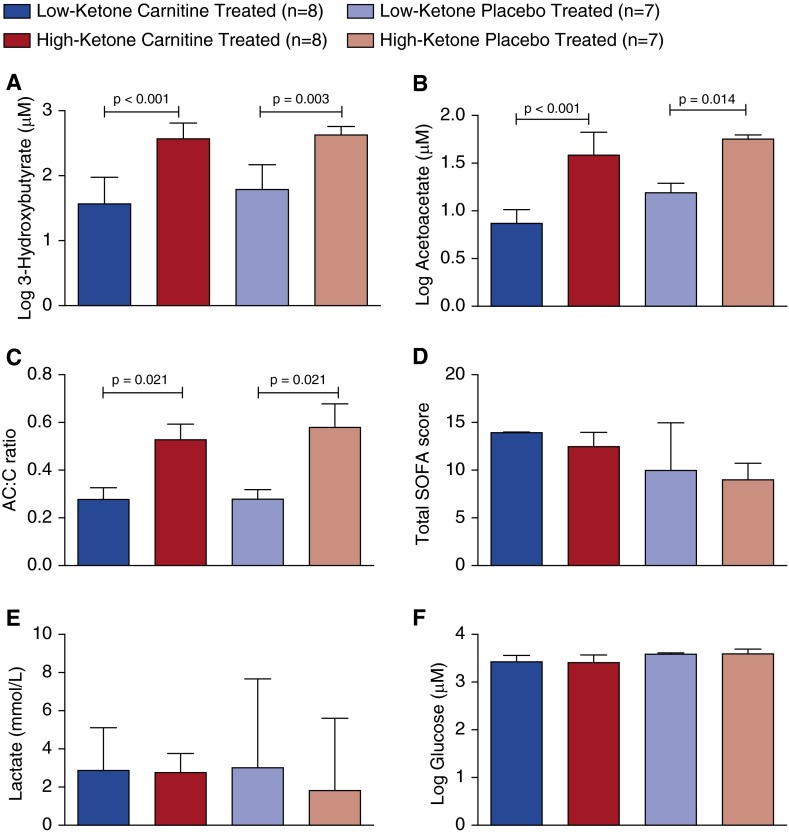
Figure 4.
(A) 3-Hydroxybutyrate (3-OHB) and (B) acetoacetate concentrations and (C) acetylcarnitine–carnitine (AC:C) ratios of placebo- and l-carnitine-treated patients who were categorized as either high or low ketone, based on the 3-OHB concentration at T0 (before treatment) (see text). The higher levels of ketone bodies and AC:C ratios suggest that high-ketone patients had a greater disruption in metabolic homeostasis than did low-ketone patients. This was not differentiated by (D) the T0 total SOFA scores, (E) point-of-care lactate concentrations, or (F) glucose concentrations as measured by nuclear magnetic resonance because they were not different across groups. SOFA = Sequential Organ Failure Assessment.
Table 1.
Patient demographics and clinical characteristics of patients categorized as low or high ketone before administration of l-carnitine or placebo
| Variable | Low Ketones (n = 15) | High Ketones (n = 15) | P Value |
|---|---|---|---|
| Age, yr (IQR) | 60 (52, 68) | 69 (60, 74) | 0.096 |
| Race, n (%) | |||
| White | 11 (73.33) | 13 (86.67) | 0.410 |
| African American | 4 (26.67) | 2 (13) | |
| Male, n (%) | 12 (80) | 9 (60) | 0.268 |
| Comorbidities, n (%) | |||
| Coronary artery disease | 5 (33) | 2 (13) | 0.235 |
| Congestive heart failure | 2 (13) | 4 (27) | 0.410 |
| COPD | 4 (27) | 4 (27) | 0.999 |
| DNR | 0 (0) | 0 (0) | 0.999 |
| Hypertension | 10 (67) | 11 (73) | 0.714 |
| Diabetes | 4 (27) | 6 (40) | 0.473 |
| Chronic renal insufficiency | 2 (13) | 4 (27) | 0.410 |
| Baseline laboratory values (IQR) | |||
| Platelets, cells/mm3 | 159 (82, 232) | 196 (158, 335) | 0.280 |
| Creatinine, mg/dl | 1.8 (1.4, 2.4) | 1.7 (1.5, 2.7) | 0.945 |
| Total bilirubin, mg/dl | 2.1 (0.6, 3.1) | 1.2 (0.9, 1.9) | 1.000 |
| Lactate, mmol/L | 2.9 (2.2, 5.1) | 1.9 (1.6, 4.0) | 0.085 |
| SOFA, score (IQR) | 10 (9, 14) | 12.5 (8, 14) | 0.871 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; DNR = Do Not Resuscitate order; SOFA = Sequential Organ Failure Assessment.
Note: Data represent medians and interquartile ranges (IQRs) or absolute numbers with percentages (%), as appropriate.
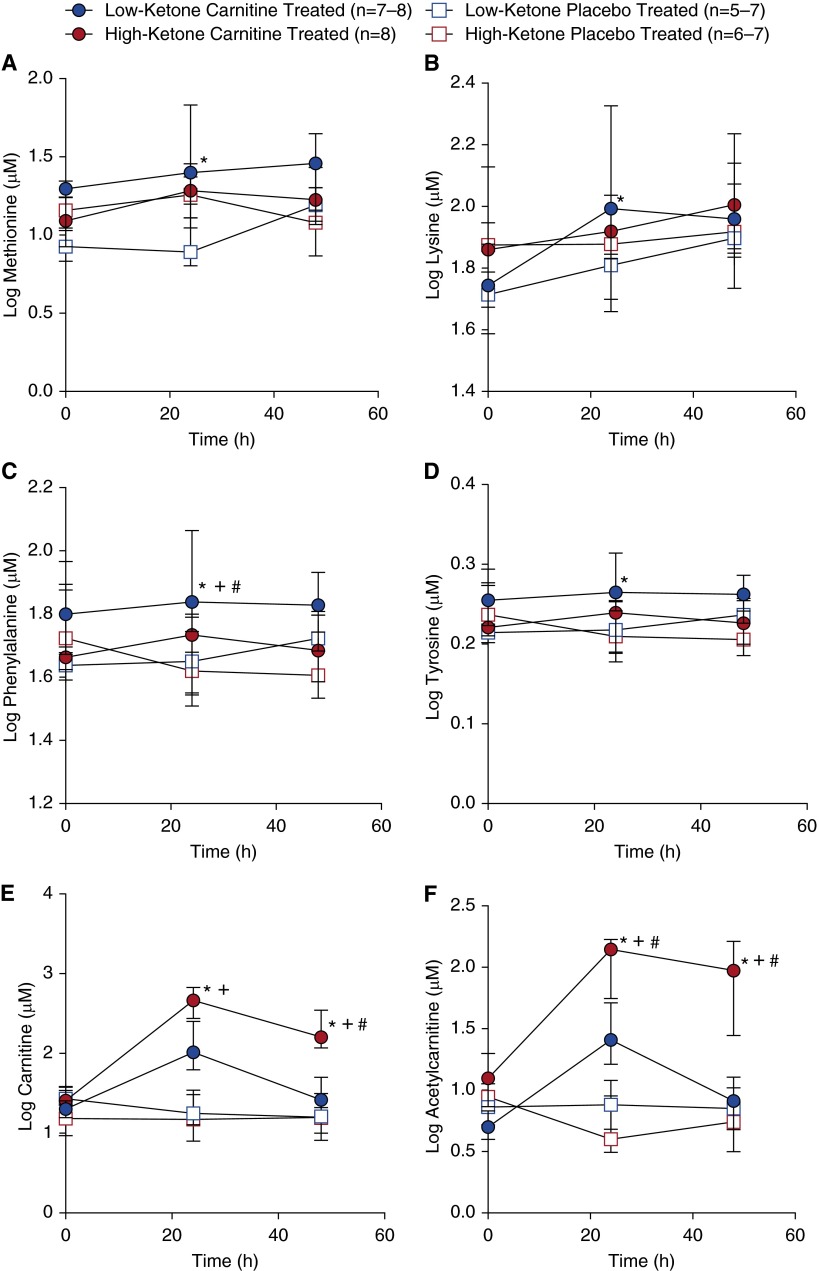
Temporal changes in the levels of several key metabolites are illustrated by subgroup in Figure 5. Methionine and lysine (carnitine precursors; Figure 1 and Figures 5A and 5B) and phenylalanine and tyrosine (catecholamine precursors; Figure 1 and Figures 5C and 5D) tended to be higher in low-ketone, l-carnitine-treated patients as compared with the other groups, suggesting that carnitine supplementation had a temporal treatment effect not only on carnitine-related metabolic pathways, but also on pathways that may be relevant to the pathophysiology of septic shock. Conversely, carnitine and acetylcarnitine (Figures 5E and 5F) were higher in high-ketone, l-carnitine-treated patients, suggesting less carnitine use in these patients. This was further evidenced by the AC:C ratio (Figure E6A), which was consistently lower in high-ketone patients regardless of treatment but remained relatively stable in low-ketone, l-carnitine-treated patients. The trends in changes in the ketone bodies 3-OHB and acetoacetate were also similar in low-ketone patients (Figures E6B and E6C) and increased over time whereas they declined over time in high-ketone patients; glucose remained relatively stable across all four patient groups (Figure E6D).
Figure 5.
Metabolite profiles of patients with septic shock and high or low ketones treated with l-carnitine were different from those of placebo-treated patients. (A) Methionine was increased in low-ketone, l-carnitine-treated patients compared with low-ketone, placebo-treated patients at T24 (24 [±4] h after treatment) (*P = 0.021). There was also a trend toward (B) increased lysine in the low-ketone, l-carnitine-treated patients (*P = 0.078 vs. low-ketone, placebo-treated patients). Methionine and lysine are precursors of endogenous carnitine synthesis. At T24, (C) phenylalanine trended higher (T24) in low-ketone, l-carnitine-treated patients than in high-ketone, l-carnitine-treated patients (*P = 0.051), low-ketone, placebo-treated patients (+P = 0.078), and high-ketone, placebo-treated patients (#P = 0.010). (D) Tyrosine followed a similar trend to phenylalanine in which low-ketone, l-carnitine-treated patients had higher levels at T24 compared with high-ketone, l-carnitine-treated patients (*P = 0.008). Phenylalanine and tyrosine, which can be synthesized from phenylalanine, are proteinogenic amino acids and participate in the synthesis of catecholamines. High-ketone, l-carnitine-treated patients had higher carnitine (E) levels compared with low-ketone, L-carnitine-treated patients (*P = 0.003) and high- and low-ketone, placebo-treated patients (+P < 0.001) at T24 and low-ketone L-carnitine, low-ketone placebo, and high-ketone placebo at T48 (*+#P < 0.0001). A similar pattern was evident in acetylcarnitine (F) concentration in which the T24 and T48 level in high-ketone, L-carnitine-treated patients was higher compared with low-ketone L-carnitine-treated patients (*P = 0.001 at T24; *P < 0.0001 at T48), low-ketone, placebo-treated patients (+P = 0.008 at T24; +P < 0.0001 at T48) and high-ketone, placebo-treated patients (#P < 0.001 at both T24 and T48). At no time were there differences between the low- and high-ketone, placebo-treated groups. There were also no differences in these metabolite levels between groups at T0 (before treatment). Data represent medians ± the interquartile range.
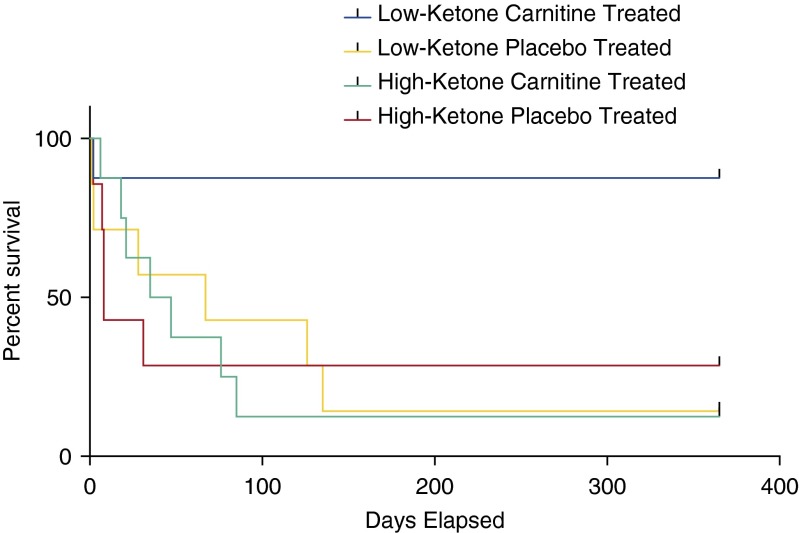
There was a significant difference between the low- and high-ketone groups in time to complete cessation of vasopressors (1,653 min [IQR 1,080, 2,210] vs. 2,918 min [IQR 2,385, 4,380; P = 0.039) as well as a significant difference in the change in cumulative vasopressor index (30) from T0 to T24 (–3 [IQR –4, –2] vs. –1 [IQR –2, 0]; P = 0.019), where negative numbers signify a decreasing vasopressor requirement. Both of these findings support faster improvement in cardiovascular derangement in the low-ketone group. Further analysis demonstrated that this difference was primarily due to the subgroup of patients treated with l-carnitine, as there was no difference in time to vasopressor withdrawal in the high- and low-ketone groups in placebo patients (P = 0.317) but a significant reduction among l-carnitine-treated patients (P = 0.021). Most critically, however, these four subgroups demonstrated significantly different clinical outcomes. Using the mortality end points of the parent clinical trial, the treatment benefit of carnitine was evident in both high- and low-ketone patients at 28 days (Figure E7). However, over time, survival analysis yielded curves that demonstrated a marked trend toward greater patient survival in l-carnitine-treated patients with low ketones (P = 0.007) compared with l-carnitine-treated patients with high ketones (Figure 6). These data demonstrate the potential usefulness of pharmacometabolomics to more fully inform l-carnitine treatment response phenotypes than those that can be detected using more traditional end points such as SOFA scores.
Figure 6.
Low-ketone, l-carnitine-treated patients had a trend toward lower 1-year mortality compared with the other groups of patients (chi square P = 0.038).
Discussion
Pharmacometabolomics, employing 1-D 1H-NMR quantitative metabolomics coupled with a robust analysis strategy, enabled the identification of two distinct l-carnitine treatment response phenotypes. Collectively, this initial exploratory study demonstrates how this discovery, systems biology science, can be effectively applied to complex human illnesses such as sepsis to find drug response phenotypes that were not otherwise readily apparent. This exemplifies precision medicine and in this instance, the pretreatment metabolome held information about the differentiation of l-carnitine treatment responders that was not evident from traditional clinical phenotype markers such as lactate levels and SOFA scores. From this preliminary study, we learned that the extent of the elevation of ketone bodies, 3-OHB and acetoacetate, may have predictive value in defining l-carnitine treatment response. It has been reported that the accumulation of ketones may be indicative of impaired metabolic function in sepsis (39). This is likely due to ketogenesis rather than an impairment in ketone metabolism because ketone levels declined over time in high-ketone patients (see Figure E5). As such, these findings provide some insight into potential underlying mechanisms and the nature of the sepsis-induced disruption in metabolism as well as the mechanisms of l-carnitine treatment response. More generally, this study demonstrates the feasibility of pharmacometabolomics for identifying subgroups of patients more likely to respond to therapy, potentially increasing the likelihood of clinical trial success because it will permit more homogeneous, targeted enrollment criteria. This could have a significant impact on the design and implementation of clinical drug trials and aid in driving precision medicine strategies in sepsis.
The idea that metabolomics has utility in differentiating sepsis phenotypes has gained attention. A metabolomics study of sepsis showed that levels of carnitine and carnitine esters in sepsis survivors were similar to those in uninfected patients, but significantly increased in nonsurvivors (19). In our study, we applied pharmacometabolomics to sepsis and showed that although the pretreatment levels of 3-OHB and acetoacetate, and the AC:C ratios, in high and low serum ketone groups were similar in both the placebo and interventional arms, lower levels of ketone bodies were attributable to a more favorable long-term (1-yr) outcome in l-carnitine-treated patients. This is critically important, because both the level of ketone bodies and carnitine treatment explain the differences in survival between the groups. This is evidenced by the fact that high-ketone patients derived an early benefit from l-carnitine treatment and that the long-term (1-year) outcome of patients in the placebo arm were similar regardless of whether they were categorized as high or low ketone. As such, these data suggest that l-carnitine treatment had an early impact on rescuing the metabolism of high-ketone patients but that this was not maintained over time, implying that these patients may have a unique, and possibly greater, disruption in metabolism compared with low-ketone patients. Conversely, the low-ketone l-carnitine-treated patients had lower 28-day mortality and sustained 1-year survival compared with patients in any of the other groups. However, we cannot dismiss the possibility that the younger age of the low-ketone group influenced outcome and acknowledge that given the small number of patients in this study, these data only show possible trends in survival that need verification in a larger study. Differences in SOFA score likely did not influence outcome because, despite a 2.5-fold difference between low- and high-ketone groups (Table 1), the low-ketone carnitine-treated patients had a higher median SOFA score than any of the other groups (Figure 4).
l-Carnitine is required for the transport of long-chain fatty acids (LCFAs) into the mitochondria for their subsequent use in β-oxidation (Figure 1) (40, 41). This process, involving a number of enzymes and transporters, ultimately leads to the generation of the acetyl-CoA that is needed for the tricarboxylic acid cycle. Sepsis is known to lead to a disruption in metabolism and mitochondrial function, although the specific deficit(s) is/are unknown and are often a point of debate (3, 26, 27, 42, 43). Metabolically, it is not readily apparent why high-ketone patients derived an early benefit of l-carnitine treatment that was lost over time. Compared with high-ketone patients, low-ketone patients demonstrated lower carnitine and acetylcarnitine levels after supplementation, suggesting that low-ketone patients may be better able to use carnitine (as renal function was not different between the groups). This is substantiated by two other findings in low-ketone, l-carnitine-treated patients: (1) the trends of increased methionine and lysine concentrations (Figures 5A and 5B), and (2) the AC:C ratio (Figure E6A). Methionine and lysine are precursors of endogenous carnitine production (Figure 1) and the trend of increasing levels of these metabolites may signify a reduced need for carnitine. The AC:C ratio is viewed as an indicator of carnitine homeostasis (Figure 1) and in low-ketone, l-carnitine-treated patients it remained relatively stable during the 48-hour study period. Furthermore, data showing the poor prognostic significance of high levels of carnitine and its esters in sepsis nonsurvivors (19) are reiterated in this study as high-ketone, l-carnitine-treated patients had higher carnitine levels and lower long-term survival than low-ketone, l-carnitine-treated patients. As such, it is tempting to hypothesize that l-carnitine reverses or at least mitigates further deterioration of sepsis-induced mitochondrial dysfunction in this subset of patients, although we acknowledge that this hypothesis was not specifically tested in our study. However, given that the primary underlying mitochondrial deficit in sepsis remains unknown and that there are numerous potential sites of perturbation, we can suggest that there may be a group of patients with “rescuable” metabolism or with sufficient “metabolic flexibility” (38) (as has been described in type 2 diabetes mellitus) who are able to effectively respond to l-carnitine therapy (44, 45). To further elaborate these data and to test this hypothesis will require a larger, validation study with subsequent prospective testing that could include investigations of the ability of l-carnitine supplementation to either rescue or mitigate mitochondrial dysfunction, and the extent of association of these changes with changes in the serum metabolome.
Regarding other effects on the metabolome, previous investigations in nonseptic patients have demonstrated that l-carnitine therapy can improve glucose use particularly in skeletal muscle (46, 47). We did not specifically measure glucose use but there were no differences or notable trends in glucose levels across the groups and the prevalence of diabetes mellitus was similar across low- and high-ketone patients (see Table 1). However, there were other findings on the influence of l-carnitine on metabolism. Specifically, trends of increasing levels of tyrosine and phenylalanine are particularly intriguing. One previous clinical trial of acetyl-l-carnitine treatment in sepsis demonstrated improvements in right atrial and systolic blood pressures (48). As these two metabolites are precursors of endogenous catecholamines, it is conceivable that carnitine supplementation increased their bioavailability, thus serving to rescue patients from shock. This is substantiated by the observed differences in time to vasopressor withdrawal between high- and low-ketone groups treated with l-carnitine in which low-ketone, l-carnitine-treated patients tended to have a shorter time to cessation of vasopressor therapy and a lower vasopressor index. These data are hypothesis generating, but raise the possibility that the drug action of l-carnitine extends beyond LCFA transport. Should its efficacy be confirmed in an ongoing phase 2 clinical trial (https://clinicaltrials.gov/ct2/show/NCT01665092), this may be an area that warrants additional investigation.
We acknowledge that there are limitations to our study, most notably its preliminary and exploratory nature. We assayed existing samples from a relatively small number of patients that were collected as part of a clinical trial and employed a statistical and pathway data analysis strategy (Figure E1) to demonstrate the feasibility of pharmacometabolomics in a complex critical illness. This is an example of one type of strategy, and although there may be others, we acknowledge that it is not all encompassing as there may be additional metabolites that could further differentiate l-carnitine treatment response phenotypes. These might include those involved in glycine, serine, and threonine metabolism, and also arginine and proline metabolism as found by pathway analysis (Table E2 and Figure E3). We recognize that additional testing and validation using a larger cohort as well as a prospective, multicenter study are required to verify our findings particularly because, given the small sample size in this study, we were not able to test 3-OHB as a continuous variable. Nevertheless, to the best of our knowledge, this is the first pharmacometabolomics study in sepsis and it raises the prospect of the use of this discovery, systems biology science, to drive precision medicine in complex, critical illnesses for which there is a paucity of effective pharmacotherapies. Our findings also illustrate the informativeness of the often dismissed aqueous metabolites of sepsis (49) and how metabolomics-based drug response phenotyping could significantly impact the design of future clinical drug trials in sepsis. Ultimately, the integration of pharmacometabolomics and pharmacogenomics may be particularly informative in lending insight into the mechanistic underpinnings of drug mechanism and response (50, 51).
In conclusion, this pilot study used quantitative untargeted 1-D 1H-NMR pharmacometabolomics and demonstrated good feasibility in monitoring changes in the metabolome of patients with septic shock treated with a novel metabolic therapy, l-carnitine. A pathway analysis strategy allowed for the identification of a subgroup of patients with a unique metabolic signature that had a more favorably long-term response to therapy that was not readily apparent from the parent clinical trial. Overall, these data suggest that the pretreatment metabolome might hold much-needed knowledge to direct precision medicine strategies in sepsis.
Acknowledgments
Acknowledgment
Portions of this work were presented at the American Thoracic Society Meeting, San Diego, May 16–21, 2014. The authors thank Larisa Yeomans, Ph.D., for help with the construction of Figure E4.
Footnotes
Supported by the University of Michigan’s College of Pharmacy and its Biochemical Nuclear Magnetic Resonance Core and in part by the Michigan Regional Comprehensive Metabolomics Research Core (AK and Chenomx software), which is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; DK097153). The clinical trial was supported by a grant from the American Heart Association (10POST3560001) and the Cannon Foundation (SRG10-004). Dr. Jones’s effort was supported by a grant from the National Institute of General Medical Sciences (NIGMS; R01GM103799). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK, NIGMS, or the National Institutes of Health.
Author Contributions: M.A.P. conducted the clinical trial, analyzed data, and wrote the manuscript; M.A.F. conducted the NMR assays and performed NMR spectral analysis, including the identification and quantification of serum metabolites, using Chenomx software; and assisted in writing the manuscript; A.K. analyzed data and assisted in the writing of the manuscript; A.E.J. conducted the clinical trial, assisted in writing the manuscript; and reviewed the manuscript; J.T. assisted in sample processing and the acquisition of NMR metabolomics data, and contributed to the writing of the manuscript; B.N.H. assisted in writing the manuscript; K.A.S. conceived and directed the pharmacometabolomics component of the study, analyzed data, and wrote the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, et al. ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wunderink RG, Walley KR. Update in sepsis and pulmonary infections 2013. Am J Respir Crit Care Med. 2014;190:25–31. doi: 10.1164/rccm.201403-0577UP. [DOI] [PubMed] [Google Scholar]
- 4.Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, Iwashyna TJ. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Vincent JL, Marshall J, Reinhart K. Important issues in the design and reporting of clinical trials in severe sepsis and acute lung injury. J Crit Care. 2008;23:493–499. doi: 10.1016/j.jcrc.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 6.National Research Council (US) Washington, DC: National Academies Press; 2011. Committee on a Framework for Developing a New Taxonomy of Disease. Toward precision medicine: building a knowledge network for biomedical research and a new taxonomy of disease. [PubMed] [Google Scholar]
- 7.Katsnelson A. Momentum grows to make ‘personalized’ medicine more ‘precise’. Nat Med. 2013;19:249. doi: 10.1038/nm0313-249. [DOI] [PubMed] [Google Scholar]
- 8.Christaki E, Giamarellos-Bourboulis EJ. The beginning of personalized medicine in sepsis: small steps to a bright future. Clin Genet. 2014;86:56–61. doi: 10.1111/cge.12368. [DOI] [PubMed] [Google Scholar]
- 9.Man M, Close SL, Shaw AD, Bernard GR, Douglas IS, Kaner RJ, Payen D, Vincent JL, Fossceco S, Janes JM, et al. Beyond single-marker analyses: mining whole genome scans for insights into treatment responses in severe sepsis. Pharmacogenomics J. 2013;13:218–226. doi: 10.1038/tpj.2012.1. [DOI] [PubMed] [Google Scholar]
- 10.Clayton TA, Lindon JC, Cloarec O, Antti H, Charuel C, Hanton G, Provost JP, Le Net JL, Baker D, Walley RJ, et al. Pharmaco-metabonomic phenotyping and personalized drug treatment. Nature. 2006;440:1073–1077. doi: 10.1038/nature04648. [DOI] [PubMed] [Google Scholar]
- 11.Clayton TA, Baker D, Lindon JC, Everett JR, Nicholson JK. Pharmacometabonomic identification of a significant host–microbiome metabolic interaction affecting human drug metabolism. Proc Natl Acad Sci USA. 2009;106:14728–14733. doi: 10.1073/pnas.0904489106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everett JR, Loo RL, Pullen FS. Pharmacometabonomics and personalized medicine. Ann Clin Biochem. 2013;50:523–545. doi: 10.1177/0004563213497929. [DOI] [PubMed] [Google Scholar]
- 13.Serkova NJ, Standiford TJ, Stringer KA. The emerging field of quantitative blood metabolomics for biomarker discovery in critical illnesses. Am J Respir Crit Care Med. 2011;184:647–655. doi: 10.1164/rccm.201103-0474CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, et al. The human serum metabolome. PLoS One. 2011;6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia J, Broadhurst DI, Wilson M, Wishart DS. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics. 2013;9:280–299. doi: 10.1007/s11306-012-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaddurah-Daouk R, Weinshilboum RM Pharmacometabolomics Research Network. Pharmacometabolomics: implications for clinical pharmacology and systems pharmacology. Clin Pharmacol Ther. 2014;95:154–167. doi: 10.1038/clpt.2013.217. [DOI] [PubMed] [Google Scholar]
- 17.Collino S, Martin FP, Rezzi S. Clinical metabolomics paves the way towards future healthcare strategies. Br J Clin Pharmacol. 2013;75:619–629. doi: 10.1111/j.1365-2125.2012.04216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puskarich MA, Kline JA, Krabill V, Claremont H, Jones AE. Preliminary safety and efficacy of L-carnitine infusion for the treatment of vasopressor-dependent septic shock: a randomized control trial. JPEN J Parenter Enteral Nutr. 2013;38:736–743. doi: 10.1177/0148607113495414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langley RJ, Tsalik EL, van Velkinburgh JC, Glickman SW, Rice BJ, Wang C, Chen B, Carin L, Suarez A, Mohney RP, et al. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci Transl Med. 2013;5:195ra195. doi: 10.1126/scitranslmed.3005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seymour CW, Yende S, Scott MJ, Pribis J, Mohney RP, Bell LN, Chen YF, Zuckerbraun BS, Bigbee WL, Yealy DM, et al. Metabolomics in pneumonia and sepsis: an analysis of the GenIMS cohort study. Intensive Care Med. 2013;39:1423–1434. doi: 10.1007/s00134-013-2935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mickiewicz B, Duggan GE, Winston BW, Doig C, Kubes P, Vogel HJ Alberta Sepsis Network. Metabolic profiling of serum samples by 1H nuclear magnetic resonance spectroscopy as a potential diagnostic approach for septic shock. Crit Care Med. 2014;42:1140–1149. doi: 10.1097/CCM.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 22.Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA Emergency Medicine Shock Research Network (EMShockNet) Investigators. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones AE. Lactate clearance for assessing response to resuscitation in severe sepsis. Acad Emerg Med. 2013;20:844–847. doi: 10.1111/acem.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Víctor VM, Espulgues JV, Hernández-Mijares A, Rocha M. Oxidative stress and mitochondrial dysfunction in sepsis: a potential therapy with mitochondria-targeted antioxidants. Infect Disord Drug Targets. 2009;9:376–389. doi: 10.2174/187152609788922519. [DOI] [PubMed] [Google Scholar]
- 25.Corbucci GG, Gasparetto A, Antonelli M, Bufi M, Crimi G, Conti G, De Blasi RA, Candiani A, Cooper MB, Jones DA, et al. Effects of L-carnitine administration on mitochondrial electron transport activity present in human muscle during circulatory shock. Int J Clin Pharmacol Res. 1985;5:237–241. [PubMed] [Google Scholar]
- 26.Crouser ED. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion. 2004;4:729–741. doi: 10.1016/j.mito.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Harrois A, Huet O, Duranteau J. Alterations of mitochondrial function in sepsis and critical illness. Curr Opin Anaesthesiol. 2009;22:143–149. doi: 10.1097/ACO.0b013e328328d1cc. [DOI] [PubMed] [Google Scholar]
- 28.Levy B, Sadoune LO, Gelot AM, Bollaert PE, Nabet P, Larcan A. Evolution of lactate/pyruvate and arterial ketone body ratios in the early course of catecholamine-treated septic shock. Crit Care Med. 2000;28:114–119. doi: 10.1097/00003246-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Stringer KA, Puskarich MA, Finkel MA, Karnovsky A, Jones AE. L-Carnitine treatment impacts amino acid and energy metabolism in sepsis as detected by untargeted 1H-nuclear magnetic resonance (NMR) pharmacometabolomics [abstract] Am J Respir Crit Care Med. 2014;189:A3932. [Google Scholar]
- 30.Trzeciak S, McCoy JV, Phillip Dellinger R, Arnold RC, Rizzuto M, Abate NL, Shapiro NI, Parrillo JE, Hollenberg SM Microcirculatory Alterations in Resuscitation and Shock (MARS) Investigators. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med. 2008;34:2210–2217. doi: 10.1007/s00134-008-1193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. The SOFA (Sepsis-Related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 32.Stringer KA, Serkova NJ, Karnovsky A, Guire K, Paine R, III, Standiford TJ. Metabolic consequences of sepsis-induced acute lung injury revealed by plasma ¹H-nuclear magnetic resonance quantitative metabolomics and computational analysis. Am J Physiol Lung Cell Mol Physiol. 2011;300:L4–L11. doi: 10.1152/ajplung.00231.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serkova NJ, Brown MS. Quantitative analysis in magnetic resonance spectroscopy: from metabolic profiling to in vivo biomarkers. Bioanalysis. 2012;4:321–341. doi: 10.4155/bio.11.320. [DOI] [PubMed] [Google Scholar]
- 34.Tiziani S, Emwas AH, Lodi A, Ludwig C, Bunce CM, Viant MR, Günther UL. Optimized metabolite extraction from blood serum for 1H nuclear magnetic resonance spectroscopy. Anal Biochem. 2008;377:16–23. doi: 10.1016/j.ab.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 35.Lacy P, McKay RT, Finkel M, Karnovsky A, Woehler S, Lewis MJ, Chang D, Stringer KA. Signal intensities derived from different NMR probes and parameters contribute to variations in quantification of metabolites. PLoS One. 2014;9:e85732. doi: 10.1371/journal.pone.0085732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mercier P, Lewis MJ, Chang D, Baker D, Wishart DS. Towards automatic metabolomic profiling of high-resolution one-dimensional proton NMR spectra. J Biomol NMR. 2011;49:307–323. doi: 10.1007/s10858-011-9480-x. [DOI] [PubMed] [Google Scholar]
- 37.Šidák Z. Rectangular confidence regions for the means of multivariate normal distributions. J Am Stat Assoc. 1967;62:626–633. [Google Scholar]
- 38.Miyata Y, Shimomura I. Metabolic flexibility and carnitine flux: the role of carnitine acyltransferase in glucose homeostasis. J Diabetes Investig. 2013;4:247–249. doi: 10.1111/jdi.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura K, Inokuchi R, Doi K, Fukuda T, Tokunaga K, Nakajima S, Noiri E, Yahagi N. Septic ketoacidosis. Intern Med. 2014;53:1071–1073. doi: 10.2169/internalmedicine.53.1791. [DOI] [PubMed] [Google Scholar]
- 40.Famularo G, De Simone C, Trinchieri V, Mosca L. Carnitines and its congeners: a metabolic pathway to the regulation of immune response and inflammation. Ann N Y Acad Sci. 2004;1033:132–138. doi: 10.1196/annals.1320.012. [DOI] [PubMed] [Google Scholar]
- 41.Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab (Lond) 2010;7:30. doi: 10.1186/1743-7075-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belikova I, Lukaszewicz AC, Faivre V, Damoisel C, Singer M, Payen D. Oxygen consumption of human peripheral blood mononuclear cells in severe human sepsis. Crit Care Med. 2007;35:2702–2708. doi: 10.1097/01.ccm.0000295593.25106.c4. [DOI] [PubMed] [Google Scholar]
- 43.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 44.Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:92–103. doi: 10.1038/nrendo.2011.138. [DOI] [PubMed] [Google Scholar]
- 45.Seiler SE, Martin OJ, Noland RC, Slentz DH, DeBalsi KL, Ilkayeva OR, An J, Newgard CB, Koves TR, Muoio DM. Obesity and lipid stress inhibit carnitine acetyltransferase activity. J Lipid Res. 2014;55:635–644. doi: 10.1194/jlr.M043448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noland RC, Koves TR, Seiler SE, Lum H, Lust RM, Ilkayeva O, Stevens RD, Hegardt FG, Muoio DM. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem. 2009;284:22840–22852. doi: 10.1074/jbc.M109.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stephens FB, Constantin-Teodosiu D, Greenhaff PL. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol. 2007;581:431–444. doi: 10.1113/jphysiol.2006.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gasparetto A, Corbucci GG, De Blasi RA, Antonelli M, Bagiella E, D’Iddio S, Trevisani C. Influence of acetyl-L-carnitine infusion on haemodynamic parameters and survival of circulatory-shock patients. Int J Clin Pharmacol Res. 1991;11:83–92. [PubMed] [Google Scholar]
- 49.Patti GJ, Tautenhahn R, Rinehart D, Cho K, Shriver LP, Manchester M, Nikolskiy I, Johnson CH, Mahieu NG, Siuzdak G. A view from above: cloud plots to visualize global metabolomic data. Anal Chem. 2013;85:798–804. doi: 10.1021/ac3029745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis JP, Yerges-Armstrong LM, Ellero-Simatos S, Georgiades A, Kaddurah-Daouk R, Hankemeier T. Integration of pharmacometabolomic and pharmacogenomic approaches reveals novel insights into antiplatelet therapy. Clin Pharmacol Ther. 2013;94:570–573. doi: 10.1038/clpt.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yerges-Armstrong LM, Ellero-Simatos S, Georgiades A, Zhu H, Lewis JP, Horenstein RB, Beitelshees AL, Dane A, Reijmers T, Hankemeier T, et al. Purine pathway implicated in mechanism of resistance to aspirin therapy: pharmacometabolomics-informed pharmacogenomics. Clin Pharmacol Ther. 2013;94:525–532. doi: 10.1038/clpt.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]