Abstract
Brain stimulation, in the form of electroconvulsive therapy (ECT), has long been a gold standard treatment for depression, but today, the field of neuromodulation is rapidly changing with the advent of newer and more precise tools to alter neuroplasticity and to treat brain-based disorders. Now there are new means to induce focal seizures, as with magnetic seizure therapy (MST), or modifications to ECT. There are also surgical approaches to target brain circuits via implanted stimulators placed in the brain or on cranial nerves. Finally, there are noninvasive subconvulsive approaches for the transcranial application of either electric or magnetic fields. Collectively, these tools have transformed the face of neurotherapeutics and informed our understanding of the brain basis of complex neurobehavioral conditions.
Introduction
Major depressive disorder is a leading public health problem that contributes to significant morbidity and mortality, affecting approximately 14 million American adults each year [1]. Epidemiological surveys estimated that depression-related absenteeism and low work performance costs US workplaces $52 billion [2]. Presently, the first line management of depression consists of antidepressant medications and/or psychotherapy. However, as found in the landmark Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, 63% of patients failed to achieve remission with their first antidepressant medication and despite subsequent antidepressant augmentations or switches, 33% failed to achieve remission [3]. For treatment-resistant depression patients, various neuromodulation approaches are currently in use or in development.
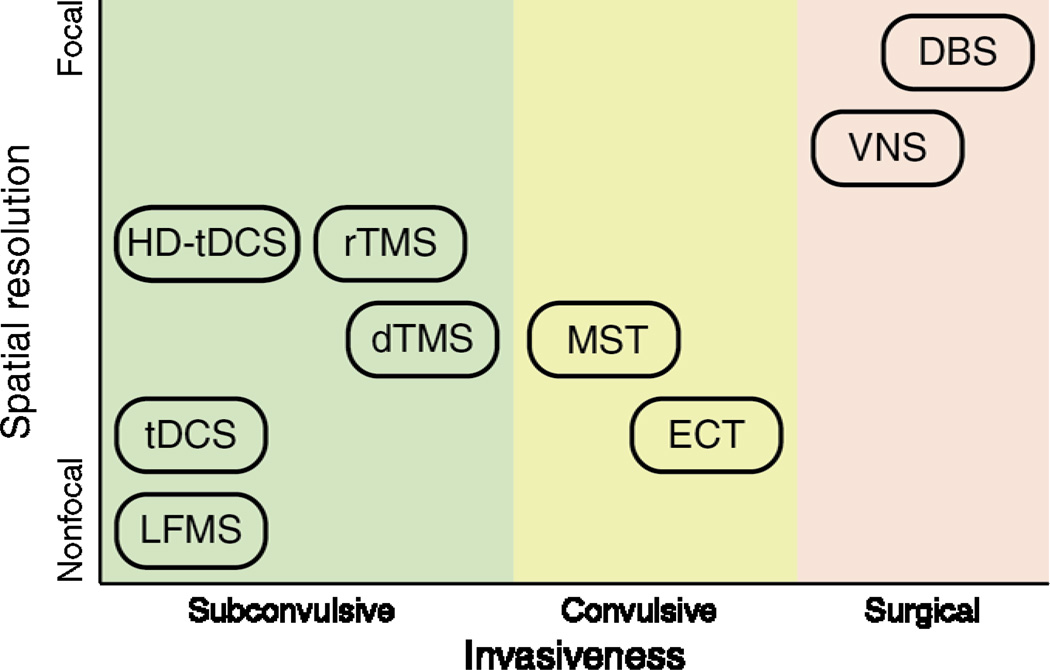
Neuromodulation approaches encompass a wide spectrum of strategies that can be categorized into convulsive, subconvulsive, and neurosurgical implantation therapies. The convulsive therapies involve the induction of a therapeutic generalized seizure in patients under anesthesia, either via the direct injection of electrical currents through scalp electrodes, as in the case of electroconvulsive therapy (ECT), or via electromagnetic induction, as in the case of magnetic seizure therapy (MST). The therapeutic mechanism is hypothesized to be related to the nature of the induced seizure, although there is evidence that stimulus parameters, and hence the induced electric field, may also effect clinical outcome. Subconvulsive therapies involve the application of electromagnetic fields at levels below seizure threshold. Such subconvulsive therapies include transcranial direct current stimulation (tDCS), high definition-transcranial direct current stimulation (HD-tDCS) transcranial magnetic stimulation (TMS), repetitive TMS (rTMS), deep TMS (dTMS), and low field magnetic stimulation (LFMS). The neurosurgical implantation therapies involve the implantation of battery-powered devices to deliver chronic or intermittent electrical stimulation, such as deep brain stimulation (DBS) and vagus nerve stimulation (VNS). Figure 1 summaries the spatial resolution and invasiveness profile of the various neuromodulation therapies. This review describes salient features and the state of the art of each intervention.
Figure 1.
Spatial resolution and invasiveness profile of various neuromodulation therapies for mood disorders. Subconvulsive therapies include transcranial direction current stimulation (tDCS), high definition-transcranial direct current stimulation (HD-tDCS), low field magnetic stimulation (LFMS), repetitive transcranial magnetic stimulation (rTMS), and deep transcranial magnetic stimulation (dTMS). Convulsive therapies include magnetic seizure therapy (MST) and electroconvulsive therapy (ECT). Neurosurgical therapies include vagus nerve stimulation (VNS) and deep brain stimulation (DBS).
Convulsive therapies
Electroconvulsive therapy (ECT)
Electroconvulsive therapy is administered by delivering electricity directly to the brain via scalp electrodes to induce a generalized tonic-clonic seizure. Modern ECT consists of delivering constant current (800 or 900mA) rectangular pulses with alternating polarity. Pulse widths in the 0.5–2 ms range are referred to as “brief”, and pulse widths < 0.5 ms are termed “ultrabrief”. The practice of ECT dates back to the 1930’s, and its modern version is considered the “gold standard” brain stimulation treatment for severe major depressive disorder. It is estimated that 1–2 million individuals receive ECT each year worldwide and its use has increased over the years [4]. The speed of response and clinical efficacy with ECT are unparalleled, with a remission rate of approximately 70% [5]. The adverse cognitive effects of ECT; however, substantially reduce its tolerability. Some of the adverse cognitive effects include post-ictal disorientation, learning and memory difficulties, and retrograde amnesia [6].
Among the approaches to mitigate side effects are alterations of the dosing of the electrical stimulus, which includes electrode placement, pulse amplitude, shape, and width, and pulse train frequency, duration, directionality and polarity. Ultrabrief pulse ECT has been shown to significantly reduce cognitive side effects while maintaining efficacy [7]. The geometry of the ECT electrodes and their placement on the patient’s head, as well the head anatomy, determine the spatial distribution of the induced electric field in the brain [8]. Electrode configuration plays a critical role in the therapeutic action and adverse effects of ECT. Sackeim et al. showed in a series of studies that right unilateral electrode placement, combined with proper stimulus dosing, can be as effective as bilateral ECT while having more benign cognitive side effects [9]. In a recent feasibility study with a novel electrode configuration, focal electrically administered seizure therapy (FEAST) demonstrated tolerability and clinically meaningful antidepressant improvement [10]. Refinements of electrode configuration are key to maximizing benefits of ECT, and remains an area of active study. Work on novel electrode placements for ECT may be informed by computational modeling of the electric field distributions in the brain [11,12], such that dosing paradigms could be designed to target brain regions implicated in depression while avoiding those associated with adverse side effects. Another aspect of the ECT stimulus that could be further optimized is the pulse current amplitude [8]. High current amplitude used in conventional ECT devices exposes the entire brain to suprathreshold stimulation that far exceeds neural activation threshold [8]. This may contribute to the cognitive side effects of ECT. Further, the seizure threshold titration procedure for individualizing ECT dosage adjusts only the duration of the stimulus pulse train, which unlike amplitude, are unrelated to the spatial distribution of the induced electric field. Thus, conventional titration procedures do not compensate for anatomy-dependent variability in the strength and focality of stimulation [13]. Thus, individualized low current amplitude has been proposed as a means to compensate for anatomical variability and improve stimulation focality (e.g., reducing the level of direct electrical stimulation in the hippocampus) [12].
Magnetic seizure therapy (MST)
Magnetic seizure therapy involves inducing a series of generalized seizures under anesthesia using high-dose repetitive transcranial magnetic stimulation (rTMS) [14]. Evidence shows that the intracerebral electric field induced by ECT is unfocal and variable due to the high electrical impedance of the skull, current shunting in the scalp, and variation of head tissue anatomy [15]. On the other hand, MST offers greater control of the induced electric field due to the lack of impedance of brain tissue to magnetic fields. In vivo studies in preclinical studies with nonhuman primates demonstrated the safety of MST and supported the hypothesis that the MST-induced current and resultant seizure are more focal than ECT [16]. This enhanced control represents a means to focus the treatment in targeted cortical structures thought to mediate antidepressant effects and to reduce spread to medial temporal structures implicated in the amnestic side effects of ECT. Studies support the safety of MST [17] and provide open-label evidence of antidepressant action. Clinical trials in human have suggested comparable efficacy of MST and ECT [18], and further research is warranted. A recently completed multi-center, randomized, controlled trial that contrasted the safety and efficacy MST and ultrabrief pulse width right unilateral ECT will provide further information.
Subconvulsive therapies
Transcranial magnetic stimulation
Transcranial magnetic stimulation is a non-invasive technique used for numerous research and therapeutic applications, including the study of normal and pathological brain function, and the treatment of neuropsychiatric diseases [19]. The technique involves the use of brief, intense pulses of electric current delivered to a coil placed on the subject’s head to generate an electric field in the brain via electromagnetic induction. The locus of activation in the brain is approximately in the area where the induced electrical field is maximal; this location, in turn, depends on the stimulating coil geometry and placement [20]. rTMS to left dorsolateral prefrontal cortex (DLPFC) was approved by the FDA for the treatment of major depression. However, the efficacy in the pivotal trial was modest (24% response, 14% remission at 6 weeks) [21]. Studies to date have targeted the DLPFC based on evidence of dysregulation in this region and connectivity between prefrontal cortex and the limbic system in depression [22]. However, the limited therapeutic effect size of rTMS to the DLPFC suggests that this region may not be the optimal target for depression, and has motivated the development of a TMS coil that could access deeper brain structures [23]. Work with deep TMS (dTMS) to date has demonstrated feasibility and safety [24] and significant antidepressant benefits which are double those reported with conventional rTMS, which led to its recent FDA approval as a treatment for depression. To date, only a limited range of TMS treatment parameters has been examined in clinical trials. Since neurophysiologic understanding of TMS on the neuronal level is limited in clinical studies, ongoing preclinical studies with TMS-compatible intracerebral recordings at single-neuron resolution will help inform the interactions between the induced electric field and neuronal response, which is fundamental to understanding mechanisms of action of TMS [25].
Low field magnetic stimulation (LFMS)
There is some evidence that the subthreshold electric field induced in the brain by the gradient coils in magnetic resonance imaging scanners can have mood altering properties. Thus, low field magnetic stimulation employs very weak magnetic fields applied uniformly throughout the brain. Research has suggested that LFMS influences glucose metabolism in the brain [26] and exerts antidepressant effects in humans [27]. A preclinical controlled also demonstrated an antidepressant-life effect of LFMS in a rodent model of learned helplessness [28]. The gradient coils used in LFMS studies induce a more diffuse electric field compared to TMS coils with comparable depth of field penetration [20]. Another LFMS technique called synchronized TMS (sTMS) employs rotating permanent magnets over a broad scalp area, with rotational frequency synchronized to the subject’s individual alpha frequency. A randomized, sham controlled, double-blind study showed that the sTMS approach relative to sham achieved greater reduction in depression severity [29].
Transcranial direct current stimulation (tDCS)
Transcranial direct current stimulation uses constant, low-amplitude direct current (typically 1–2 mA) delivered to the brain via scalp electrodes to modulate cortical excitability that has been used to treat depression as well as enhance neurocognitive performance. Preliminary antidepressant efficacy of tDCS is supported by two randomized, sham-controlled studies [30,31]. However, another study with similar parameters was unable to replicate the effect sizes [32]. Thus, the efficacy tDCS in treating mood disorders warrants further investigation since published data are inconsistent, and indeed there is a multi-center randomized controlled trial presently underway. There are also opportunities to refine the stimulation technique to improve its neuromodulation potency. Several computational studies have described novel tDCS electrode designs, such as high definition-tDCS [33], and electric field targeting strategy through multi-electrode optimization algorithms [34,35].
Neurosurgical implantation therapies
Deep brain stimulation (DBS)
Deep brain stimulation is a neurosurgical technique that involves the implantation of a pulse generator and electrodes to deliver chronic electrical stimulation to focal deep brain regions, which has provided therapeutic benefits for a variety of neurological and psychiatric conditions. As implicated in neuroimaging studies and theoretical pathways for depressive symptoms, a number of neuroanatomical targets have been proposed for the treatment of depression. Such targets include the subgenual cingulate cortex, nucleus accumbens, ventral caudate/ventral striatum, and lateral habenula [36]. For example, DBS of the white matter underlying the subgenual anterior cingulate cortex showed marked antidepressant effects in open-label studies of patients with severe treatment-resistant major depression [37]. Further, DBS was found to have no neurocognitive adverse effects in patients with depression who showed no change in general intellectual ability, processing speed, verbal and visual memory, or executive functions [38]. Technical innovations with DBS include battery power management through stimulation waveform optimization [39] and electrode design for more efficient neuromodulation [40]. To date, however, large randomized controlled trials seeking to demonstrate antidepressant efficacy did not lead to a positive result.
Vagus nerve stimulation (VNS)
Vagus nerve stimulation involves the surgical implantation of a pulse generator to deliver intermittent electrical stimulation to the left vagus nerve via helical electrodes. The use of VNS for both unipolar and bipolar depression was approved by the FDA for patients who failed to respond to at least four antidepressant trials. Although VNS showed no statistically significant acute antidepressant effect compared to sham treatment, there was a clinically meaningful antidepressant effect after 12 months of VNS [41], which suggests a potential long-term growing benefit of VNS. In terms of neurocognitive effects, patients receiving open-label VNS showed no impairment in the domains of motor speed, verbal fluency, attention, or memory, and showed improved performance on measures of psychomotor function, language, and executive function [42]. The mechanisms by which VNS modulates mood is not fully understood, but theories include alteration of norepinephrine by projections to the locus coeruleus, elevated levels of inhibitory GABA, and enhanced cortical inhibition [43].
Understanding biological mechanisms
As clinical neuromodulation becomes more sophisticated, efforts to elucidate inherent biological mechanisms underlying both therapeutic and adverse effects become more important in order to design safer and more effective interventions. Many different theories have been formulated to explain how ECT acts to modulate brain function that spans chemical, endocrinological, immunological, and electrophysiological frameworks. Two unifying yet seemingly contradictory hypotheses emerged: the anticonvulsant hypothesis which posited that hypoperfusion, decreased seizure duration and inhibition of neural activity were associated with therapeutic effects [44], and the neurotrophic effect hypothesis that postulated increased growth factors, transcription factors and neurogenesis accounted for the clinical benefits [45]. Longitudinal neuroimaging studies over the past decade have demonstrated support for both hypotheses, leading to the intriguing proposal that an initial hypometabolic state and prolonged neurotrophic effects may both be important determinants of clinical response [46].
The single most undesirable side effect of convulsive therapeutic approaches has consistently been memory impairment [47]. To date, multiple pharmacological strategies including glutamatergic, cholinergic, and glucocorticoid manipulations have been attempted to attenuate the neurocognitive side effects of convulsive therapies. The future of the field of neuromodulation may lie in the consolidation of diverse molecular systems that parsimoniously explain a general brain-wide phenomenon, such as impaired cognitive function [48]. Perhaps the most promising development in this regard is the study of neuronal activity-dependent plasticity which has the potential to affect many of the aforementioned proposed mechanisms through control of major transcription factors and precise spatiotemporal control of gene expression [49]. By manipulating multiple genetic end-products which may have seemingly contradictory functions, a broad-ranging mechanism such as neuroepigenetics [50] can influence both the therapeutic and side effects of neuromodulatory therapy.
Conclusions and future directions
The family of neuromodulation approaches available for mood disorders is wide ranging and rapidly growing. Engineering innovations are expanding the array of stimulation modalities, and advances in neuroimaging and basic neuroscience are important for informing mechanisms of action and selection of therapeutic brain targets. To date, convulsive therapies remain the most efficacious treatment for severe major depression. Optimizing ECT electrode and MST coil configurations, as well as dosing strategies are promising ways to further maximize efficacy and enhance safety that have yet to be explored. Computational electric field modeling can help inform neurophysiological mechanisms and the development of innovative tools to refine targeting strategies. Many of these novel neuromodulation approaches have yet to be systematically evaluated in mood disorders, and may offer new hope for patients with treatment resistant depression.
Highlights.
Newly approved antidepressants include TMS and deep TMS.
Spatially and temporally precise tools can induce lasting changes in neuroplasticity.
ECT effects on mood and memory can be decoupled.
Computer simulation of induced fields provides a rational basis for device design.
Acknowledgements
This manuscript was supported in part by grants from the National Institutes of Health / National Center for Advancing Translational Sciences (KL2 TR001115-02), and National Institute of Mental Health (K23 MH087739). Dr. Deng is an inventor on patents and patent applications related to TMS coil technology. Dr. Lisanby is an inventor on patents and patent applications related to TMS coil technology and ECT dosing procedure. Dr. Lisanby has served as Principal Investigator on industry-sponsored research grants to Columbia/RFMH or Duke (Neuronetics (past), Brainsway, ANS/St. Jude Medical (past), Cyberonics (past)); equipment loans to Columbia or Duke (Magstim, MagVenture); is supported by grants from NIH (R01MH091083-01, 5U01MH084241-02, 5R01MH060884-09), Stanley Medical Research Institute, National Alliance for Research on Schizophrenia and Depression, and the Wallace H. Coulter Foundation; and has no consultancies, speakers bureau memberships, board affiliations, or equity holdings in related industries.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Drs. McClintock and Luber, and Mr. Oey have no conflicts of interest to declare.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush A, Walters EE, Wang PS. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R) JAMA: Journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 3.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 4.Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357:1939–1945. doi: 10.1056/NEJMct075234. [DOI] [PubMed] [Google Scholar]
- 5.Husain MM, Rush AJ, Fink M, Knapp R, Petrides G, Rummans T, Biggs MM, O'Connor K, Rasmussen K, Litle M, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry. 2004;65:485–491. doi: 10.4088/jcp.v65n0406. [DOI] [PubMed] [Google Scholar]
- 6.Prudic J. Strategies to minimize cognitive side effects with ECT: Aspects of ECT technique. J ECT. 2008;24:46–51. doi: 10.1097/YCT.0b013e31815ef238. [DOI] [PubMed] [Google Scholar]
- 7.Sackeim HA, Prudic J, Nobler MS, Fitzsimons L, Lisanby SH, Payne N, Berman RM, Brakemeier E-L, Perera T, Devanand DP. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1:71–83. doi: 10.1016/j.brs.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deng Z-D, Lisanby SH, Peterchev AV. Electric field strength and focality in electroconvulsive therapy and magnetic seizure therapy: a finite element simulation study. J Neural Eng. 2011;8:016007. doi: 10.1088/1741-2560/8/1/016007. This is the first computational study to systematically compare the electric field induced by various existing forms of electroconvulsive therapy (ECT) and magnetic seizure therapy (MST). It was found that MST provides 3–6 times weaker and 10–60 times more focal stimulation compared to ECT. Conventional ECT with fixed high current amplitude induces electric field field that exceeds neural activation threshold by more than 6 times, which is higher than necessary for seizure induction, and potentially contributing to the side effect of ECT. This motivates the use lower current as a means of diminishing side effects. This study also demonstrates the utility of computational electric field modeling in explaining the biophysics of seizure therapy.
- 9. Sackeim HA, Prudic J, Devanand DP, Nobler MS, Lisanby SH, Peyser S, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57:425–434. doi: 10.1001/archpsyc.57.5.425. This study demonstrated that high dose right unilateral ECT has a comparable efficacy to bilateral ECT, with a significant decrease in neurocogntive side effects. This suggests that different brain circuits are responsible for the efficacy and side effects of ECT, for example, seizure activity in the medial temporal lobe may be related to retrograde amnesia post ECT, while focal seizure induction in the prefrontal cortex may be related to efficacy. The notion that the efficacy and side effects of ECT can be dissociated motivated the exploration of focal seizure induction techniques, such as magnetic seizure therapy, and focal electrically administered seizure therapy.
- 10.Nahas Z, Short B, Burns C, Archer M, Schmidt M, Prudic J, Nobler MS, Devanand DP, Fitzsimons L, Lisanby SH, et al. A feasibility study of a new method for electrically producing seizures in man: focal electrically administered seizure therapy [FEAST] Brain Stimul. 2013;6:403–408. doi: 10.1016/j.brs.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Loo CK, Bai S, MClinNeuro MD, Gálvez V, Dokos S. Revisiting frontoparietal montage in electroconvulsive therapy clinical observations and computer modeling: a future treatment option for unilateral electroconvulsive therapy. J ECT. doi: 10.1097/YCT.0000000000000147. In press. [DOI] [PubMed] [Google Scholar]
- 12.Deng Z-D, Lisanby SH, Peterchev AV. Controlling stimulation strength and focality in electroconvulsive therapy via current amplitude and electrode size and spacing: comparison with magnetic seizure therapy. J ECT. 2013;29:325–335. doi: 10.1097/YCT.10.1097/YCT.0b013e3182a4b4a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng ZD, Lisanby S, Peterchev A. Effect of anatomical variability on electric field characteristics of electroconvulsive therapy and magnetic seizure therapy: a parametric modeling study. IEEE Trans Neural Syst Rehabil Eng. 2014 doi: 10.1109/TNSRE.2014.2339014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lisanby SH, Schlaepfer TE, Fisch HU, Sackeim HA. Magnetic seizure therapy of major depression. Arch Gen Psychiatry. 2001;58:303–305. doi: 10.1001/archpsyc.58.3.303. [DOI] [PubMed] [Google Scholar]
- 15.Sackeim HA, Long J, Luber B, Moeller JR, Prohovnik I, Devanand DP, Nobler MS. Physical properties and quantification of the ECT stimulus: I. Basic principles. Convuls Ther. 1994;10:93–123. [PubMed] [Google Scholar]
- 16.Lisanby SH, Moscrip TD, Morales O, Luber B, Schroeder C, Sackeim HA. Neurophysiological characterization of magnetic seizure therapy (MST) in non-human primates. Clin Neurophysiol Suppl. 2003;56:81–99. doi: 10.1016/s1567-424x(09)70212-0. [DOI] [PubMed] [Google Scholar]
- 17.Lisanby SH, Luber B, Schlaepfer TE, Sackeim HA. Safety and feasibility of magnetic seizure therapy (MST) in major depression: randomized within-subject comparison with electroconvulsive therapy. Neuropsychopharmacology. 2003;28:1852–1865. doi: 10.1038/sj.npp.1300229. [DOI] [PubMed] [Google Scholar]
- 18. Kayser SBBH, Grubert C, Hadrysiewicz BL, Axmacher N, Schlaepfer TE. Antidepressant effects, of magnetic seizure therapy and electroconvulsive therapy, in treatment-resistant depression. J Psychiatr Res. 2011;45:569–576. doi: 10.1016/j.jpsychires.2010.09.008. In this open-label study of the effect of MST in treatment resistant depression. The antidepressant resposne was found to be similar between MST and ECT. Patients received MST had quicker recovery and reorientation times compared to ECT, had no cognitive side effects. This suggests that MST can be a potential alternative for patients with treatment resistant depression.
- 19.Fitzgerald PB, Daskalakis ZJ. The effects of repetitive transcranial magnetic stimulation in the treatment of depression. Expert Rev Med Devices. 2011;8:85–95. doi: 10.1586/erd.10.57. [DOI] [PubMed] [Google Scholar]
- 20. Deng Z-D, Lisanby SH, Peterchev AV. Electric field depth–focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul. 2013;6:1–13. doi: 10.1016/j.brs.2012.02.005. This paper presents the most comprehensive comparison of transcranial magnetic stimulation (TMS) coil electric field characteristics to date. This work shows that there all TMS coils are subject to a consistent depth–focality tradeoff, i.e. deeper electric field penetration is achieved at the expense of reduced focality.
- 21.George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, Anderson B, Nahas Z, Bulow P, Zarkowski P, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67:507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 22.Mottaghy FM, Keller CE, Gangitano M, Ly J, Thall M, Parker JA, Pascual-Leone A. Correlation of cerebral blood flow and treatment effects of repetitive transcranial magnetic stimulation in depressed patients. Psychiatry Res. 2002;115:1–14. doi: 10.1016/s0925-4927(02)00032-x. [DOI] [PubMed] [Google Scholar]
- 23.Deng Z-D, Lisanby SH, Peterchev AV. Coil design considerations for deep transcranial magnetic stimulation. Clin Neurophysiol. 2014;125:1202–1212. doi: 10.1016/j.clinph.2013.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levkovitz Y, Roth Y, Harel EV, Braw Y, Sheer A, Zangen A. A randomized controlled feasibility and safety study of deep transcranial magnetic stimulation. Clin Neurophysiol. 2007;118:2730–2744. doi: 10.1016/j.clinph.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 25.Mueller JK, Grigsby EM, Prevosto V, Petraglia FW, III, Rao H, Deng Z-D, Peterchev AV, Sommer MA, Egner T, Platt ML, et al. Simultaneous transcranial magnetic stimulation and single-neuron recording in alert non-human primates. Nat Neurosci. 2014;17:1130–1136. doi: 10.1038/nn.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volkow ND, Tomasi D, Wang GJ, Fowler JS, Telang F, Wang R, Alexoff D, Logan J, Wong C, Pradhan K, et al. Effects of low-field magnetic stimulation on brain glucose metabolism. Neuroimage. 2010;51:623–628. doi: 10.1016/j.neuroimage.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohan M, Parow A, Stoll AL, Demopulos C, Friedman S, Dager S, Hennen J, Cohen BM, Renshaw PF. Low-field magnetic stimulation in bipolar depression using an MRI-based stimulator. Am J Psychiatry. 2004;161:93–98. doi: 10.1176/appi.ajp.161.1.93. [DOI] [PubMed] [Google Scholar]
- 28.Carlezon WAJ, Rohan ML, Mague SD, Meloni EG, Parsegian A, Cayetano K, Tomasiewicz HC, Rouse ED, Cohen BM, Renshaw PF. Antidepressant-like effects of cranial stimulation within a low-energy magnetic field in rats. Biol Psychiatry. 2005;57:571–576. doi: 10.1016/j.biopsych.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Jin Y, Phillips B. A pilot study of the use of EEG-based synchronized transcranial magnetic stimulation (sTMS) for treatment of major depression. BMC Psychiatry. 2014;14:13. doi: 10.1186/1471-244X-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fregni F, Boggio PS, Nitsche MA, Marcolin MA, Rigonatti SP, Pascual-Leone A. Treatment of major depression with transcranial direct current stimulation. Bipolar Disorders. 2006;8:203–204. doi: 10.1111/j.1399-5618.2006.00291.x. This is the first randomized, double-blind, sham-controlled study of transcranial direct current stimulation (tDCS) for the treatment of major depression. After five sessions with tDCS, four out of five patients benefited from active treatment and none in the sham group. On average, the active group achieved a 60%–70% reduction in the depression scores. These encouraging results sparked subsequent studies on tDCS in depression.
- 31.Boggio PS, Rigonatti SP, Ribeiro RB, Myczkowski ML, Nitsche MA, Pascual-Leone A, Fregni F. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychopharmacol. 2008;11:249–254. doi: 10.1017/S1461145707007833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loo CK, Sachdev P, Martin D, Pigot M, Alonzo A, Malhi GS, Lagopoulos J, Mitchell P. A double-blind, sham-controlled trial of transcranial direct current stimulation for the treatment of depression. Int J Neuropsychopharmacol. 2010;13:61–69. doi: 10.1017/S1461145709990411. [DOI] [PubMed] [Google Scholar]
- 33.Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2:201–207. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dmochowski JP, Datta A, Bikson M, Su Y, Parra LC. Optimized multi-electrode stimulation increases focality and intensity at target. J Neural Eng. 2011;8:046011. doi: 10.1088/1741-2560/8/4/046011. [DOI] [PubMed] [Google Scholar]
- 35.Park J-H, Hong S-B, Kim D-W, Suh M, Im C-H. A novel array-type transcranial direct current stimulation (tDCS) system for accurate focusing on targeted brain areas. IEEE Trans Magn. 2011;47:882–885. [Google Scholar]
- 36.Anderson RJ, Frye MA, Abulseoud OA, Lee KH, McGillivray JA, Berk M, Tye SJ. Deep brain stimulation for treatment-resistant depression: efficacy, safety and mechanisms of action. Neurosci Biobehav Rev. 2012;36:1920–1933. doi: 10.1016/j.neubiorev.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 38.Malone DA, Jr, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, Rauch SL, Rasmussen SA, Machado AG, Kubu CS, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wongsarnpigoon A, Grill WM. Energy-efficient waveform shapes for neural stimulation revealed with a genetic algorithm. J Neural Eng. 2010;7:046009. doi: 10.1088/1741-2560/7/4/046009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howell B, Naik S, Grill WM. Influences of interpolation error, electrode geometry, and the electrode-tissue interface on models of electric fields produced by deep brain stimulation. IEEE Trans Biomed Eng. 2014;61:297–307. doi: 10.1109/TBME.2013.2292025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rush AJ, George MS, Sackeim HA, Marangell LB, Husain MM, Giller C, Nahas Z, Haines S, Simpson RKJ, Goodman R. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47:276–286. doi: 10.1016/s0006-3223(99)00304-2. [DOI] [PubMed] [Google Scholar]
- 42.Sackeim HA, Keilp JG, Rush AJ, George MS, Marangell LB, Dormer JS, Burt T, Lisanby SH, Husain M, Cullum CM, et al. The effects of vagus nerve stimulation on cognitive performance in patients with treatment-resistant depression. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:53–62. [PubMed] [Google Scholar]
- 43.Kosel M, Schlaepfer TE. Mechanisms and state of the art of vagus nerve stimulation. J ECT. 2002;18:189–192. doi: 10.1097/00124509-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Sackeim HA, Decina P, Prohovnik I, Malitz S, Resor SR. Anticonvulsant and antidepressant properties of electroconvulsive therapy: a proposed mechanism of action. Biol Psychiatry. 1983;18:1301–1310. [PubMed] [Google Scholar]
- 45.Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, Duman RS. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J Neurosci. 2003;23:10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abbott CC, Gallegos P, Rediske N, Lemke NT, Quinn DK. A review of longitudinal electroconvulsive therapy: neuroimaging investigations. J Geriatr Psychiatry Neurol. 2014;27:33–46. doi: 10.1177/0891988713516542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClintock SM, Choi J, Deng ZD, Appelbaum LG, Krystal AD, Lisanby SH. Multifactorial determinants of the neurocognitive effects of electroconvulsive therapy. J ECT. 2014;30:165–176. doi: 10.1097/YCT.0000000000000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frais AT. Electroconvulsive therapy: a theory for the mechanism of action. J ECT. 2010;26:60–61. doi: 10.1097/YCT.0b013e3181a92e8f. [DOI] [PubMed] [Google Scholar]
- 49.de Jong JO, Arts B, Boks MP, Sienaert P, van den Hove DL, Kenis G, van Os J, Rutten BP. Epigenetic effects of electroconvulsive seizures. J ECT. 2014;30:152–159. doi: 10.1097/YCT.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 50.Saab BJ, Mansuy IM. Neuroepigenetics of memory formation and impairment: the role of microRNAs. Neuropharmacology. 2014;80:61–69. doi: 10.1016/j.neuropharm.2014.01.026. [DOI] [PubMed] [Google Scholar]