Abstract
Rationale: The effect of weight loss on asthma in obese adults warrants rigorous investigation.
Objectives: To examine an evidence-based, practical, and comprehensive lifestyle intervention targeting modest weight loss and increased physical activity for asthma control.
Methods: The trial randomized 330 obese adults with uncontrolled asthma to receive usual care enhanced with a pedometer, a weight scale, information about existing weight management services at the participating clinics, and an asthma education DVD, or with these tools plus the 12-month intervention.
Measurements and Main Results: The primary outcome was change in Asthma Control Questionnaire (ACQ) scores from baseline to 12 months. Participants (mean [SD] age, 47.6 [12.4] yr) were 70.6% women, 20.0% non-Hispanic black, 20.3% Hispanic/Latino, and 8.2% Asian/Pacific Islander. At baseline, they were obese (mean [SD] body mass index, 37.5 [5.9] kg/m2) and had uncontrolled asthma (Asthma Control Test score, 15.1 [3.8]). Compared with control subjects, intervention participants achieved significantly greater mean weight loss (±SE) (intervention, −4.0 ± 0.8 kg vs. control, −2.1 ± 0.8 kg; P = 0.01) and increased leisure-time activity (intervention, 418.2 ± 110.6 metabolic equivalent task–min/wk vs. control, 178.8 ± 109.1 metabolic equivalent task–min/wk; P = 0.05) at 12 months. But between-treatment mean (±SE) differences were not significant for ACQ changes (intervention, –0.3 ± 0.1 vs. control, –0.2 ± 0.1; P = 0.92) from baseline (mean [SD], 1.4 [0.8]), nor for any other clinical asthma outcomes (e.g., spirometric results and asthma exacerbations). Among all participants regardless of treatment assignment, weight loss of 10% or greater was associated with a Cohen d effect of 0.76 and with 3.78 (95% confidence interval, 1.72–8.31) times the odds of achieving clinically significant reductions (i.e., ≥0.5) on ACQ as stable weight (<3% loss or gain from baseline). The effects of other weight change categories were small.
Conclusions: Moderately and severely obese adults with uncontrolled asthma can safely participate in evidence-based lifestyle intervention for weight loss and active living. The modest average weight and activity improvements are comparable to those shown to reduce cardiometabolic risk factors in studies of similar interventions in other populations but are not associated with significant net benefits for asthma control or other clinical asthma outcomes in the current population. Instead, weight loss of 10% or greater may be required to produce clinically meaningful improvement in asthma.
Clinical trial registered with www.clinicaltrials.gov (NCT00901095).
Keywords: weight loss, exercise, adults, asthma, clinical trial
Obesity and asthma affect diverse populations of all age groups—78 million U.S. adults are currently obese (1), and 19 million have asthma (2). Since the first prospective study in 1999 implicating increasing body mass index (BMI) in adult-onset asthma (3), a body of evidence has emerged linking obesity to incident asthma, with risk ratios of 1.1 to 3.5 (4, 5). The preponderance of the evidence suggests obese asthma may be a distinct phenotype in some cases where obesity contributes to asthma development but a disease modifier in others where obesity complicates preexisting asthma, possibly through multifactorial mechanisms (e.g., mechanical, inflammatory, and immunologic factors) (4, 6). Furthermore, asthma in obesity is more difficult to control with standard controller therapy (7). In addition to confirming obesity as a risk factor for incident asthma and worsened control of prevalent asthma, evidence of causality must establish whether weight loss per se, or what magnitude of weight loss, improves asthma outcomes. Inquiries into the possible benefits of weight loss on asthma have been limited to nonrandomized studies of bariatric surgery (8, 9) and randomized pilot studies of nonsurgical interventions (10, 11).
The Breathe Easier through Weight Loss Lifestyle (BE WELL) Intervention study is a rigorous randomized controlled trial (RCT) of an evidence-based, practical, and comprehensive lifestyle intervention targeting modest weight loss and increased physical activity among obese adults with uncontrolled asthma. We hypothesized that, compared with the control group, the 12-month BE WELL intervention would significantly improve asthma control, as measured by the Asthma Control Questionnaire (ACQ) (12). We also hypothesized that intervention participants would achieve greater improvements in other asthma clinical outcomes (e.g., spirometric results, asthma exacerbations, and healthcare use) and in body weight and physical activity than control subjects.
Methods
An Institutional Review Board of the Kaiser Foundation Research Institute in Northern California and the BE WELL Data and Safety Monitoring Board approved the study. All participants provided written informed consent. The trial protocol was published previously (13).
Study Participants
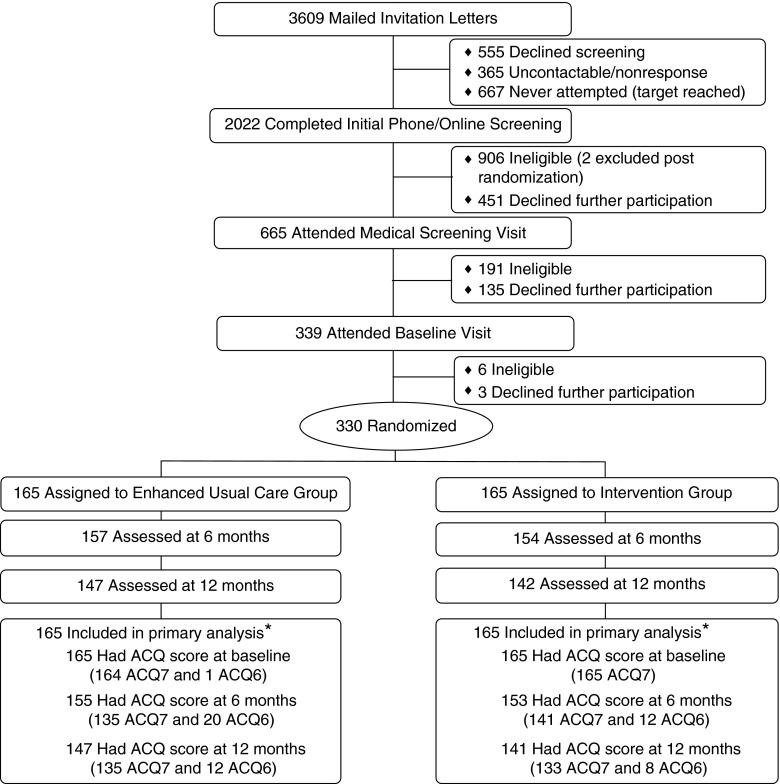
Participants were recruited (April 16, 2010 through August 13, 2012) from eight medical centers of Kaiser Permanente in Northern California (KPNC). Inclusion criteria included age 18 to 70 years, BMI greater than or equal to 30 kg/m2, and confirmation of uncontrolled persistent asthma through a multistage screening process (i.e., electronic asthma registry queries; completion of the Asthma Control Test (ACT); pre- and post-bronchodilator spirometry; and, if necessary, medical chart reviews by an asthma specialist) (see Table E1 in the online supplement). Exclusion criteria included serious medical or psychiatric conditions (e.g., chronic obstructive pulmonary disease, stroke, and psychosis) or special life circumstances (e.g., pregnancy and planned relocation). Of the 3,609 patients mailed recruitment letters after their primary care provider approval for study contact, 1,144 explicitly refused participation or missed visit appointments despite multiple staff attempts to reschedule, 1,103 were ineligible, 365 were unreachable, 667 were not screened because of recruitment success, and 330 (vs. 324 projected) were fully eligible and randomized (Figure 1).
Figure 1.
Participant flow. *The Asthma Control Questionnaire (ACQ) score was computed as the mean of the complete seven-item scores (ACQ7) or the six-item scores (ACQ6) if FEV1 was missing because spirometry was not performed or not usable per American Thoracic Society standards.
Randomization
We applied our published dynamic block randomization method (14) to assure better than chance between-treatment balance across six prognostic factors (study site, age, sex, race/ethnicity, BMI, and ACQ score). The method automatically ensures allocation concealment. Participants were randomly assigned to one of two treatment conditions: usual care enhanced with a pedometer, a weight scale, and general information about weight management and asthma; or with these tools plus the BE WELL intervention (n = 165 per treatment). The trial design precluded blinding participants or interventionists to treatment assignment; however, the investigators, Data and Safety Monitoring Board members, outcome assessors, and data analyst were masked throughout the trial.
Treatment Arms
Control
All participants continued to receive standard medical care from their providers, who were not informed by the study of participants’ treatment assignment. Each participant received a pedometer, a body weight scale, a list of routinely offered KPNC weight management services, and a KPNC standard asthma self-management educational DVD. The research team made no other attempts to intervene with control participants.
Intervention
Intervention participants received a 12-month lifestyle intervention adapted from the landmark Diabetes Prevention Program (DPP) trial (15). The intervention dually targeted modest weight loss and increased physical activity and had three successive stages: Intensive (13 weekly in-person group sessions over 4 months using a published curriculum [16, 17]), Transitional (two monthly in-person individual sessions), and Extended (three bimonthly or more frequent phone consultations depending on participant needs, preferences, and availability). The intervention was theory-based and goal-oriented. It was grounded in Social Cognitive Theory (18) and used proven behavior change strategies (e.g., self-monitoring, action planning, and problem solving) to help participants achieve and maintain realistic, clinically meaningful weight loss (7–10% of baseline) and physical activity (at least 150 min/wk of moderate-intensity physical activity) goals. Following a structured, comprehensive protocol similar to that recommended in the latest obesity treatment guideline (19), the BE WELL intervention staff monitored and responded to participants’ individual weight-loss needs, preferences, and personal circumstances by counseling them on healthy eating with moderate calorie reductions (by 500–1,000 kcal/d, but daily total calories no less than 1,200 kcal), moderate-intensity physical activity (e.g., brisk walking), and behavioral self-management skills suited to what each participant was eating and doing, and the changes (s)he was willing and able to make. We ensured intervention fidelity through standard training, systematic interventionist record keeping, audit and feedback based on audio recordings of sessions, and ongoing supervision. (For more detail on the intervention, see the protocol [13].)
Outcome Measures
Assessments occurred at baseline and at 6 and 12 months post randomization. Of the 330 randomized participants, 311 (94.2%) were assessed at 6 months and 289 (87.6%) at 12 months (Figure 1). All outcome assessors were trained and continuously monitored.
The ACQ (12, 20) integrates seven components of asthma control, including five patient-reported asthma symptoms, reported need for bronchodilators, and measured pulmonary function; all are scored on a 7-point scale (0–6), with a higher score indicating worse asthma. A decrease of at least 0.5 points on ACQ is regarded as clinically significant (21). Participants also completed the Asthma Control Test (22) and Mini Asthma-specific Quality of Life Questionnaire (23). Trained research staff performed spirometry according to the American Thoracic Society standards (24), with ongoing quality monitoring. Two independent respiratory therapists rated all spirograms. A valid test required a minimum of two maneuvers meeting the American Thoracic Society acceptability and reproducibility criteria, from among which the best parameter values were chosen and used in data analyses. Published protocols were used to obtain height (baseline only), weight, waist circumference, and blood pressure measurements (25, 26), and for the Stanford 7-Day Physical Activity Recall interview (27). The latter provided estimates of weekly leisure-time physical activity (metabolic equivalent task [MET] minutes) and total daily energy expenditure (kcal/kg). Data abstracted from KPNC electronic health records included encounters, diagnoses, and pharmacy dispensing events during the 12 months before and after randomization. Participants were interviewed at each follow-up visit about possible adverse events during the past 6 months, and the study physician adjudicated the events per study safety protocol.
The primary outcome was the ACQ score, computed as the mean of the complete seven-item scores (ACQ7) or the six-item scores (ACQ6) if FEV1 was missing because spirometry was not performed or the test was invalid. Both measures were previously validated (12, 21). There were 330 participants with ACQ scores (329 ACQ7 and 1 ACQ6) at baseline, 308 (276 and 32) at 6 months, and 288 (268 and 20) at 12 months.
Statistical Analyses
Intention-to-treat analyses of between-treatment differences in primary and secondary outcomes tested for treatment-by-time interactions in repeated-measures mixed-effects linear (for continuous outcomes) or logistic models (for categorical outcomes). The fixed effects of each model consisted of the baseline value of the outcome of interest, randomization balancing factors, treatment, time point (6 or 12 mo), and treatment-by-time interaction. The random effects accounted for repeated measures with a first-order autoregressive covariance matrix and for clustering of patients within randomization block and recruitment cohort. Missing data were handled directly through maximum-likelihood estimation via mixed modeling. Least-square means (±SE) were obtained from the models.
All analyses were conducted using SAS, version 9.2 (SAS Institute Inc., Cary, NC). The targeted sample size of 162 participants per arm provided 90% power to detect a 0.4-SD between-treatment mean difference (medium effect by Cohen standards [28]) in the primary outcome, using t tests at 5% α (two-sided) and assuming up to a 20% loss to follow-up through 12 months. We based our estimates on the lone RCT of weight loss (through a 14-week program involving very-low-calorie diet and behavior change) in asthma published at the time of our study design, which reported improvements in various asthma outcomes, corresponding to Cohen ds of 0.4 to 0.6, at 12 months of follow-up (29).
Results
Baseline Characteristics
Participants were middle-aged, mostly women, never smokers, and racially and ethnically diverse (Table 1). At baseline, participants were moderately or severely obese (mean [SD] BMI, 37.5 [5.9] kg/m2), had highly variable leisure-time physical activity levels (874.2 [1,369.5] MET-min/wk), had poor asthma control (ACT, 15.1 [3.8]; prebronchodilator FEV1 to FVC ratio, 68.1% [11.6%]), and ACQ averaged 1.4 (SD, 0.8). Of them, 27.9% had Class III obesity (BMI ≥ 40), 31.5% Class II (BMI 35 to <40), and 38.5% Class I (BMI 30 to <35).
Table 1.
Baseline characteristics of the study participants
| Characteristic |
All Participants |
Enhanced Usual
Care |
Intervention |
|---|---|---|---|
| (n = 330) | (n = 165) | (n = 165) | |
| Age, mean (SD), yr | 47.6 (12.4) | 47.7 (12.1) | 47.5 (12.6) |
| Female | 70.6 | 70.9 | 70.3 |
| Race/ethnicity | |||
| Non-Hispanic white | 49.7 | 49.7 | 49.7 |
| Non-Hispanic black | 20.0 | 19.4 | 20.6 |
| Asian/Pacific Islander | 8.2 | 8.5 | 7.9 |
| Hispanic/Latino | 20.3 | 20.6 | 20.0 |
| Education,* n = 327 | |||
| High school graduate or less | 16.2 | 13.5 | 18.9 |
| Some college | 41.6 | 44.2 | 39.0 |
| College graduate or above | 42.2 | 42.3 | 42.1 |
| Employment status,* n = 327 | |||
| Full time | 64.2 | 69.3 | 59.2 |
| Part time | 11.6 | 9.8 | 13.4 |
| Unemployed/retired/disabled | 24.2 | 20.9 | 27.4 |
| Family annual income,* n = 316 | |||
| <$35,000 | 13.0 | 10.3 | 15.5 |
| $35,000 to <$55,000 | 22.5 | 20.7 | 24.2 |
| $55,000 to <$75,000 | 23.4 | 23.2 | 24.2 |
| $75,000 to <$100,000 | 12.3 | 12.9 | 11.8 |
| $100,000+ | 28.8 | 32.9 | 24.8 |
| Current smokers,* n = 329 | 5.8 | 4.3 | 7.3 |
| Former smokers,* n = 329 | 28.9 | 28.1 | 29.7 |
| Pack-years of current or former smokers, mean (SD) | 12.1 (13.4) | 13.9 (15.5) | 10.5 (10.9) |
| Asthma onset age < 12 yr by self-report,* n = 317 | 43.2 | 45.3 | 41.1 |
| Self-reported positive response to aeroallergens as asthma triggers,*,† n = 327 | 90.5 | 91.4 | 89.6 |
| ACQ, mean (SD) | 1.4 (0.8) | 1.4 (0.7) | 1.4 (0.8) |
| FEV1, mean (SD), L | 2.6 (0.8) | 2.6 (0.8) | 2.6 (0.8) |
| FVC, mean (SD), L | 3.8 (1.0) | 3.8 (1.0) | 3.8 (1.1) |
| FEV1/FVC, mean (SD), % | 68.1 (11.6) | 67.8 (11.7) | 68.6 (11.5) |
| ACT, mean (SD) | 15.1 (3.8) | 15.3 (3.7) | 14.9 (3.8) |
| MiniAQLQ, overall, mean (SD) | 5.3 (1.0) | 5.2 (1.0) | 5.3 (1.0) |
| MiniAQLQ, symptoms, mean (SD) | 5.2 (1.1) | 5.1 (1.1) | 5.2 (1.1) |
| MiniAQLQ, environment, mean (SD) | 4.8 (1.5) | 4.7 (1.5) | 4.8 (1.5) |
| MiniAQLQ, emotions, mean (SD) | 5.0 (1.5) | 4.9 (1.5) | 5.1 (1.4) |
| MiniAQLQ, activities, mean (SD) | 5.9 (1.0) | 5.9 (1.0) | 5.8 (1.1) |
| Weight, mean (SD), kg | 104.2 (19.6) | 104.2 (20.1) | 104.2 (19.1) |
| BMI, mean (SD), kg/m2 | 37.5 (5.9) | 37.6 (5.7) | 37.4 (6.0) |
| BMI, obesity category | |||
| Class I obesity (BMI 30 to <35) | 38.5 | 37.6 | 39.4 |
| Class II obesity (BMI 35 to <40) | 31.5 | 31.5 | 31.5 |
| Class III obesity (BMI ≥40) | 27.9 | 29.1 | 26.7 |
| Waist circumference, mean (SD), cm | 117.8 (14.2) | 117.7 (14.5) | 118.0 (13.9) |
| Leisure-time physical activity, mean (SD), MET-min/wk‡ | 874.2 (1,369.5) | 907.1 (1,650.9) | 841.0 (1,012.2) |
| Total energy expenditure, mean (SD), kcal/kg/d§ | 33.7 (2.9) | 34.0 (5.3) | 33.5 (2.1) |
| Systolic blood pressure, mean (SD), mm Hg | 118.7 (11.8) | 118.9 (11.4) | 118.5 (12.2) |
| Diastolic blood pressure, mean (SD), mm Hg | 73.7 (9.3) | 73.9 (8.9) | 73.4 (9.7) |
Definition of abbreviations: ACQ = Asthma Control Questionnaire; ACT = Asthma Control Test; BMI = body mass index; MET = metabolic equivalent task; MiniAQLQ = Mini Asthma Quality of Life Questionnaire.
Data are expressed % unless otherwise indicated. ACQ, range 0–6 with higher scores indicating worse asthma control; ACT, range 5–25 with higher scores indicating better asthma control; mAQLQ, range 1–7 with higher scores indicating less impairment. Differences in baseline characteristics between treatment arms were not significant (P > 0.05).
The denominator is less than the total number (330) of the randomized participants due to missing data.
Aeroallergens included pollen, house dust mites, cats, dogs, cockroaches, and molds.
Physical activity levels were measured by the interview-administered Stanford 7-day Physical Activity Recall (27). Leisure-time physical activity = non–work-related moderate activity min/wk × 4 METs + hard activity min/wk × 6 METs + very hard activity min/wk × 10 METs. One MET is defined as the energy expenditure for sitting quietly.
The Stanford 7-day Physical Activity Recall data also provided estimates of total daily energy expenditures. Total energy expenditure = sleep hours × 1 MET + light activity hours × 1.5 METs + moderate activity hours × 4 METs + hard activity hours × 6 METs + very hard activity hours × 10 METs.
Weight Loss and Physical Activity
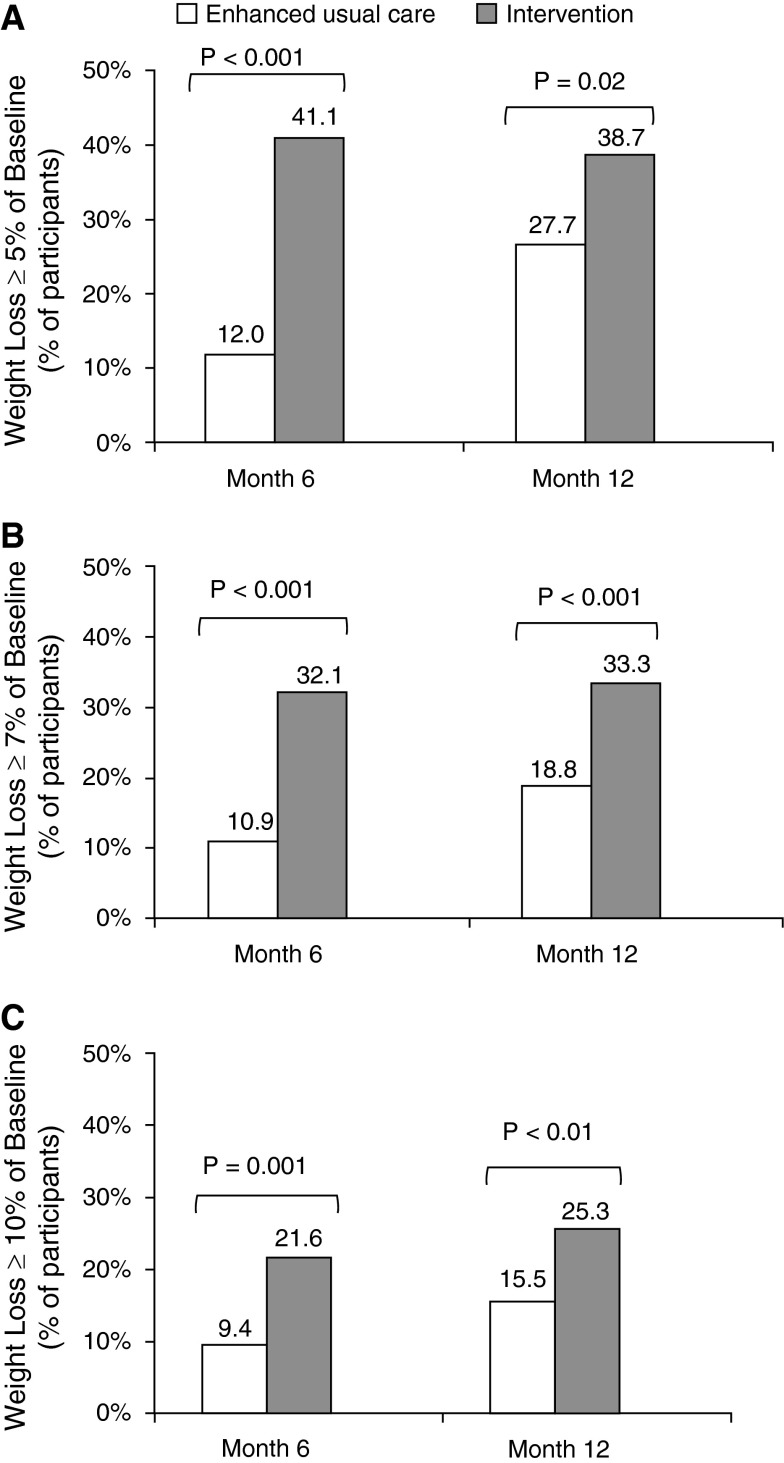
The mean weight change was –5.0 kg (5.0% of baseline) in intervention participants versus –1.1 kg (1.3%) in control subjects at 6 months (P < 0.001) and –4.0 kg (4.1%) versus –2.1 kg (2.1%) at 12 months (P < 0.01). The between-treatment mean differences were –3.8 kg (95% confidence interval [CI], −5.3 to –2.4) at 6 months and –1.9 kg (95% CI, −3.3 to –0.5) at 12 months. Intervention participants also had significantly greater reductions in BMI and waist circumference than control subjects at 6 and 12 months (Table 2). At 12 months, the percentage of intervention participants meeting clinically meaningful weight-loss thresholds was 38.7% (vs. 27.7% of control subjects) for greater than or equal to 5% loss of baseline weight, 33.3% (vs. 18.8%) for greater than or equal to 7% loss, and 25.3% (vs. 15.5%) for greater than or equal to 10% loss (P < 0.05 for all) (Figure 2). Complete fitted distributions of percent weight changes are shown in Figure E1. Mean BMI at 12 months was 37.0 (SD, 6.2) in control subjects and 36.1 (6.6) in intervention participants, who also had 1.6 (95% CI, 1.0 to 2.6) times the odds of moving to a lower BMI category from baseline (e.g., from Class III to Class II or I obesity) as control subjects.
Table 2.
Estimated mean (SE) changes in weight, physical activity, and asthma outcomes from baseline to 6 and 12 months in the intention-to-treat population
| |
Change from Baseline to 6 mo |
Change from Baseline to 12 mo |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline* | Enhanced Usual Care | Intervention | P Value | Enhanced Usual Care | Intervention | P Value | ||
| Intervention goal measures: body weight and physical
activity | ||||||||
| Weight, kg | 104.2 (19.6) | −1.1 (0.8) | −5.0 (0.8) | <0.001 | −2.1 (0.8) | −4.0 (0.8) | 0.01 | |
| Weight, % | 100 | −1.3 (0.7) | −5.0 (0.7) | <0.001 | −2.1 (0.7) | −4.1 (0.7) | 0.005 | |
| BMI, kg/m2 | 37.5 (5.9) | −0.4 (0.3) | −1.8 (0.3) | <0.001 | −0.7 (0.3) | −1.4 (0.3) | 0.008 | |
| Waist circumference, cm | 117.8 (14.2) | −1.6 (1.2) | −4.9 (1.2) | <0.001 | −2.7 (1.2) | −4.3 (1.2) | 0.04 | |
| Leisure-time physical activity, MET-min/wk | 874.2 (1,369.5) | 136.4 (107.5) | 257.5 (107.9) | 0.30 | 178.8 (109.1) | 418.2 (110.6) | 0.05 | |
| Total energy expenditure, kcal/kg/d | 33.7 (2.9) | 0.3 (0.2) | 0.5 (0.2) | 0.39 | 0.3 (0.2) | 0.7 (0.2) | 0.05 | |
| Asthma control, lung function, and functional
status | ||||||||
| ACQ | 1.4 (0.8) | −0.2 (0.1) | −0.3 (0.1) | 0.15 | −0.2 (0.1) | −0.3 (0.1) | 0.92 | |
| FEV1, L | 2.6 (0.8) | 0.1 (0.0) | 0.1 (0.0) | 0.12 | 0.1 (0.0) | 0.2 (0.0) | 0.34 | |
| FVC, L | 3.8 (1.0) | 0.1 (0.1) | 0.1 (0.1) | 0.31 | 0.2 (0.1) | 0.2 (0.1) | 0.21 | |
| FEV1/FVC | 68.1 (11.6) | 0.0 (0.8) | 0.9 (0.8) | 0.30 | 0.5 (0.8) | −0.0 (0.8) | 0.52 | |
| ACT | 15.1 (3.8) | 3.2 (0.5) | 3.9 (0.5) | 0.17 | 4.0 (0.5) | 4.5 (0.5) | 0.35 | |
| MiniAQLQ, overall | 5.3 (1.0) | 0.2 (0.1) | 0.3 (0.1) | 0.28 | 0.4 (0.1) | 0.4 (0.1) | 0.42 | |
| MiniAQLQ, symptoms | 5.2 (1.1) | 0.4 (0.1) | 0.4 (0.1) | 0.59 | 0.5 (0.1) | 0.6 (0.1) | 0.67 | |
| MiniAQLQ, environment | 4.8 (1.5) | 0.1 (0.1) | 0.1 (0.1) | 0.70 | 0.3 (0.1) | 0.2 (0.1) | 0.94 | |
| MiniAQLQ, emotions | 5.0 (1.5) | 0.2 (0.1) | 0.4 (0.1) | 0.22 | 0.4 (0.1) | 0.5 (0.1) | 0.25 | |
| MiniAQLQ, activities | 5.9 (1.0) | 0.1 (0.1) | 0.3 (0.1) | 0.15 | 0.2 (0.1) | 0.3 (0.1) | 0.24 | |
Values are covariate-adjusted, mixed-model–based mean (SE) estimates for the intention-to-treat population unless otherwise indicated.
For definition of abbreviations, see Table 1.
Values are mean (SD).
Figure 2.
Categorical weight loss at 6 and 12 months in the intention-to-treat population. (A) Percentage of participants with ≥5% loss of baseline weight. (B) Percentage of participants with ≥7% loss of baseline weight. (C) Percentage of participants with ≥10% loss of baseline weight.
The mean increases in leisure-time physical activity of at least moderate intensity were 257.5 and 418.2 MET-min/wk at 6 and 12 months, respectively, among intervention participants. The latter was significantly higher than a mean increase of 178.8 MET-min/wk among control subjects (net difference, 239.4; 95% CI, 2.3 to 476.5 MET-min/wk) at 12 months, corresponding to a significant between-treatment difference in change of total daily energy expenditure (P < 0.05) (Table 2).
Asthma Control, Symptoms, and Lung Function
The mean (±SE) adjusted change in ACQ score from baseline was –0.3 ± 0.1 in intervention participants versus –0.2 ± 0.1 in control subjects at 12 months (P = 0.92). Between-treatment differences in mean adjusted changes from baseline also did not reach statistical significance for ACQ at 6 months, nor for ACT, Mini Asthma-specific Quality of Life Questionnaire (overall and subscales), or spirometric parameters (FEV1, FVC, and FEV1/FVC) at 6 or 12 months (Table 2).
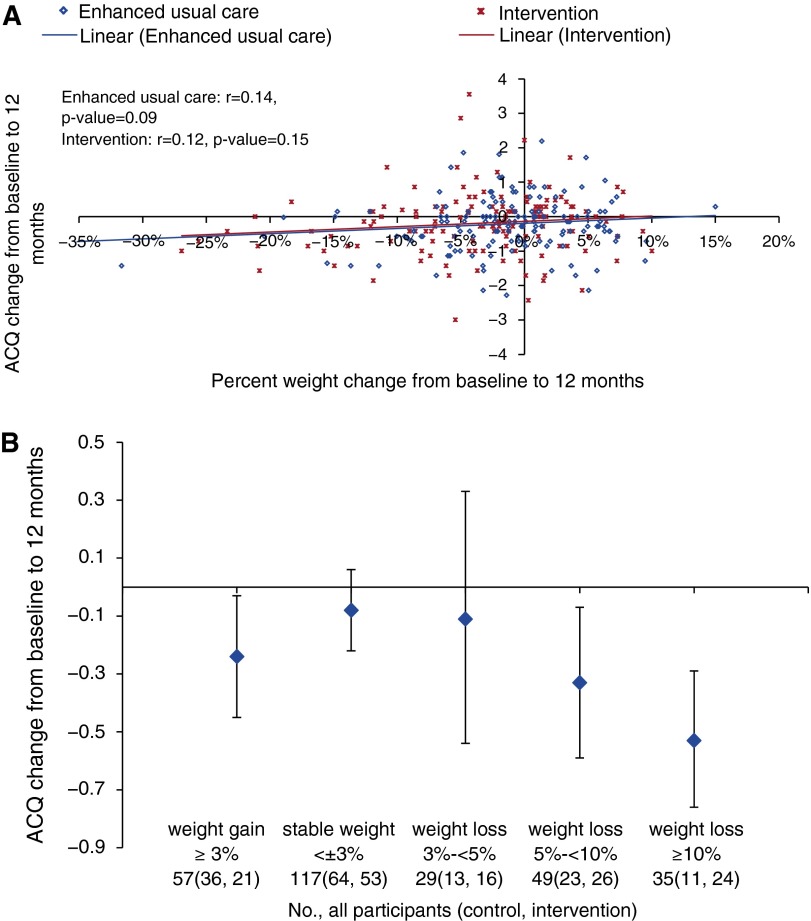
Among all participants regardless of treatment assignment, ACQ change from baseline to 12 months was not linearly correlated with percent weight change (Figure 3A) or change in weekly leisure-time MET-minutes (Figures E2 and E3). However, ACQ change showed a graded relationship with categories of percentage weight change where participants with 5% to less than 10% or greater than or equal to 10% weight loss achieved statistically significantly improved ACQ scores compared with those whose weight was stable defined by less than 3% loss or gain (Figure 3B and Table 3). Weight loss of greater than or equal to 10% was associated with a large one-sample effect size, Cohen d = 0.76, for ACQ change and with 3.78 (95% CI, 1.72–8.31) times the odds of achieving clinically significant (i.e., 0.5-point or greater) reductions on ACQ as stable weight. The effect sizes of other weight change categories were small (Table 3).
Figure 3.
Correlations between change in Asthma Control Questionnaire (ACQ) scores and percentage weight change from baseline to 12 months among all participants. (A) Correlation between change in ACQ scores and continuous percent weight change. (B) Correlation between change in ACQ scores and categorical percent weight change.
Table 3.
Effect estimates for Asthma Control Questionnaire change from baseline to 12 months by weight change category among all participants regardless of treatment assignment
| Weight Change, % | All Participants (Control, Intervention), n | ACQ Change, Mean (95% CI) | 1-Sample Cohen d | ACQ Decrease ≥ 0.5, OR (95% CI) |
|---|---|---|---|---|
| Weight gain ≥ 3% | 57 (36, 21) | −0.24 (−0.45, −0.03) | 0.30 | 1.24 (0.61, 2.54) |
| Stable weight < ±3% | 117 (64, 53) | −0.08 (−0.22, 0.06) | 0.10 | Reference |
| Weight loss 3 to <5% | 29 (13, 16) | −0.11 (−0.54, 0.33) | 0.10 | 1.67 (0.70, 4.02) |
| Weight loss 5 to <10% | 49 (23, 26) | −0.33 (−0.59, −0.07) | 0.36 | 2.19 (1.08, 4.46) |
| Weight loss ≥ 10% | 35 (11, 24) | −0.53 (−0.76, −0.29) | 0.76 | 3.78 (1.72, 8.31) |
Definition of abbreviations: ACQ = Asthma Control Questionnaire; CI = confidence interval; OR = odds ratio.
The intervention effects on ACQ change and weight loss at 12 months were comparable among subgroups defined by sex, race/ethnicity, age of asthma onset, or positive response to aeroallergens as asthma triggers (e.g., pollens, house dust mites, molds, or animal dander), all of which were measured by self-report (Table E2).
Medication Acquisition and Healthcare Encounters
Based on pharmacy dispensing records during the 12 months before randomization, 8.5% of the 330 participants did not acquire any asthma controller medications, 32.1% had asthma exacerbations requiring systemic corticosteroids, 3.0% had asthma-related hospitalizations, and 18.8% had asthma-related emergency department visits. The corresponding numbers were 17.9, 24.8, 4.2, and 18.5, respectively, during the 12 months after randomization. The mean beclomethasone canister equivalents (a measure of the antiinflammatory equipotency [30]) of the total acquired controllers (excluding theophylline and maintenance systemic corticosteroids used for >15 days) were 15.1 (SD, 13.0) in the prerandomization year and 13.6 (13.2) in the postrandomization year. The mean albuterol canister equivalents (a measure of the bronchodilator equipotency) of the total acquired short-acting β-agonists were 5.0 (SD, 4.4) and 4.6 (4.6) in the same 2 years. The intervention and control groups did not differ significantly in any of these measures in each yearly period or in the changes of these measures from the pre- to postrandomization year, nor did they differ in any other healthcare use measures (Table 4).
Table 4.
Medication acquisition and healthcare encounters among all participants
| 12 mo before Randomization, % |
12 mo after Randomization, % |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| All Participants (n = 330) | Enhanced Usual Care (n = 165) | Intervention (n = 165) | P Value | All Participants (n = 330) | Enhanced Usual Care (n = 165) | Intervention (n = 165) | P Value | P Value* | |
| Controller user | |||||||||
| Yes | 91.5 | 90.9 | 92.1 | 0.69 | 82.1 | 83.0 | 81.2 | 0.67 | 0.53 |
| No | 8.5 | 9.1 | 7.9 | 17.9 | 17.0 | 18.8 | |||
| Type of controllers† | |||||||||
| ICS alone | 40.3 | 41.8 | 38.8 | 30.0 | 29.7 | 30.3 | |||
| ICS + LABA only | 33.0 | 30.3 | 35.8 | 35.5 | 34.6 | 36.4 | |||
| LTRA alone | 2.1 | 2.4 | 1.8 | 3.0 | 4.2 | 1.8 | |||
| ICS + LTRA | 3.5 | 3.7 | 3.6 | 0.84 | 2.4 | 3.0 | 1.8 | 0.86 | 0.78 |
| ICS + LABA+LTRA | 11.2 | 12.1 | 10.3 | 10.0 | 10.3 | 9.7 | |||
| Methylxanthines/>15 d of systemic corticosteroids | 1.2 | 0.6 | 1.8 | 1.2 | 1.2 | 1.2 | |||
| None | 8.5 | 9.1 | 7.9 | 17.9 | 17.0 | 18.8 | |||
| Beclomethasone canister equivalents, mean (SD)‡ | 15.1 (13.0) | 15.7 (13.9) | 14.5 (12.2) | 0.42 | 13.6 (13.2) | 14.1 (14.0) | 13.1 (12.3) | 0.51 | 0.86 |
| SABA user | 93.3 | 92.1 | 94.6 | 0.38 | 84.6 | 81.8 | 87.3 | 0.17 | 0.92 |
| Albuterol canister equivalents, mean (SD)§ | 5.0 (4.4) | 5.2 (5.1) | 4.8 (3.6) | 0.39 | 4.6 (4.6) | 4.6 (5.2) | 4.5 (4.0) | 0.89 | 0.34 |
| No. of asthma exacerbations requiring systemic corticosteroids|| | |||||||||
| 0 | 67.9 | 66.1 | 69.7 | 75.2 | 77.6 | 72.7 | |||
| 1 | 18.2 | 17.6 | 18.8 | 0.59 | 15.5 | 15.2 | 15.8 | 0.59 | 0.16 |
| 2 | 7.3 | 9.1 | 5.5 | 6.1 | 4.9 | 7.3 | |||
| 3+ | 6.7 | 7.3 | 6.1 | 3.3 | 2.4 | 4.2 | |||
| Total d of systemic corticosteroid supply for asthma exacerbations, mean (SD)|| | 13.5 (9.7) | 13.6 (7.8) | 13.4 (11.6) | 0.60 | 13.1 (9.9) | 12.4 (9.7) | 13.6 (10.2) | 0.87 | 0.74 |
| Healthcare encounters | |||||||||
| Asthma-related¶ | |||||||||
| None | 19.1 | 18.8 | 19.4 | 30.6 | 34.6 | 26.7 | |||
| Hospitalization | 3.0 | 3.0 | 3.0 | 0.89 | 4.2 | 3.6 | 4.9 | 0.40 | 0.26 |
| ED visit | 18.8 | 17.6 | 20.0 | 18.5 | 15.8 | 21.2 | |||
| Outpatient visit | 78.2 | 80.0 | 76.4 | 67.6 | 64.2 | 70.9 | |||
| Respiratory-related** | |||||||||
| None | 16.1 | 15.8 | 16.4 | 25.2 | 27.9 | 22.4 | |||
| Hospitalization | 3.0 | 3.0 | 3.0 | 0.99 | 4.6 | 4.2 | 4.9 | 0.59 | 0.28 |
| ED visit | 20.9 | 20.6 | 21.2 | 18.8 | 16.4 | 21.2 | |||
| Outpatient visit | 81.5 | 83.0 | 80.0 | 73.0 | 70.9 | 75.2 | |||
| Total†† | |||||||||
| None | 2.4 | 1.2 | 3.6 | 3.9 | 4.9 | 3.0 | |||
| Hospitalization | 3.0 | 3.0 | 3.0 | 0.56 | 5.2 | 4.9 | 5.5 | 0.83 | 0.68 |
| ED visit | 29.1 | 29.7 | 28.5 | 25.5 | 24.9 | 26.1 | |||
| Outpatient visit | 97.3 | 98.2 | 96.4 | 96.1 | 95.2 | 97.0 | |||
Definition of abbreviations: ED = emergency department; ICD-9-CM = International Classification of Diseases, 9th revision, Clinical Modification; ICS = inhaled corticosteroid; LABA = long-acting, inhaled β2-agonists; LTRA = leukotriene modifiers; SABAs = short-acting β-agonists.
Extracted healthcare use data from electronic health records included all visits by service type (ED, hospital inpatient, or outpatient department and specialty) to Kaiser Permanente and its contracted facilities, and pharmacy dispensing records. Data are expressed as % unless otherwise indicated.
Mixed model-based P values for between-treatment differences in change from 12 months before to 12 months after randomization.
Percentages of participants with any or no asthma controller medications dispensed in each 12-month period.
The estimated total number of canisters of beclomethasone to which the asthma controller medications dispensed for a patient in each 12-month period was equivalent in terms of the antiinflammatory effectiveness. Each dispensing of a controller medication (excluding theophylline and systemic corticosteroids used for >15 d), in any form or quantity, was assigned a weight representing its equivalent in fractional or multiple canisters of beclomethasone 80 mg (30). The weighted values were summed for each patient to obtain a cumulative measure of controller medication dose dispensed during the 12 months before and after randomization separately.
The estimated total number of canisters of albuterol to which the amount of all SABAs dispensed in each 12-month period was equivalent in terms of the bronchodilator effectiveness. Each SABA medication, in any type or delivery mode, was assigned a weight representing its equivalent in canister of albuterol (30). The weighted values were summed for each patient to obtain a cumulative measure of dose dispensed during the 12 months before and after randomization separately.
Defined as systemic corticosteroids (by tablet, suspension, or injection) dispensed for 3 to 15 days of use.
Asthma-related healthcare encounters were visits with ICD-9-CM code 493 as a primary or secondary diagnosis.
Respiratory-related healthcare encounters were visits with any of the following ICD-9-CM codes as a primary or secondary diagnosis: 422, 427–428, 460–466, 470–474, 480–487, and 490–519.
Total healthcare encounters included all visits regardless of diagnosis.
Intervention Participation Rates
Intervention participants attended a mean 77.1% (SD, 26.7%) of the 13 weekly group sessions (median number of sessions attended, 11; interquartile range, 9–13) during the 4-month Intensive stage; 63.6% attended both monthly individual sessions, 11.5% one, and 24.9% no session during the 2-month Transitional stage; and 49.0% received at least three phone consultations (median, two calls; interquartile range, zero to six) during the 6-month Extended stage.
Adverse Events
One serious adverse event that may have been related to the study was detected in an intervention participant who incurred a fall and fractured wrist while walking for exercise. Nine hospitalizations in nine intervention participants and nine in eight control subjects occurred during the 12-month trial, none of which appeared to be study related. There were no deaths.
Discussion
This trial shows that an evidence-based, practical, comprehensive lifestyle intervention that produced modest weight and activity improvements did not improve asthma control better than a comparison treatment involving enhanced usual care in a racially and ethnically diverse sample of obese adults with uncontrolled asthma. ACQ scores improved modestly in both arms, but the between-treatment difference in change from baseline did not reach statistical significance, nor was it significant for any of the additional measures indicative of asthma-associated current impairment (e.g., FEV1 and symptoms) and future risk (e.g., exacerbations treated with systemic corticosteroids) through 12 months of follow-up. In both treatments there was not a worsening of asthma impairment or risk.
The existence of a dose–response relationship between increasing BMI and risk of developing asthma (3–5) and having poorer control among those with asthma (7) does not automatically imply that there is a similar dose–response relationship between weight loss and improvements in asthma outcomes. Observational and quasi-experimental studies dominate the literature on weight loss in asthma (11, 31). A 2012 Cochrane review (10) found only four completed RCTs, all of which tested short-term (≤6 mo) dietary interventions among obese adults with asthma. Three of the four trials used meal replacements to achieve stringent calorie restrictions (<1,200 kcal/d) (29, 32, 33), and the fourth one combined a low-calorie diet with weight-loss medications (34). Only the trial by Scott and colleagues (33) explicitly included physical activity, comparing a diet-alone (limited to 885–1,170 kcal/d through meal replacement), exercise-alone, and combined intervention. The interventions in the four trials produced reductions of 7.5 to 14.5% of baseline weight and were associated with improvements in asthma control, symptoms, lung function, and/or asthma medication use. However, as concluded in the Cochrane review, these trials were limited by small sample sizes (33 to 96), short intervention durations (40 d to 6 mo), unclear adequacy of allocation concealment, and a high risk of performance and detection bias (10). Several nonrandomized studies also reported considerable improvements in asthma outcomes after bariatric surgery (8, 9). All these published studies show improved asthma after losing more substantial weight than observed in the current trial.
BE WELL intervention participants did achieve significantly greater, albeit modest, improvements in weight loss and physical activity—the dual intervention goals—than control subjects. On average, however, intervention participants did not reach the targeted 7 to 10% weight loss program goal. The BE WELL lifestyle intervention was based on the multicenter DPP efficacy trial (15), and we previously demonstrated the effectiveness of interventions using the same adapted DPP curriculum as in BE WELL for weight loss and cardiometabolic risk reduction among high-risk overweight or obese adults in primary care (17). A metaanalysis of 28 U.S.-based studies adapting the efficacious, but resource-intensive, DPP lifestyle intervention into various “real-world” settings reported an average 4.0% (95% CI, 2.8–5.2%) loss of baseline weight at 12 months among intervention participants with complete follow-up data (35). Intention-to-treat analyses in three RCTs (36–38) of DPP-based lifestyle interventions in the U.S. primary care setting showed net-of-usual-care weight losses ranging from –1.1 kg (1.1%) to –4.5 kg (4.7%) at 12 months. In a recent review (39) of pragmatic lifestyle intervention programs in the United States and abroad, a metaanalysis of 11 RCTs showed a pooled net treatment effect of –1.62 (95% CI, –2.37 to –0.86) kg weight lost at 12 months. Compared with these findings, the BE WELL intervention similarly achieved a modest net loss of 1.9 kg (2.0%) of baseline weight, which, however, is notably smaller than the above-mentioned weight loss studies among obese adults with asthma. Importantly, our subgroup analysis results revealed a graded relationship between weight loss and ACQ change where there may not be clinically significant impact on ACQ improvement until 10% weight loss. Together with other weight loss evidence in asthma, they support the value of substantial weight loss for asthma control. In other words, weight loss works in asthma, but it appears different than for diabetes and cardiovascular risk factors where even modest amounts of 3 to 5% can afford clinical benefits (19). Modest weight loss might also help with asthma, but asthma outcomes are perhaps less sensitive than cardiometabolic outcomes such as glycemic or blood pressure control.
These findings have important clinical and mechanistic implications. Future research is needed to identify the minimal weight loss for clinically meaningful improvement in asthma. If effective and empirically supported lifestyle interventions are safe for obese adults with uncontrolled asthma, but they alone are unlikely to produce sufficient weight loss for asthma control, then the efficacy of stepped-up treatments (e.g., medically supervised very-low-calorie diet, weight loss drug therapy, and bariatric surgery) must be rigorously evaluated. Mechanistically, the possibility of a higher clinically important threshold of weight loss for asthma control than for cardiometabolic risk factor control may have important implications for understanding the effects of obesity, or the reversal of such effects through weight loss, on lung mechanics versus systemic and airway inflammation.
This study has limitations. It was a single-center trial of only 12 months in duration, although we included eight medical centers across five geographically distant counties in the Northern California Bay Area, and, to our knowledge, it is the first rigorous RCT of an evidence-based, practical, and comprehensive lifestyle intervention targeting weight loss and physical activity for asthma control. Second, lipid profile and glucose levels were not measured, although we did not design the trial to reconfirm the well-established benefits from weight reduction and increased physical activity on these common cardiometabolic risk factors. Third, BE WELL participants had access to quality healthcare, and the net weight reductions from the intervention were modest, but, as noted, the magnitude of the reductions compares favorably with those of similar lifestyle interventions in diverse settings and populations (35–38). Also, the control subjects in this research study received tools and information that may not be included in standard care for obese patients with asthma in routine care settings. Although we found no evidence of dose-dependent improvement in ACQ with weight loss, our results suggest the possibility of a threshold effect whereby weight loss as large as 10% or more may be required to actually improve asthma control. RCTs are needed to investigate the effects of large weight loss achievable through combined behavioral and pharmacological treatment or bariatric surgery on asthma outcomes. Equally important, research on weight loss or weight gain prevention for reducing incident asthma is needed.
In conclusion, few RCTs have attempted weight loss for asthma control, and none have implemented a comprehensive lifestyle intervention similar to that tested in the BE WELL trial. Its diverse sample, low attrition, and high methodological rigor are strengths. The results show that on a background of quality asthma care, a 1-year, evidence-based lifestyle intervention does not on average improve asthma control among moderately or severely obese adults with uncontrolled asthma. The minimally necessary weight loss for clinically important improvement in asthma may be greater than the accepted modest amounts for diabetes and cardiovascular risk factor control. Weight loss greater than or equal to 10% may be required to produce clinically meaningful improvement in asthma. The currently demonstrated intervention effectiveness for modest weight loss and increased physical activity are nonetheless noteworthy, because it indicates that obese adults with uncontrolled asthma can successfully participate in lifestyle interventions that have proven cardiometabolic benefits.
Acknowledgments
Acknowledgment
The authors thank the following individuals for their instrumental contributions to the conduct of the study: Veronica Luna, B.S., Andrea Blonstein, M.B.A., R.D., Jodi Thirtyacre, Elizabeth Jameiro, M.D., and Debbie Miller, M.B.A. They also thank the BE WELL Data and Safety Monitoring Board members (Randall S. Stafford, M.D., Ph.D., Ware G. Kuschner, M.D., and Manisha Desai, Ph.D.) and extend special thanks to the BE WELL participants and their families who made this study possible. They also acknowledge the Diabetes Prevention Support Center (DPSC) of the University of Pittsburgh for training and support in the Group Lifestyle Balance program; the current intervention program is a derivative of this material.
Footnotes
The BE WELL study was supported by grant R01HL094466 from the National Heart, Lung, and Blood Institute and internal funding from the Palo Alto Medical Foundation Research Institute. Dr. Lavori acknowledges support by the Clinical and Translational Science Award 1 UL1 RR025744 for the Stanford Center for Clinical and Translational Education and Research (Spectrum) from the National Center for Research Resources.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute. No sponsor or funding source had a role in the design or conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review, or approval of the manuscript.
Author Contributions: Conception and design: J.M., P.S., P.W.L., C.A.C., S.R.W., C.D.G., A.S.B., and W.L.H.; oversight of data acquisition: J.M. and P.S.; data analysis: L.X. and P.W.L.; manuscript writing and/or critical revisions for important intellectual content: J.M., P.S., L.X., P.W.L., C.A.C., S.R.W., C.D.G., A.S.B., W.L.H., and N.L.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Liu X.Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010 Hyattsville, MD: National Center for Health Statistics; 2012. 1–8 [PubMed] [Google Scholar]
- 3.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 4.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, Celedón JC, Shore SA American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7:325–335. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 5.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutherland ER. Linking obesity and asthma. Ann N Y Acad Sci. 2014;1311:31–41. doi: 10.1111/nyas.12357. [DOI] [PubMed] [Google Scholar]
- 7.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, Pedersen SE GOAL Investigators Group. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170:836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 8.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128:508–515.e1–2. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy RC, Baptist AP, Fan Z, Carlin AM, Birkmeyer NJ. The effects of bariatric surgery on asthma severity. Obes Surg. 2011;21:200–206. doi: 10.1007/s11695-010-0155-6. [DOI] [PubMed] [Google Scholar]
- 10.Adeniyi FB, Young T. Weight loss interventions for chronic asthma. Cochrane Database Syst Rev. 2012;7:CD009339. doi: 10.1002/14651858.CD009339.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Moreira A, Bonini M, Garcia-Larsen V, Bonini S, Del Giacco SR, Agache I, Fonseca J, Papadopoulos NG, Carlsen KH, Delgado L, et al. Weight loss interventions in asthma: EAACI evidence-based clinical practice guideline (part I) Allergy. 2013;68:425–439. doi: 10.1111/all.12106. [DOI] [PubMed] [Google Scholar]
- 12.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 13.Ma J, Strub P, Camargo CA, Jr, Xiao L, Ayala E, Gardner CD, Buist AS, Haskell WL, Lavori PW, Wilson SR. The Breathe Easier through Weight Loss Lifestyle (BE WELL) Intervention: a randomized controlled trial. BMC Pulm Med. 2010;10:16. doi: 10.1186/1471-2466-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao L, Lavori PW, Wilson SR, Ma J. Comparison of dynamic block randomization and minimization in randomized trials: a simulation study. Clin Trials. 2011;8:59–69. doi: 10.1177/1740774510391683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer MK, Kriska AM, Venditti EM, Miller RG, Brooks MM, Burke LE, Siminerio LM, Solano FX, Orchard TJ. Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am J Prev Med. 2009;37:505–511. doi: 10.1016/j.amepre.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Ma J, Yank V, Xiao L, Lavori PW, Wilson SR, Rosas LG, Stafford RS. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med. 2013;173:113–121. doi: 10.1001/2013.jamainternmed.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bandura A. Englewood Cliffs, NJ: Prentice Hall; 1986. Social foundations of thought and action: a social cognitive theory. [Google Scholar]
- 19.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juniper EF, Bousquet J, Abetz L, Bateman ED GOAL Committee. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100:616–621. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99:553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32–38. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 25.Measures from the PhenX Toolkit version February 4, 2011. Ver 4.2 [accessed 2015 Jan 15]. Available fromhttp://www.phenxtoolkit.org
- 26.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 27.Blair SN, Haskell WL, Ho P, Paffenbarger RS, Jr, Vranizan KM, Farquhar JW, Wood PD. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J. Hillsdale, NJ: Erlbaum; 1988. Statistical power analysis for the behavioral sciences, 2nd ed. [Google Scholar]
- 29.Stenius-Aarniala B, Poussa T, Kvarnström J, Grönlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ. 2000;320:827–832. doi: 10.1136/bmj.320.7238.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schatz M, Nakahiro R, Crawford W, Mendoza G, Mosen D, Stibolt TB. Asthma quality-of-care markers using administrative data. Chest. 2005;128:1968–1973. doi: 10.1378/chest.128.4.1968. [DOI] [PubMed] [Google Scholar]
- 31.Eneli IU, Skybo T, Camargo CA., Jr Weight loss and asthma: a systematic review. Thorax. 2008;63:671–676. doi: 10.1136/thx.2007.086470. [DOI] [PubMed] [Google Scholar]
- 32.Hernández Romero A, Matta Campos J, Mora Nieto A, del Rivero L, Andrés Dionicio AE, Aguilar Ramírez P, Barthell Solís C, García González A, Carreta Macías L, Murguía Corral R, et al. Clinical symptom relief in obese patients with persistent moderate asthma secondary to decreased obesity [in Spanish] Rev Alerg Mex. 2008;55:103–111. [PubMed] [Google Scholar]
- 33.Scott HA, Gibson PG, Garg ML, Pretto JJ, Morgan PJ, Callister R, Wood LG. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: a randomized trial. Clin Exp Allergy. 2013;43:36–49. doi: 10.1111/cea.12004. [DOI] [PubMed] [Google Scholar]
- 34.Dias-Júnior SA, Reis M, de Carvalho-Pinto RM, Stelmach R, Halpern A, Cukier A. Effects of weight loss on asthma control in obese patients with severe asthma. Eur Respir J. 2014;43:1368–1377. doi: 10.1183/09031936.00053413. [DOI] [PubMed] [Google Scholar]
- 35.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood) 2012;31:67–75. doi: 10.1377/hlthaff.2011.1009. [DOI] [PubMed] [Google Scholar]
- 36.Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, Miller ER, III, Dalcin A, Jerome GJ, Geller S, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365:1959–1968. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, Kumanyika S, Schmitz KH, Diewald LK, Barg R, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365:1969–1979. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett GG, Warner ET, Glasgow RE, Askew S, Goldman J, Ritzwoller DP, Emmons KM, Rosner BA, Colditz GA Be Fit, Be Well Study Investigators. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Arch Intern Med. 2012;172:565–574. doi: 10.1001/archinternmed.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, Khunti K. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care. 2014;37:922–933. doi: 10.2337/dc13-2195. [DOI] [PubMed] [Google Scholar]