Abstract
Rationale: Incidental pulmonary nodule detection is postulated to cause distress, but the frequency and magnitude of that distress have not been reported. The quality of patient–clinician communication and the perceived risk of lung cancer may influence distress
Objectives: To evaluate the association of communication processes with distress and the perceived risk of lung cancer using validated instruments.
Methods: We conducted a prospective cohort study of patients with incidentally detected nodules who received care at one Department of Veterans Affairs Medical Center. We measured distress with the Impact of Event Scale and patient-centered communication with the Consultation Care Measure, both validated instruments. Risk of lung cancer was self-reported by participants. We used multivariable adjusted logistic regression to measure the association of communication quality with distress.
Measurements and Main Results: Among 122 Veterans with incidental nodules, 23%, 12%, and 4% reported experiencing mild, moderate, and severe distress, respectively, at the time they were informed of the pulmonary nodule. Participant-reported risk of lung cancer was not associated with distress. In the adjusted model, high-quality communication was associated with decreased distress (odds ratio [OR] = 0.28, 95% confidence interval [CI] = 0.08–1.00, P = 0.05). Among participants who reported a risk of malignancy of 30% or less, high-quality communication was associated with decreased distress (OR = 0.15, 95% CI = 0.02–0.92, P = 0.04), but was not associated with distress for those who reported a risk greater than 30% (OR = 0.12 (95% CI = 0.00–3.97, P = 0.24), although the P value for interaction was not significant.
Conclusions: Veterans with incidental pulmonary nodules frequently reported inadequate information exchange regarding their nodule. Many patients experience distress after they are informed that they have a pulmonary nodule, and high-quality patient–clinician communication is associated with decreased distress. Communication strategies that only target improved knowledge of the risk of malignancy may not be sufficient to reduce the distress associated with nodule detection.
Keywords: pulmonary nodule, patient-clinician communication, patient-centered outcomes
Every year, hundreds of thousands of patients are diagnosed with incidental pulmonary nodules (1, 2), fueled by the large and growing number of patients who undergo radiologic imaging studies (3–6). The number of patients with pulmonary nodules is likely to substantially increase, as multiple organizations, including the U.S. Preventive Services Task Force, now recommend annual low-dose computed tomography (CT) screening for adults at high risk of developing lung cancer (7–12). These recommendations are mainly based on the National Lung Screening Trial, which showed that low-dose CT screening resulted in substantial mortality benefits, but at the cost of very many false positives, usually due to detection of a pulmonary nodule (13).
Harris and colleagues (14) recently developed a taxonomy of harms from screening that are also applicable to the detection of pulmonary nodules. One of the harms is the resultant psychosocial consequences. Limited evidence from screening trials suggests that distress increases in the short term, and qualitative studies from patients in routine care settings indicate that nodule detection is associated with distress as well (15–19). The screening trials have not evaluated methods to mitigate these harms, but the qualitative work has identified communication processes as a potentially modifiable factor that might be associated with distress.
Based on this work, and guided by a theoretical model of patient-centered communication processes (20), we evaluated the occurrence and magnitude of distress among a cohort of Veterans with incidental pulmonary nodules. We hypothesized that distress would be common, and higher general communication quality and lower perceived risk of lung cancer would be associated with decreased levels of distress.
Some of the results from this article were presented at the May 2014 International Conference of the American Thoracic Society as an abstract.
Methods
Overview and Setting
We conducted a prospective study at the Portland Veterans Affairs Medical Center (PVAMC), an academic-affiliated hospital with outlying primary care clinics, among patients with an incidentally detected (not from screening) nodule. At the PVAMC, thoracic radiology images with nodules are electronically flagged (2). In general, primary care providers are responsible for notifying the patient and determining the evaluation, usually without guidance from pulmonologists. Study participants were recruited between February 2012 and December 2013.
At the PVAMC, all patients found to have a nodule are entered into a clinical registry regardless of nodule number and characteristics. Medical records from patients enrolled in this registry were reviewed, and those with newly reported, incidental nodules less than 3 cm were potentially eligible for this study. Patients with a plan for, but who had not yet obtained, nonurgent imaging follow up were potentially eligible. After approval from the treating clinician and mental health clinician (if relevant), we contacted the patient by mail to participate. The Institutional Review Board of the PVAMC approved this study, and all patients provided written informed consent.
We excluded patients who scored less than 17/30 on the St. Louis University Mental Status Examination (21), who resided in skilled nursing care facilities, had previously been diagnosed with lung cancer, were diagnosed with psychotic or cognitive disorders, or had a terminal illness (including a current diagnosis of cancer). Participants completed surveys in person with trained research coordinators.
Primary Outcome Measure
We used the Impact of Event Scale (IES) to measure symptoms of emotional distress as a result of nodule detection experienced in the 7 days before the survey. Several studies have reported that the IES has high internal consistency among patients with cancer (22). For the primary analyses, we used a score of 8 or more to indicate at least mild distress (23).
Secondary Outcome Measure
The patient-centered communication model includes the exchange of information as a key component of high-quality communication. We assessed appropriate information exchange by measuring participants’ perceptions of lung cancer malignancy risk. A similar scale was used in a previous trial of lung cancer screening (24). We used an 11-point Likert scale (with 10-point incremental percentage increases from 0 to 100%) to measure the participants’ estimates that the nodule was caused by lung cancer (25). We used an identical scale to determine the participants’ perceptions of the clinicians’ estimate of risk. To analyze self-reported lung cancer malignancy risk, we dichotomized the responses to 30% or less chance or greater than 30% chance. We also calculated the objective risk of lung cancer based on the Mayo model (26).
Primary Exposure Measure
The primary exposure variable was the overall quality of self-reported communication regarding the nodule. Participants were asked to answer the communication questions regarding the clinician who would be responsible for coordinating nodule care (usually the primary care provider) or the clinician who first notified them if they had not communicated with the responsible clinician about the nodule. The primary measure of the perception of communication was the statement “The overall quality of communication with your provider is excellent,” which was rated on a seven-point Likert scale, from “very strongly agree” to “very strongly disagree.” This question is from the Consultation Care Measure, which is based on the patient-centered communication model and was recommended in an analysis of multiple communication instruments (27). For the primary analysis, we dichotomized this variable as “high quality” if participants indicated they agreed, strongly agreed, or very strongly agreed, and “low quality” if the response was neutral to very strongly disagreed. Missing responses were coded as missing and not included in the primary statistical analyses, but were included in sensitivity analyses. We also used this variable measured continuously in sensitivity analyses.
Covariates
We used self-report to measure sex, education level, marital status, income, smoking status, and self-report of mental health conditions. We abstracted information from the electronic medical record regarding: age at entry to the study; nodule details, including size, location (upper versus non-upper lobe), characteristics (spiculated versus nonspiculated); indication for the imaging study that best characterized the nodule and was to be used as the baseline (symptomatic versus asymptomatic [e.g., a chest X-ray for cough noted a small nodule, and subsequent CT was performed for nodule visualization, not the cough, was categorized as “asymptomatic”]); and time from the imaging study to the date of survey administration. We did not independently review the radiologic images. We reviewed the electronic medical record to identify how the participant was informed about the study results (letter, phone, in person) and who informed her/him, but used self-report as our measure. We collected information on reasons why participants did not seek more information about nodules from their clinicians and suggestions for improving communication processes.
We used a Likert scale (0–10 from “very worst” to “very best”) to measure how informed the participant was about the nodule and how satisfied she/he was with the clinician’s explanation. We categorized responses as “not at all,” “somewhat,” and ”very” for scores of 0, 1–7, and 8–10, respectively. Participants were permitted to report “I don’t know” as well.
Participants reported if they were satisfied with care regarding the pulmonary nodule and if that care could be better. These items used a five-point Likert scale (strongly disagree to strongly agree). For the satisfaction variable, we dichotomized responses as either satisfied (agree or strongly agree) versus not satisfied (neutral, disagree, or strongly disagree). For perceived quality of care, we dichotomized responses as either “care could not be better” (disagree or strongly disagree) or “care could be better” (neutral, agree, or strongly agree).
Participants reported if their clinician was skilled and knowledgeable about caring for patients with nodules using a five-point Likert scale (not at all knowledgeable to extremely knowledgeable) (28). Responses were dichotomized as “skilled/knowledgeable” if the participant reported “very” or “extremely knowledgeable (an expert),” or “not skilled/knowledgeable” otherwise. If participants could not estimate the clinicians’ skill and knowledge, their response was recorded as missing, and was included as “not skilled/knowledgeable” in the univariate analyses.
Analysis
We used logistic regression to report odds ratios (ORs) as measures of the association of patient, nodule, and communication characteristics with distress and self-reported risk of lung cancer. Because there are limited data about potential confounders of the associations with distress, models were constructed parsimoniously, excluding variables that were not confounders if they did not change the threshold level of significance and/or point estimates by more than 10%. A priori, we adjusted for age, smoking status, and self-reported depression. Education, marital status, self-reported post-traumatic stress disorder (PTSD), nodule characteristics, time from nodule identification to survey administration, and who informed the participant, and the method used to inform the participant did not confound the relation between general communication and the distress, so were not included in the final model. Income and indication for the imaging study did confound the association, so the final model included these variables plus age, smoking status, and self-reported depression. We did not include possible intermediate outcomes between communication and distress, such as information obtained, satisfaction with the explanation, and nodule care/clinician variables, in the adjusted model.
We performed multiple sensitivity analyses. First, we used linear regression to measure univariate associations by using continuous outcome measures of risk and distress. Second, we performed an analysis with communication measured continuously for the association with distress. Third, because our outcome of distress was common, we used generalized linear model regression to estimate the univariate relative risks. Because of our relatively small sample size, we could not measure the multivariable-adjusted relative risk of communication with distress.
The patient-centered communication theoretic model emphasizes adequate information exchange as a core domain, and we hypothesized that the association of communication quality with distress may be modified by the assessment of adequate information, that is, perceived risk of lung cancer. We assessed the association between general communication quality and distress for effect modification by this variable. Likelihood ratio tests were conducted to assess the interaction between communication and distress and the risk perception subgroup. P values for interaction were obtained to compare the fit of the models with and without the interaction terms.
All tests were two tailed using robust standard errors to minimize assumptions about equal variance, and a P value of less than 0.05 was considered statistically significant. Analyses were conducted using Stata/IC 11.0 (StataCorp, College Station, TX).
Results
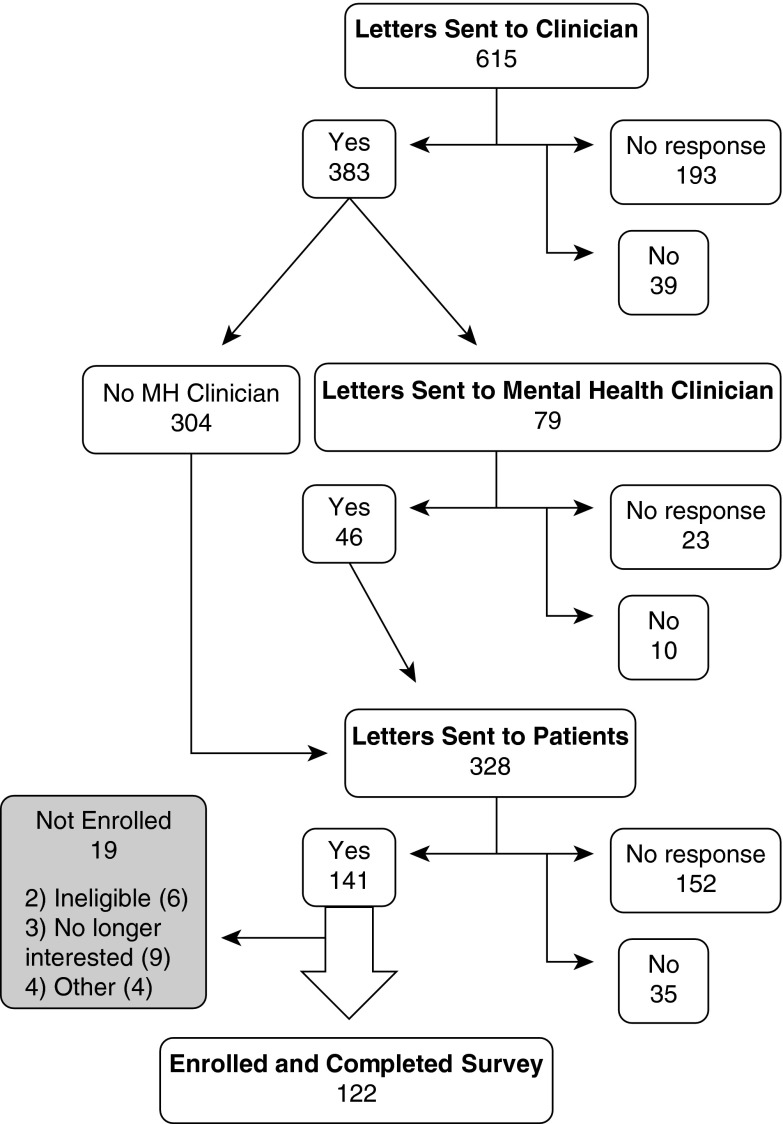
We collected data from 122 participants out of 328 contacted Veterans (response rate = 37%; 20% of total potentially eligible; Figure 1). Few clinicians or patients actively declined to participate, and the most common reason for nonparticipation was inability to contact the clinician or patient (classified as “no response” in Figure 1). Participants were mostly older men who often had concomitant self-reported depression and PTSD diagnoses (Table 1). The average nodule size was 6 mm (SD = 3 mm; range = 2–18 mm). Distribution of the nodule size was: 50 nodules of 4 mm or less, 42 nodules of greater than 4–6 mm, 19 nodules greater than 6–8 mm, and 11 nodules greater than 8 mm.
Figure 1.
Flow chart of patient enrollment. MH = mental health.
Table 1.
Cohort characteristics
| |
n (%) or Mean ± SD |
|---|---|
| Characteristic | (n = 122) |
| Age, yr | 64.1 ± 8.7 |
| Sex (% male) | 114 (93) |
| Socioeconomic status | |
| Education (≥college) | 77 (63) |
| Currently married | 64 (53) |
| Income (≥$30k/yr) | 70 (61) |
| Smoking status | |
| Never | 27 (22) |
| Past | 58 (48) |
| Current | 37 (30) |
| Mental health | |
| Depression (self-reported) | 60 (49) |
| PTSD (self-reported) | 39 (32) |
| Nodule/imaging characteristics | |
| Nodule size, mm | 5.8 ± 2.7 |
| Location in upper lobe | 46 (41) |
| Spiculated nodule | 2 (2) |
| Symptomatic indication* | 99 (82) |
| Detection to study visit, d | 122 ± 76 |
| Nodule information processes/satisfaction | |
| How the participant was informed | |
| Letter | 62 (52) |
| Visit | 32 (27) |
| Phone | 26 (22) |
| Who informed the participant | |
| Primary care provider | 41 (34)† |
| Research | 44 (36) |
| Other | 37 (30) |
| How informed about what a nodule is | |
| Not at all informed | 39 (32) |
| Somewhat informed | 65 (54) |
| Very informed | 14 (12) |
| Unknown | 3 (2) |
| How satisfied with clinician’s explanation | |
| Not at all satisfied | 7 (6) |
| Somewhat satisfied | 23 (19) |
| Very satisfied | 32 (26) |
| Unknown | 21 (17) |
| Missing | 39 (32) |
| Nodule care | |
| Satisfied with care | 78 (69) |
| Care could not be better | 24 (21) |
| Clinician is very skilled/knowledgeable | 21 (17) |
| Participant-reported risk of lung cancer | |
| ≤30% | 50 (41) |
| >30% | 56 (46) |
| Missing | 16 (13) |
| Average self-reported risk of lung cancer | 39% ± 26% |
| Participant’s assessment of the clinician-estimated risk of lung cancer | |
| ≤30% | 36 (30) |
| >30% | 45 (37) |
| Missing | 41 (34) |
| Average calculated risk of lung cancer (Mayo model) | 10% ± 12% |
| Communication processes | |
| Low-quality communication | 28 (23) |
| High-quality communication | 68 (56) |
| Missing quality communication | 26 (21) |
| Average communication quality score (lower scores better; max score = 7) | 3.0 ± 1.9 |
| Distress‡ | |
| No distress | 59 (48) |
| At least mild distress | 48 (39) |
| Missing distress | 15 (12) |
| Average distress score | 12.8 ± 15.1 |
Definition of abbreviation: PTSD = post-traumatic stress disorder.
Percentages may not add to 100% secondary to rounding; percentages are of nonmissing data unless there were more than 10% missing data, in which case this category is included.
For example, nodules found on a computed tomography (CT) performed for cough or hemoptysis, for example, were categorized as “symptomatic.” If a chest X-ray for cough noted a small nodule and subsequent CT was performed for nodule visualization, not the cough, this was categorized as “asymptomatic.”
A total of 28 unique primary care providers.
Distress measured on Impact of Event Scale with scores ≥8 classified as mild or greater distress.
Information Obtained
Veterans participated only if their clinician gave assent and indicated that the patient was informed about the nodule. Over one-third (36%) of participants could not recall receiving this information before our contact, and classified as receiving notification from the research staff (Table 1). These participants were still surveyed about their reactions and communication about the nodule after our initial contact.
Participants often reported being ill informed about their nodule, and only 12% felt very informed. They were more often satisfied with their clinicians’ explanation of the nodule, although 32% could not answer this question, as they felt they had not received any explanation, and 17% reported this as unknown, as they felt they had no basis for comparison to report satisfaction.
Although 13% of participants could not estimate a risk that the nodule was lung cancer, on average, they reported a 39% risk of malignancy (SD = 26%), and 46% of the participants reported a risk greater than 30%. Average calculated risk using the Mayo model was 10% (SD = 12%).
Distress
As a result of the nodule diagnosis, 23%, 12%, and 4% of participants reported mild, moderate, and severe distress, respectively. Fifteen participants (12%) felt unable to report on their level of distress, most often because they could not recall previously being informed about the nodule. Of the participants who reported, the average score on the IES was 13 (SD = 15; Table 1).
Table 2 shows the univariate associations of each characteristic with at least mild distress. Table E1 reports the cohort characteristics stratified by distress. Notably, neither a self-perceived risk of lung cancer over 30% (OR = 1.73, 95% CI = 0.74–4.03, P = 0.21) nor inability to estimate that risk (OR = 2.31, 95% CI = 0.71–7.51, P = 0.16) were associated with distress. Characteristics associated with known risk for malignancy, such as increased age, current or past history of smoking, and nodule size, were either not associated with increased distress or inversely associated. Mental health comorbidities of self-reported depression and PTSD were strongly associated with increased distress (OR = 8.31, 95% CI = 3.44–20.07, P < 0.001 and OR = 3.26, 95% CI = 1.40–7.54, P = 0.006, respectively). The mode of communication and the role of the clinician who notified the participant were not associated with distress. Higher satisfaction with the clinician’s explanation and reporting that care could not be better were associated with decreased distress.
Table 2.
Univariate associations with at least mild distress and a participant-reported risk of lung cancer greater than 30%
| |
Distress ≥Mild* |
Participant-Reported Risk of Lung Cancer >30% |
|---|---|---|
| Characteristic | OR (95% CI) | OR (95% CI) |
| Age (per year) | 0.93 (0.87–1.00)† | 0.99 (0.94–1.03) |
| Sex (male) | 1.39 (0.31–6.18) | 0.83 (0.18–3.93) |
| Socioeconomic status | ||
| Education (≥college) | 1.37 (0.62–3.04) | 0.52 (0.23–1.17) |
| Currently married | 1.01 (0.47–2.18) | 0.81 (0.38–1.76) |
| Income (≥$30k/yr) | 0.72 (0.31–1.63) | 0.81 (0.36–1.85) |
| Smoking status | ||
| Never | Ref | Ref |
| Past | 0.46 (0.17–1.23) | 1.80 (0.65–5.02) |
| Current | 0.43 (0.15–1.26) | 4.79 (1.50–15.27)† |
| Mental health | ||
| Depression (self-reported) | 8.31 (3.44–20.07)† | 1.00 (0.46–2.15) |
| PTSD (self-reported) | 3.26 (1.40–7.54)† | 0.42 (0.18–0.97)† |
| Nodule/imaging characteristics | ||
| Nodule size (in mm) | 1.02 (0.89–1.18) | 1.31 (1.08–1.59)† |
| Location in upper lobe | 1.14 (0.51–2.55) | 1.41 (0.62–3.20) |
| Symptomatic indication‡ | 3.00 (1.00–9.03)† | 0.80 (0.29–2.19) |
| Detection to visit (per days) | 1.00 (0.99–1.00) | 1.00 (1.00–1.00) |
| Nodule information processes/satisfaction | ||
| How the participant was informed | ||
| Letter | Ref | Ref |
| Visit | 0.41 (0.14–1.23) | 1.57 (0.62–4.00) |
| Phone | 0.76 (0.30–1.95) | 0.96 (0.36–2.55) |
| Who informed the participant | ||
| Primary care provider | Ref | Ref |
| Research | 1.09 (0.42–2.78) | 0.48 (0.19–1.23) |
| Other | 0.77 (0.31–1.93) | 0.63 (0.23–1.69) |
| How informed about what a nodule is | ||
| Not at all informed | Ref | Ref |
| Somewhat informed | 1.38 (0.57–3.33) | 1.42 (0.59–3.37) |
| Very informed | 0.77 (0.21–2.86) | 0.66 (0.18–2.48) |
| How satisfied with clinician’s explanation | ||
| Not at all satisfied | Ref | Ref |
| Somewhat satisfied | 0.22 (0.02–2.13) | 1.33 (0.11–16.78) |
| Very satisfied | 0.10 (0.01–0.95)† | 0.31 (0.03–3.42) |
| Unknown | 0.06 (0.01–0.68)† | 0.24 (0.02–2.83) |
| Nodule care | ||
| Satisfied w/ care | 0.40 (0.17–0.98)† | 0.91 (0.38–2.20) |
| Care could not be better | 0.26 (0.09–0.78)† | 0.85 (0.33–2.22) |
| Clinician is very skilled/knowledgeable | 1.15 (0.44–3.00) | 4.21 (1.28–13.78)† |
| Participant-reported risk of lung cancer | ||
| ≤30% | Ref | NA |
| >30% | 1.73 (0.74–4.03) | NA |
| Missing | 2.31 (0.71–7.51) | NA |
| Communication processes | ||
| High-quality communication | 0.45 (0.18–1.15) | 0.97 (0.37–2.51) |
| Communication quality (for every 1 point improvement) | 0.82 (0.65–1.04) | 0.83 (0.67–1.05) |
Definition of abbreviations: CI = confidence interval; NA = not applicable; OR = odds ratio; PTSD = post-traumatic stress disorder.
Distress measured on Impact of Event Scale with scores ≥8 classified as mild or greater distress.
P < 0.05.
Nodules found on a computed tomography (CT) performed for cough or hemoptysis, for example, were categorized as “symptomatic.” If a chest X-ray for cough noted a small nodule and subsequent CT was performed for nodule visualization, not the cough, this was categorized as “asymptomatic.”
Patient-Centered Communication and Distress
Quality of communication was not associated with distress (OR = 0.45, 95% CI = 0.18–1.15, P = 0.10) on univariate analysis, but, in multivariable-adjusted analysis, high-quality communication was associated with decreased distress (OR = 0.28, 95% CI = 0.08–1.00, P = 0.05). Among participants who reported a risk of lung cancer to 30% or less, high-quality communication was associated with decreased distress (OR = 0.15 (95% CI = 0.02–0.92 P = 0.04), but was not associated with distress for those who reported a risk of greater than 30% (OR = 0.12, 95% CI = 0.00–3.97, P = 0.24). The P value for interaction was 0.14.
Risk of Lung Cancer
Table 2 shows the univariate associations of the patient, nodule, and communication characteristics with a greater than 30% self-reported risk that the pulmonary nodule was lung cancer. Table E1 reports the cohort characteristics stratified by perceived risk. Known predictors of malignancy, such as current smoking status and nodule size, were associated with increased perceived risk (OR = 4.79, 95% CI = 1.50–15.27, P = 0.008 and OR = 1.31, 95% CI = 1.08–1.59, P = 0.007, respectively). PTSD was associated with a decreased self-reported risk (OR = 0.42, 95% CI = 0.18–0.97, P = 0.04), and reporting that the clinician was very skilled/knowledgeable at caring for patients with pulmonary nodules was associated with increased risk perception (OR = 4.21, 95% CI = 1.28–13.78, P = 0.02).
Sensitivity Analyses
The direction and significance of the univariate associations reported in Table 2 were not appreciably different when we analyzed distress and perceived risk of lung cancer measured continuously. Similarly, the analysis of the association of communication measured continuously with distress did not appreciably alter the direction or significance of the association. Finally, the direction and significance of the univariate associations between distress and participant-perceived risk of lung cancer were similar when measured as relative risks, although, as expected, the magnitude of those associations decreased.
Seeking Information
Table 3 lists reasons why participants did not seek more information and suggestions for improving communication processes. Among the participants who did not seek more information, the most common reason was satisfaction with the information already received. Other commonly reported reasons included that they had more important problems to worry about, the clinician had not informed them about the nodule, and the clinician was not worried, so they were not worried. In terms of potential changes to communication processes, one participant (1%) felt nothing could be improved. A majority of participants wanted more information about the frequency of nodule detection, more information about the causes of nodules, more discussion with the clinician about the patients’ worries and fears, more information about the risk of lung cancer, and to always be informed in person.
Table 3.
Information seeking and communication recommendations
| |
Times Reported |
|---|---|
| n (%) | |
| Reasons for not seeking more information from the clinician (n = 85) | |
| Satisfaction with the information previously provided | 24 (28) |
| There were other/more important problems to worry about | 13 (15) |
| My clinician did not inform me about my nodule diagnosis | 12 (14) |
| My clinician didn’t seem worried about it so I wasn’t | 11 (13) |
| I had a visit scheduled with my clinician already | 10 (12) |
| I didn’t think about seeking more information | 7 (8) |
| I wasn’t worried or concerned about the diagnosis | 6 (7) |
| I thought my clinician would call | 5 (6) |
| I assumed my clinician was too busy to answer my questions | 4 (5) |
| I don’t know how to contact my clinician | 3 (4) |
| I have had previous experience with my clinician not calling me back | 3 (4) |
| I was scared of the possible answer | 1 (1) |
| Other responses | 15 (18) |
| Recommendations for improving communication processes (n = 121) | |
| More information about how common nodules are | 112 (93) |
| More information about what a nodule is | 108 (89) |
| More discussion regarding patients’ worries and fears | 90 (74) |
| More information about the risk of lung cancer | 85 (70) |
| Tell the patient in person about their nodule diagnosis | 85 (70) |
| Decrease the time interval from imaging study to information about results | 78 (66) |
| More patient involvement in determining the follow-up interval | 72 (60) |
| More information about the risks of radiation from planned computed tomography scans | 67 (55) |
| More information about everything, in letter or phone call (including: how to contact clinician, explanation of follow-up plan, other resources, etc.) | 17 (14) |
| Ensure clinician is being honest and giving facts | 11 (9) |
| More interaction with the clinician regarding nodule | 11 (9) |
| Show pictures of scan and explain image | 6 (5) |
| Other responses | 17 (14) |
Discussion
We found that Veterans with incidental pulmonary nodules frequently reported inadequate information exchange regarding their nodules. Almost half experienced at least mild distress. Many Veterans substantially overestimated their risk of lung cancer. Interestingly, current smoking status and nodule size were associated with increased perceived risk, but these characteristics and self-perceived risk itself were not associated with increased distress. High-quality communication was associated with decreased distress that was not modified by self-perceived risk.
Similar to our previous qualitative findings among Veterans and non-Veterans with incidental pulmonary nodules (17–19), we found that information exchange was often inadequate and patients frequently experienced distress. That work suggested that self-perceived risk of lung cancer influenced distress, but that finding was not substantiated in this study. Communication quality was associated with distress only among participants with a lower self-perceived risk of lung cancer, but the P value for interaction was nonsignificant. Thus, more study is necessary to determine if self-perceived risk, a surrogate of adequate information exchange, actually acts as an effect modifier of this association.
A core component of patient-centered communication processes is accurate information exchange about the diagnosis and risks and benefits of treatment options (29) that includes plain language, describes absolute instead of relative risks, and uses tables and graphs (30, 31). In this study and our previous work, almost all patients reported not receiving adequate information about the risk of lung cancer, and most substantially overestimated this risk (17–19). With regard to pulmonary nodules, accurate understanding of the risk of lung cancer guides subsequent decisions (32), and may impact patients’ understanding and willingness to pursue active surveillance approaches, as widely recommended for small nodules (33, 34). Adherence to recommended follow up, and thus differences in resource utilization, may be influenced by appropriate information exchange.
In addition to information exchange, high-quality patient-centered communication includes other core processes, such as discussion of individual values and preferences, engaging in shared decision making, and ensuring a therapeutic alliance (20). Given the inadequacy of information exchange, it was surprising that many participants still reported overall high-quality communication. This discrepancy may indicate that clinicians in general had demonstrated high quality in the noninformational aspects of communication processes and/or possible ceiling effects of our communication measures.
Adequate understanding may not, by itself, influence other patient-centered outcomes without attention to these other core communication processes. For example, a report from the Nederlands-Leuvens Longkanker Screenings Onderzoek (NELSON) trial that found that participants’ knowledge about screening, which was in general higher than nonparticipants (35), was not associated with patient-centered outcomes (36). Our results also suggest that risk perception may not directly influence distress. The U.S. Preventive Services Task Force and others recommend using formal tools to inform patients considering screening about the potential risks and benefits (12, 37). Utilization of such tools may be helpful, but attention to shared decision making and consideration of patients’ values and preferences may be equally, if not more, important.
Patient-centered outcomes have been evaluated among lung cancer screening trial participants, but not for patients in routine care settings (15–19). Although participants in screening trials experience increased distress as a result of nodule detection, they are unlikely representative of patients in routine care, particularly in terms of sociodemographic and psychosocial characteristics (38, 39). In addition, communication processes in trials are different than in routine care. Communication strategies are widely recognized and recommended as important mechanisms that influence patient-centered outcomes (40–42), and it will be important to continue to evaluate these strategies as screening is widely implemented. Given the strong associations between mental health conditions and increased distress that we observed, targeted communication strategies should be considered for these patients. Similarly, patients found to have larger (>8 mm) nodules may need different communication strategies, as these patients were not well represented in our cohort.
Our results have several potential limitations. First, previous qualitative results from this cohort were similar to a non-Veteran cohort from the northeastern United States, but it is not clear if our findings are generalizable to other care settings, such as for patients with nodules larger than 8 mm or those undergoing lung cancer screening. Second, this analysis is cross-sectional, so additional research is required to determine the direction of the association between communication and distress. Third, many participants were unable to report on risk of lung cancer and other variables, often as a result of inadequate communication with their clinicians. In addition, only 62% of clinicians gave us permission to discuss the study with their patients. Finally, ORs may not accurately reflect the magnitude of relative risk when an outcome is common, as in this case. Thus, changes in communication scores may not be associated with as large a change in distress as might otherwise be expected.
Conclusions
Many patients experience distress from incidental pulmonary nodule detection and seldom report receiving adequate information. With inadequate information on the risk of lung cancer, many overestimate this risk. Neither the perceived risk of lung cancer nor factors associated with increased risk were associated with increased distress, which suggests that communication strategies that target improved knowledge may not be sufficient to reduce the distress of nodule detection. Communication strategies may also need to include consideration of patients’ values and preferences, and attention to shared decision-making processes. Clinicians and healthcare systems should consider implementing processes that include all the domains of high-quality, patient-centered communication.
Footnotes
Supported by a Department of Veterans Affairs (VA) Health Services Research and Development Career Development Award CDP 11-227 (C.G.S.), and by resources from the Portland VA Medical Center, Portland, Oregon, the Puget Sound VA Healthcare System, Seattle, Washington, and the Edith Nourse Rogers Memorial VA Hospital, Bedford, Massachusetts.
The Department of Veterans Affairs did not have a role in the conduct of the study, in the collection, management, analysis, interpretation of data, or in the preparation of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the U.S. Government.
Author Contributions: All authors have made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; contributed to drafting the article for important intellectual content; and provided final approval of the version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ost D, Fein AM, Feinsilver SH. Clinical practice: the solitary pulmonary nodule. N Engl J Med. 2003;348:2535–2542. doi: 10.1056/NEJMcp012290. [DOI] [PubMed] [Google Scholar]
- 2.Holden WE, Lewinsohn DM, Osborne ML, Griffin C, Spencer A, Duncan C, Deffebach ME. Use of a clinical pathway to manage unsuspected radiographic findings. Chest. 2004;125:1753–1760. doi: 10.1378/chest.125.5.1753. [DOI] [PubMed] [Google Scholar]
- 3.Rao VM, Levin DC. The overuse of diagnostic imaging and the Choosing Wisely initiative. Ann Intern Med. 2012;157:574–576. doi: 10.7326/0003-4819-157-8-201210160-00535. [DOI] [PubMed] [Google Scholar]
- 4.Rao VM, Levin DC, Parker L, Frangos AJ, Sunshine JH. Trends in utilization rates of the various imaging modalities in emergency departments: nationwide Medicare data from 2000 to 2008. J Am Coll Radiol. 2011;8:706–709. doi: 10.1016/j.jacr.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Meer AB, Basu PA, Baker LC, Atlas SW. Exposure to ionizing radiation and estimate of secondary cancers in the era of high-speed CT scanning: projections from the Medicare population. J Am Coll Radiol. 2012;9:245–250. doi: 10.1016/j.jacr.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Smith-Bindman R, Miglioretti DL, Johnson E, Lee C, Feigelson HS, Flynn M, Greenlee RT, Kruger RL, Hornbrook MC, Roblin D, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996–2010. JAMA. 2012;307:2400–2409. doi: 10.1001/jama.2012.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, Byers T, Colditz GA, Gould MK, Jett JR, et al. Benefits and harms of CT screening for lung cancer: a systematic reviewbenefits and harms of CT screening for lung cancer. JAMA. 2012;307:1–12. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaklitsch MT, Jacobson FL, Austin JH, Field JK, Jett JR, Keshavjee S, MacMahon H, Mulshine JL, Munden RF, Salgia R, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144:33–38. doi: 10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. NCCN guidelines on lung cancer screening [accessed 2012 Jun 27]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf
- 10.Samet JM, Crowell R, San Jose Estepar R, Rand CS, Rizzo AA, Yung R.American Lung Association. Providing guidance on lung cancer screening to patients and physicians. 2012[accessed 2012 Jun 27]. Available from: http://www.lung.org/lung-disease/lung-cancer/lung-cancer-screening-guidelines/lung-cancer-screening.pdf
- 11.Wender R, Fontham ET, Barrera E, Jr, Colditz GA, Church TR, Ettinger DS, Etzioni R, Flowers CR, Gazelle GS, Kelsey DK, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63:107–117. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moyer VA. U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 13.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris RP, Sheridan SL, Lewis CL, Barclay C, Vu MB, Kistler CE, Golin CE, DeFrank JT, Brewer NT. The harms of screening: a proposed taxonomy and application to lung cancer screening. JAMA Intern Med. 2014;174:281–285. doi: 10.1001/jamainternmed.2013.12745. [DOI] [PubMed] [Google Scholar]
- 15.Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, Zakher B, Fu R, Slatore CG. Screening for lung cancer with low-dose computed tomography: a systematic review to update the U.S. Preventive Services Task Force recommendation. Ann Intern Med. 2013;159:411–420. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 16.Slatore CG, Sullivan DR, Pappas M, Humphrey LL. Patient-centered outcomes among lung cancer screening recipients with computed tomography: a systematic review. J Thorac Oncol. 2014;9:927–934. doi: 10.1097/JTO.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slatore CG, Press N, Au DH, Curtis JR, Wiener RS, Ganzini L. What the heck is a “nodule”? A qualitative study of veterans with pulmonary nodules. Ann Am Thorac Soc. 2013;10:330–335. doi: 10.1513/AnnalsATS.201304-080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA. What do you mean, a spot?: a qualitative analysis of patients’ reactions to discussions with their physicians about pulmonary nodules. Chest. 2013;143:672–677. doi: 10.1378/chest.12-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA.‘The thing is not knowing’: patients’ perspectives on surveillance of an indeterminate pulmonary nodule Health Expect 2012. Dec 16;doi: 10.1111/hex.12036 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 20.Brown J, Stewart M, Tessier S.Assessing communication between patients and doctors: a manual for scoring patient centred communication London: Thames Valley Family Practice Research Unit; 1995. Working Paper Series 952 [Google Scholar]
- 21.Tariq SH, Tumosa N, Chibnall JT, Perry MHI, Morley JE. Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14:900–910. doi: 10.1097/01.JGP.0000221510.33817.86. [DOI] [PubMed] [Google Scholar]
- 22.Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: a systematic review of assessment instruments. J Natl Cancer Inst. 2009;101:1464–1488. doi: 10.1093/jnci/djp336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joseph S. Psychometric evaluation of Horowitz’s impact of event scale: a review. J Trauma Stress. 2000;13:101–113. doi: 10.1023/A:1007777032063. [DOI] [PubMed] [Google Scholar]
- 24.Byrne MM, Weissfeld J, Roberts MS. Anxiety, fear of cancer, and perceived risk of cancer following lung cancer screening. Med Decis Making. 2008;28:917–925. doi: 10.1177/0272989X08322013. [DOI] [PubMed] [Google Scholar]
- 25.Lee SJ, Fairclough D, Antin JH, Weeks JC. Discrepancies between patient and physician estimates for the success of stem cell transplantation. JAMA. 2001;285:1034–1038. doi: 10.1001/jama.285.8.1034. [DOI] [PubMed] [Google Scholar]
- 26.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules: application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157:849–855. [PubMed] [Google Scholar]
- 27.Hudon C, Fortin M, Haggerty JL, Lambert M, Poitras ME. Measuring patients’ perceptions of patient-centered care: a systematic review of tools for family medicine. Ann Fam Med. 2011;9:155–164. doi: 10.1370/afm.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slatore CG, Feemster LC, Au DH, Engelberg RA, Curtis JR, Uman J, Reinke LF. Which patient and clinician characteristics are associated with high-quality communication among veterans with chronic obstructive pulmonary disease? J Health Commun. 2014;19:907–921. doi: 10.1080/10810730.2013.864732. [DOI] [PubMed] [Google Scholar]
- 29.Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Soc Sci Med. 2000;51:1087–1110. doi: 10.1016/s0277-9536(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 30.Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103:1436–1443. doi: 10.1093/jnci/djr318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zipkin DA, Umscheid CA, Keating NL, Allen E, Aung K, Beyth R, Kaatz S, Mann DM, Sussman JB, Korenstein D, et al. Evidence-based risk communication: a systematic reviewe. Ann Intern Med. 2014;161:270–280. doi: 10.7326/M14-0295. [DOI] [PubMed] [Google Scholar]
- 32.Ost DE, Gould MK. Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med. 2012;185:363–372. doi: 10.1164/rccm.201104-0679CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, Naidich DP, Patz EF, Jr, Swensen SJ Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237:395–400. doi: 10.1148/radiol.2372041887. [DOI] [PubMed] [Google Scholar]
- 34.Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, Wiener RS. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):e93S–120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Bergh KAM, Essink-Bot ML, van Klaveren RJ, de Koning HJ. Informed participation in a randomised controlled trial of computed tomography screening for lung cancer. Eur Respir J. 2009;34:711–720. doi: 10.1183/09031936.00098908. [DOI] [PubMed] [Google Scholar]
- 36.van den Bergh KA, Essink-Bot ML, van Klaveren RJ, de Koning HJ. Informed decision making does not affect health-related quality of life in lung cancer screening (NELSON trial) Eur J Cancer. 2010;46:3300–3306. doi: 10.1016/j.ejca.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 37.Woloshin S, Schwartz LM, Black WC, Kramer BS. Cancer screening campaigns—getting past uninformative persuasion. N Engl J Med. 2012;367:1677–1679. doi: 10.1056/NEJMp1209407. [DOI] [PubMed] [Google Scholar]
- 38.Aberle DR, Adams AM, Berg CD, Clapp JD, Clingan KL, Gareen IF, Lynch DA, Marcus PM, Pinsky PF National Lung Screening Trial Research Team. Baseline characteristics of participants in the randomized national lung screening trial. J Natl Cancer Inst. 2010;102:1771–1779. doi: 10.1093/jnci/djq434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hestbech MS, Siersma V, Dirksen A, Pedersen JH, Brodersen J. Participation bias in a randomised trial of screening for lung cancer. Lung Cancer. 2011;73:325–331. doi: 10.1016/j.lungcan.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 40.Epstein RM, Street RLJ.Patient-centered communication in cancer care: promoting healing and reducing suffering Bethesda, MD: National Cancer Institute; 2007. NIH publication no. 07-6225 [Google Scholar]
- 41.Committee on Quality of Health Care in America. Washington, D.C.: National Academies Press; 2001. Crossing the quality chasm: a new health system for the 21st century. [PubMed] [Google Scholar]
- 42.Washington, D.C.: Presidential Commission for the Study of Bioethical Issues; 2013. Anticipate and communicate: ethical management of incidental and secondary findings in the clinical, research, and direct-to-consumer contexts. [DOI] [PubMed] [Google Scholar]