Abstract
Rationale: Stakeholders seek to monitor processes and outcomes of care among patients with sepsis, but use of administrative data for sepsis surveillance is controversial. Prior studies using only principal diagnoses from claims data have shown a trend of rising sepsis incidence with falling infection incidence, implying that administrative data are inaccurate for sepsis surveillance.
Objectives: Because a sepsis diagnosis often modifies an infection site diagnosis, we sought to investigate trends in sepsis and infection using both principal and secondary diagnoses in administrative data.
Methods: This was a retrospective cohort study. We used data from the Nationwide Inpatient Sample years 2003 to 2009 to identify age-standardized, population-based trends in sepsis and infection using all available diagnosis codes. Infection sites were defined as bacteremia, pneumonia, urinary tract, skin/soft tissue, and gastrointestinal; codes for septicemia, sepsis, severe sepsis, and septic shock were used to identify “sepsis.” We identified patients with infection and mechanical ventilation to estimate incidence of severe sepsis without requiring specific claims for sepsis or acute organ failure.
Measurements and Main Results: We identified 53.9 million adult infection hospitalizations during the years 2003 to 2009; average age was 63 years, 61% of patients were women, and 70% reported white race, 14% black, and 11% Hispanic ethnicity. Incidence of hospitalizations with an infection claim increased from 3,147/100,000 U.S. residents in 2003 to 3,480/100,000 in 2009 (11% increase), whereas hospitalizations with sepsis claims increased from 359/100,000 to 535/100,000 residents during the same time frame (49% increase); P = 0.009 between infection and sepsis trends. The proportion of infection hospitalizations with a sepsis claim increased from 7.5% in 2003 to 11.5% in 2009 (54% increase). The incidence of hospitalizations with both an infection and mechanical ventilation claim during 2003 was 173/100,000 as compared with 251/100,000 in 2009 (45% increase); P = 0.76 compared with sepsis trends.
Conclusions: Sepsis claims are increasing at a greater rate than infection claims but are not inversely related. Trends in sepsis are similar to trends in infection cases requiring mechanical ventilation. Further studies should seek to identify the optimal algorithms to identify sepsis within administrative data and explore potential mechanisms for the increasing incidence of infection and sepsis in the United States.
Keywords: sepsis, health services research
Identifying accurate and efficient methods for surveillance of sepsis has public health and policy implications. Multiple studies using administrative data over the past 2 decades have identified rising rates of hospitalizations for sepsis (1–5); however, whether administrative claims data accurately capture sepsis cases is an area of controversy (6, 7). Although some argue that claims data may accurately reflect trends in sepsis incidence and outcomes (6, 8), others counter that the increasing incidence of sepsis in claims data is a spurious result, likely from financial incentives leading to increasingly attentive sepsis coding practices (7). In support of the perspective that administrative claims data inaccurately ascertain sepsis cases, recent reports using principal diagnoses from claims data have described an apparently paradoxical situation in which sepsis is increasing while infection incidence is falling (7).
Because diagnoses of sepsis (or severe sepsis or septic shock) are descriptors of infection severity, both principal and secondary diagnoses codes have generally been used for identification of sepsis through administrative claims data (2–4). To determine whether trends in sepsis and infection claims have indeed been inversely related and inappropriate for use in sepsis surveillance, we sought to explore temporal trends in sepsis and infections using all available diagnosis codes in a population-based sample of U.S. hospitalizations. We sought to further explore whether increasing claims for sepsis might be spurious by evaluating trends in patients with both infection and mechanical ventilator support claims, a strategy aimed at attenuating subjective interpretations of organ dysfunction or sepsis. We hypothesized that inverse associations between sepsis and infection observed in prior research may have been driven by use of principal diagnosis codes alone and that sepsis trends using all available diagnosis claims would be consistent with trends in infection with mechanical ventilation.
Methods
We replicated methods to identify sepsis and infection site trends in the United States as previously described by Rhee and colleagues (7), with the exception that we included both the principal diagnosis and all secondary diagnoses to identify sepsis and infection trends and included skin and soft tissue infections (International Classification of Diseases, ninth revision [ICD-9] 680, 682–686, 785.4, 728.7). Briefly, we used the Nationwide Inpatient Sample (NIS) (9) from years 2003 to 2009 to identify claims for sepsis (including codes for septicemia, sepsis, severe sepsis, and septic shock) as well as claims for specific sources of infection (7), including pneumonia, intraabdominal infection, urinary tract infection, bacteremia, and skin and soft tissue infection among hospitalized adults aged 18 years and older. The NIS is a 20% stratified sample of non-Federal community hospitals in the United States, excluding long-term care or rehabilitation hospitals. The NIS reported up to 15 hospital diagnoses throughout the study time frame. In 2003, 37 states and 997 hospitals contributed to the NIS, and in 2009, 44 states and 1,050 hospitals contributed.
We used survey-weighted methodology to determine infection and sepsis incidence. We then used U.S. census estimates to determine rates per 100,000 of U.S. population each year and directly age-standardized rates to the 2000 U.S. census distribution to determine age-adjusted incidence. We calculated the overall percent change in the age-adjusted proportion of hospitalizations with a sepsis or infection diagnosis between 2003 and 2009 as well as the age-adjusted average annual percent change (AAPC) in sepsis and infection diagnoses from 2003 to 2009.
To explore whether trends of increasing claims for sepsis might be spurious, we evaluated the proportion of patients with both infection and mechanical ventilator support claims (validated ICD-9 procedure codes for mechanical ventilation 96.7x: [86% sensitive, 99.7 specific (10)] or noninvasive ventilation 93.90: [86% sensitive, 92% specific (11)]) over time. Trends in infection with mechanical ventilator support may objectively estimate severe sepsis trends without reliance on sepsis codes or potentially subjective organ failure diagnosis codes. Similarly, we compared trends in overall sepsis incidence with trends in the incidence of sepsis with mechanical ventilation to assess whether sepsis may be “up-coded,” with fewer sepsis patients requiring mechanical ventilation over time. We used Healthcare Cost and Utilization Project software to identify Elixhauser comorbidities among patients with infections to identify trends in risk factors for sepsis (12). Trends in comorbidities over time were analyzed using Cochrane-Armitage tests or t tests, as applicable.
We used SAS version 9.3 (Cary, NC) to calculate population-weighted estimates and Joinpoint Regression Program 4.1.1.1 (Statistical Research and Applications Branch, National Cancer Institute, Bethesda, MD) to compare AAPC in incidence and mortality trends in infection and sepsis (13). Given the large number of events obtained in our national estimates, confidence intervals of proportions were not substantively different from the point estimates. All study procedures were deemed nonhuman subjects research by the Boston University Medical Center Institutional Review Board.
Results
We identified 227 million adult hospital discharges and 53.9 million infection hospitalizations in the United States during years 2003 to 2009. Among infection hospitalizations, the average age was 63 years, 61% of patients were women, and 70% reported white race, 14% black, and 11% Hispanic ethnicity. Table 1 demonstrates changes in demographics and comorbid conditions during the years 2003 to 2009, during which time the average number of comorbid conditions increased significantly from 2.1 to 2.7 per patient.
Table 1.
Characteristics of patients hospitalized with infection during 2003 and 2009
| Variable | Weighted N (%) or Mean (SD) |
|
|---|---|---|
| 2003 (N = 6,885,896) | 2009 (N = 8,396,082) | |
| Age, yr | 63.4 (19.9) | 63.5 (19.5) |
| Sex, female | 4,183,645 (61) | 4,973,846 (59) |
| Race | ||
| White | 3,601,377 (71) | 5,161,188 (72) |
| Black | 667,490 (13) | 872,389 (12) |
| Hispanic | 568,583 (11) | 725,022 (10) |
| Asian | 108,595 (2.1) | 149,366 (2.1) |
| Native American | 10,957 (0.2) | 51,626 (0.7) |
| Other | 117,932 (2.3) | 238,124 (3.3) |
| Number of comorbidities | 2.1 (1.6) | 2.7 (1.8) |
| Comorbid conditions | ||
| Heart failure | 996,781 (15) | 1,121,720 (13) |
| Valvular heart disease | 424,778 (6.2) | 318,100 (3.8) |
| Pulmonary circulation disease | 73,247 (1.1) | 222,085 (2.6) |
| Peripheral vascular disease | 322,788 (4.7) | 497,217 (5.9) |
| Paralysis | 209,557 (3.4) | 321,608 (3.8 |
| Other neurological disorders | 554,728 (8.1) | 796,066 (9.5) |
| Chronic pulmonary disease | 1,530,418 (22) | 1,855,400 (22) |
| Diabetes without chronic complications | 1,203,419 (17) | 1,733,989 (21) |
| Diabetes with chronic complications | 326,505 (4.7) | 449,265 (5.4) |
| Hypertension | 2,725,262 (40) | 4,123,106 (49) |
| Hypothyroidism | 556,377 (8.1) | 937,105 (11) |
| Chronic renal failure | 424,778 (6.2) | 1,142,375 (14) |
| Liver disease | 198,626 (2.9) | 324,177 (3.9) |
| Peptic ulcer disease | 4,296 (0.07) | 3,229 (0.04) |
| HIV | 16,840 (0.25) | 23,460 (0.28) |
| Lymphoma | 58,649 (0.85) | 87,180 (1.0) |
| Metastatic cancer | 157,225 (2.3) | 231,578 (2.8) |
| Solid tumor without metastases | 151,347 (2.2) | 225,211 (2.7) |
| Rheumatoid arthritis/collagen vascular disease | 157,759 (2.3) | 238,244 (2.8) |
| Coagulopathy | 262,485 (3.8) | 441,173 (5.3) |
| Obesity | 329,082 (4.8) | 795,239 (9.5) |
| Weight loss | 265,742 (3.9) | 616,170 (7.3) |
| Fluid and electrolyte disorders | 1,786,371 (26) | 2,680,363 (32) |
| Chronic blood loss anemia | 129,184 (1.9) | 127,610 (1.5) |
| Deficiency anemia | 998,066 (15) | 1,700,446 (20) |
| Alcohol abuse | 199,242 (2.9) | 298,403 (3.6) |
| Drug abuse | 131,321 (1.9) | 212,386 (2.5) |
| Psychoses | 197,956 (2.9) | 364,809 (4.3) |
| Depression | 456,454 (6.6) | 795,631 (9.5) |
All characteristics show P < 0.0001 for trend over time.
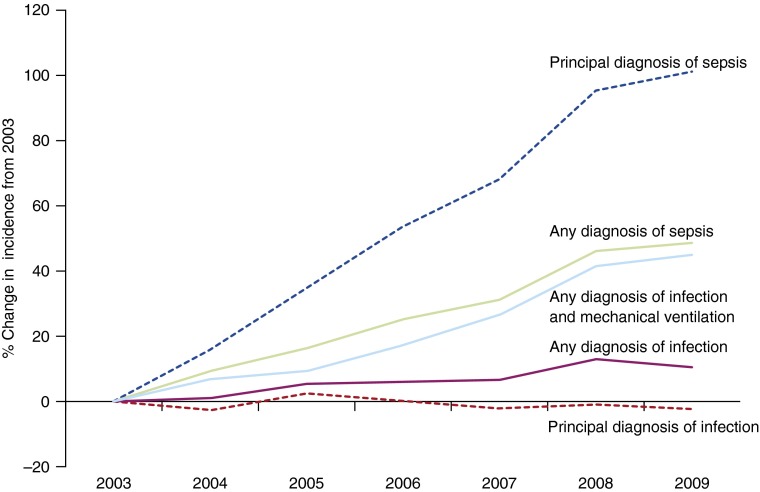
The age-adjusted incidence of hospitalizations with a principal diagnosis of sepsis increased from 161 to 324/100,000 U.S. residents (100% increase; AAPC, 12.4%; 95% confidence interval [CI], 10.1–14.7%), whereas hospitalizations with a principal diagnosis for a specific infection source declined from 1,381 to 1,349/100,000 (2% decrease; AAPC, −0.31%; 95% CI, −0.9 to 0.6%; P = 0.0002 when compared with trend for principal sepsis diagnosis). The proportion of infection hospitalizations with a sepsis claim increased from 7.5% in 2003 to 11.5% in 2009 (54% increase), whereas the proportion of sepsis hospitalizations without a concomitant infection source claim declined from 35 to 26% (25% decrease). Age-adjusted incidence of hospitalizations with any infection claim increased from 3,147/100,000 in 2003 to 3,480/100,000 in 2009 (11% increase; AAPC, 1.8%; 95% CI, 1.1–2.7%), whereas claims for sepsis increased from 359/100,000 to 535/100,000 (49% increase; AAPC, 6.9%; 95% CI, 5.9–8.0%; P = 0.009 for comparison between sepsis and infection trends; Figure 1). The incidence of infection with mechanical ventilation was 173/100,000 during 2003 as compared with 251/100,000 in 2009 (45% increase; AAPC, 6.8; 95% CI, 5.1–8.1%; Figure 1), a rate of change that was not significantly different from sepsis incidence trends (P = 0.76). The change in incidence of sepsis with mechanical ventilation (AAPC, 9.8%; 95% CI, 8.1–11.6%) was significantly greater than the increase in overall sepsis incidence (P = 0.002). The rate of decline in age-adjusted sepsis hospital mortality (from 23.8 to 19.2%) did not differ significantly from the decrease in infection mortality (from 5.1 to 4.3%; P = 0.10).
Figure 1.
Change in incidence of sepsis and specific infection sites based on principal diagnosis and any diagnosis from the International Classification of Diseases, ninth Revision, Clinical Modification codes in the United States from 2003 to 2009.
Discussion
We investigated trends in sepsis and infection site claims in administrative data from the U.S. population-based NIS during years 2003 through 2009. Contrary to results from studies using only principal diagnosis codes (7), sepsis and infection hospitalizations both increased when principal and secondary claims were used to identify diagnoses. Use of principal diagnoses alone when investigating sepsis and infection trends showed that sepsis is increasingly more likely than the infection source to be used as a principal diagnosis, a practice that may reflect higher reimbursement rates for diagnosis-related groups derived from principal sepsis codes than from infection site codes. However, the impact of changing reimbursement practices can be attenuated with use of all available diagnosis claims. Although incidence of hospitalizations with a sepsis diagnosis increased more rapidly than the incidence with an infection diagnosis, a disproportionate increase in sepsis claims may not necessarily be the result of inaccurate ICD-9 coding. For example, the proportion of hospitalized patients with infection claims who received mechanical ventilation—a common treatment for respiratory failure during severe sepsis accurately measured with claims data—increased at rates similar to the increase in sepsis claims. Our results suggest that incidence of sepsis in the United States is rising at rates similar to reports from Australia (14), mechanisms of which deserve further inquiry.
Many factors may explain a disproportionate rise in hospitalizations with sepsis as compared with infection. Our results show that comorbidities increased significantly among patients hospitalized with infection, perhaps reflecting a changing population more susceptible to sepsis. Furthermore, readmission rates for patients with sepsis are high (15, 16), with nearly half of sepsis readmissions due to recurrent infection (17). The combination of increasingly susceptible patients, improving hospital sepsis survival, and high sepsis readmission rates likely plays a role in amplifying rates of sepsis over time. Improved clinician recognition of sepsis due to local and national quality-improvement initiatives such as the Surviving Sepsis Campaign may also contribute to an appropriately increased sensitivity of ICD-9 codes for the detection of sepsis, as described in recent studies (18), as well as declining hospital mortality rates in infection and sepsis. However, despite multiple external forces potentially acting on sepsis claims, our results demonstrate that trends derived from methodologically validated uses of administrative data may yield plausible and important insights into changing sepsis epidemiology.
Prior studies have attempted to validate claims data for sepsis by comparing the accuracy of sepsis codes with chart-abstracted data in individual centers (18–22). In these studies, claims-based algorithms for sepsis, severe sepsis, and septic shock have shown high specificity (ranging from 96–100%) and positive predictive values (ranging from 70–100%). Thus, sepsis cases identified through administrative data likely reliably represent cases of sepsis identified by clinicians. However, claims data for sepsis show modest to poor sensitivities (ranging from 9–89%) for detecting sepsis when validated against chart data (18–22). Coupled with our finding that sepsis cases represent a smaller than expected fraction (11.5%) of infection hospitalizations, claims data likely underestimate sepsis incidence.
Problems with sepsis surveillance may lie less in the inaccuracies of administrative data and more with the complexities of making a sepsis diagnosis clinically. Administrative claims are ideally reflections of clinical impressions documented in the medical chart. However, impressions of severe sepsis or septic shock vary substantially between clinicians. For example, Iwashyna and colleagues (20) and Zhao and colleagues (23) showed kappa statistics of 0.70 and 0.68, respectively, (moderate agreement) between trained clinician chart reviewers regarding whether patients met criteria for severe sepsis. Difficulty in reaching clinical agreement over a sepsis diagnosis may result from (1) lack of a specific time window in which infection, systemic inflammatory response syndrome criteria, and organ failure must fall to be defined as severe sepsis; (2) different definitions used to define acute organ failures (24, 25); (3) different understandings of the terms “septicemia” or “sepsis”; or (4) varying levels of clinical suspicion for infection in the absence of unequivocally positive culture data. It is likely that variation in administrative data accuracy is reflective of the clinical complexities of a sepsis diagnosis.
Our study has limitations. As compared with Rhee and colleagues (7), who used data from 2003 through 2011, we used available data only through 2009. However, we do not suspect significant changes in ICD-9 coding trends during 2010 and 2011 as compared with 2003 to 2009. We compared trends using a variety of administrative coding algorithms but did not have chart-level data to validate our findings. The temporality between infection and mechanical ventilation using ICD-9 codes is unclear, and thus infection and mechanical ventilation may not have been etiologically related during the same hospitalization. The number of potential diagnoses reported in the NIS did not change during the time period of our study; however, it is possible that general use of more ICD-9 codes over time might confound trends in comorbidities and infection. We identified the most common infection sites to aid in comparison to prior studies but do not include all possible infection sites.
In conclusion, we show that trends in sepsis identified through use of all diagnoses from administrative data approximate trends in severe infection. Our findings suggest that ICD-9 codes may accurately identify patients with sepsis. The Centers for Medicare and Medicaid Services have recently proposed mandates for hospital reporting of severe sepsis bundle adherence (26, 27). We believe that our results support a practice of screening for severe sepsis cases using claims data, with subsequent chart review to identify specific processes of care. With the phasing out of ICD-9 and impending adoption of the more precise ICD-10 system, we are now in a unique position to proactively define, validate, standardize and further improve methodology to identify sepsis from administrative data.
Footnotes
Supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health grants K01HL116768 (A.J.W.) and K01HL114745 (T.L.).
Author Contributions: A.J.W.: Conception, design, data acquisition and analysis, drafting of manuscript, manuscript revision for intellectual content, approval of final manuscript version. T.L.: Design, manuscript revision for intellectual content, approval of final manuscript. P.K.L.: Conception, design, manuscript revision for intellectual content, approval of final manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kumar G, Kumar N, Taneja A, Kaleekal T, Tarima S, McGinley E, Jimenez E, Mohan A, Khan RA, Whittle J, et al. Milwaukee Initiative in Critical Care Outcomes Research Group of Investigators. Nationwide trends of severe sepsis in the 21st century (2000-2007) Chest. 2011;140:1223–1231. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 4.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Walkey AJ, Wiener RS, Lindenauer PK. Utilization patterns and outcomes associated with central venous catheter in septic shock: a population-based study. Crit Care Med. 2013;41:1450–1457. doi: 10.1097/CCM.0b013e31827caa89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooke CR, Iwashyna TJ. Sepsis mandates: improving inpatient care while advancing quality improvement. JAMA. 2014;312:1397–1398. doi: 10.1001/jama.2014.11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee C, Gohil S, Klompas M. Regulatory mandates for sepsis care—reasons for caution. N Engl J Med. 2014;370:1673–1676. doi: 10.1056/NEJMp1400276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med. 2014;42:625–631. doi: 10.1097/CCM.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houchens RL, Elixhauser A.Using the HCUP Nationwide Inpatient Sample to estimate trends(Updated for 1988–2004). HCUP Methods Series Report #2006-05 [updated 2006 Aug 18; accessed 2013 Jan 23]. U.S. Agency for Healthcare Research and Quality. Available from: http://www.hcup-us.ahrq.gov/reports/methods.jsp
- 10.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42:801–809. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 11.Lindenauer PK, Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Hill NS. Outcomes associated with invasive and noninvasive ventilation among patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982–1983. doi: 10.1001/jamainternmed.2014.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 15.Liu V, Lei X, Prescott HC, Kipnis P, Iwashyna TJ, Escobar GJ. Hospital readmission and healthcare utilization following sepsis in community settings. J Hosp Med. 2014;9:502–507. doi: 10.1002/jhm.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prescott HC, Langa KM, Liu V, Escobar GJ, Iwashyna TJ. Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;190:62–69. doi: 10.1164/rccm.201403-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortego A, Gaieski DF, Fuchs BD, Jones T, Halpern SD, Small DS, Sante SC, Drumheller B, Christie JD, Mikkelsen ME.Hospital-based acute care use in survivors of septic shock Crit Care Med 2014. epub Oct 31 [DOI] [PMC free article] [PubMed]
- 18.Rhee C, Murphy MV, Li L, Platt R, Klompas M Centers for Disease Control and Prevention Epicenters Program. Comparison of trends in sepsis incidence and coding using administrative claims versus objective clinical data. Clin Infect Dis. 2015;160:88–95. doi: 10.1093/cid/ciu750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306:2248–2254. doi: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwashyna TJ, Odden A, Rohde J, Bonham C, Kuhn L, Malani P, Chen L, Flanders S. Identifying patients with severe sepsis using administrative claims: patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med Care. 2014;52:e39–e43. doi: 10.1097/MLR.0b013e318268ac86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poulose JT, Cartin-Ceba R, Shoja A, Paul A, Trillo-Alvaraez C, Kashyap R, Patch R, Poulose L, Cabello-Graza J, Li G, et al. Comparison of International Classification of Disease−Ninth Revision (ICD−9) coding with retrospective case review for the diagnosis of septic shock [abstract] Am J Respir Crit Care Med. 2009;179:A4691. [Google Scholar]
- 22.Whittaker SA, Mikkelsen ME, Gaieski DF, Koshy S, Kean C, Fuchs BD. Severe sepsis cohorts derived from claims-based strategies appear to be biased toward a more severely ill patient population. Crit Care Med. 2013;41:945–953. doi: 10.1097/CCM.0b013e31827466f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao H, Heard SO, Mullen MT, Crawford S, Goldberg RJ, Frendl G, Lilly CM. An evaluation of the diagnostic accuracy of the 1991 American College of Chest Physicians/Society of Critical Care Medicine and the 2001 Society of Critical Care Medicine/European Society of Intensive Care Medicine/American College of Chest Physicians/American Thoracic Society/Surgical Infection Society sepsis definition. Crit Care Med. 2012;40:1700–1706. doi: 10.1097/CCM.0b013e318246b83a. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 25.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Medicare and Medicaid Services (CMS), HHS. Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and fiscal year 2015 rates; quality reporting requirements for specific providers; reasonable compensation equivalents for physician services in excluded hospitals and certain teaching hospitals; provider administrative appeals and judicial review; enforcement provisions for organ transplant centers; and electronic health record (EHR) incentive program. Final rule. Fed Regist. 2014;79:49853–50536. [PubMed] [Google Scholar]
- 27.National Quality Forum Statement from NQF on Review of Sepsis Measure 2014. [accessed 2014 Oct 23]. Available from: http://www.qualityforum.org/News_And_Resources/Press_Releases/2014/Statement_from_NQF_on_Review_of_Sepsis_Measure.aspx