Abstract
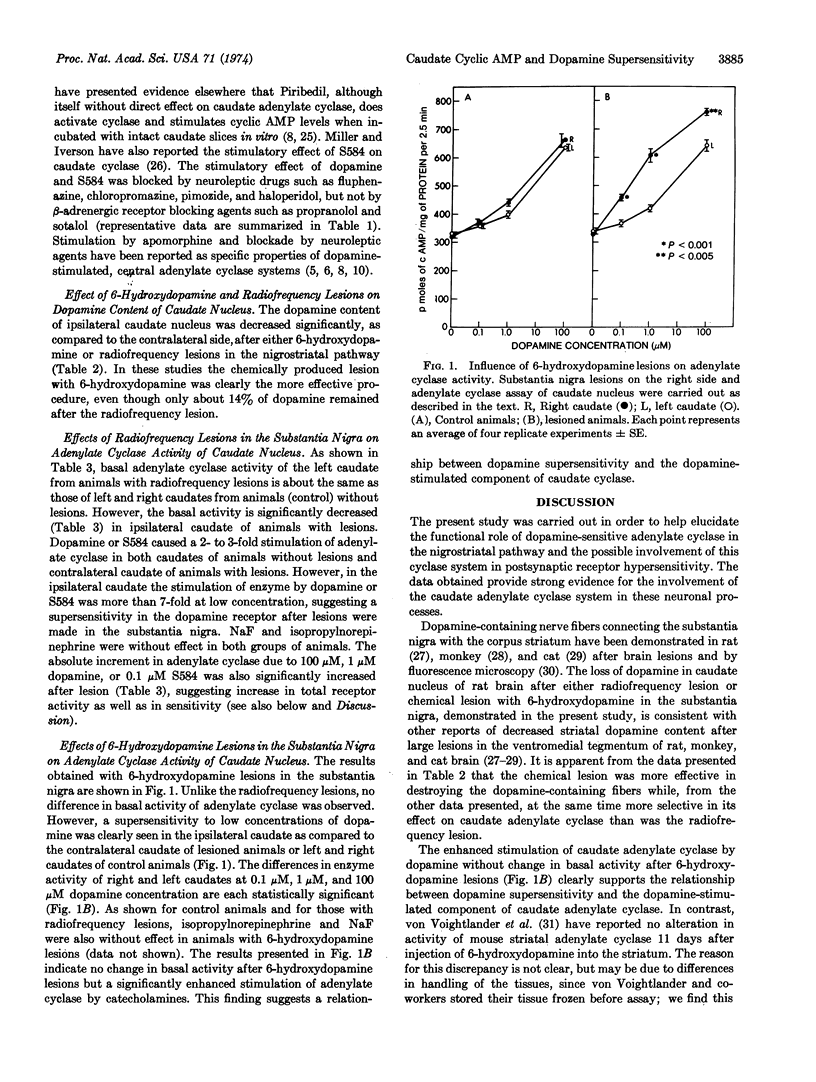
Unilateral radiofrequency lesions or chemical lesions with 6-hydroxydopamine were produced in the substantia nigra of rat brain in order to destroy dopaminergic innervations to caudate nucleus and thereby to produce functional denervation supersensitivity. Both types of lesions resulted in enhanced stimulation of caudate adenylate cyclase (EC 4.6.1.1) activity by dopamine at all dopamine concentrations tested, with more marked enhancement at the lower concentrations. Response to another dopamine agonist, 1-(3,4-dihydroxybenzyl)-4-(20pyrimidinyl) piperazine (S584) was also enhanced. 6-Hydroxydopamine lesions resulted in selective enhancement of the dopamine-stimulated component of adenylate cyclase, whereas radiofrequency lesions resulted also in a marked decrease in basal activity. It is postulated that the basal activity of caudate represents primarily an adenylate cyclase distinct from that stimulated by dopamine and destroyed only by the less selective radiofrequency lesion. The enhancement of dopamine-sensitive adenylate cyclase after lesions serves as indirect evidence for a significant role of this system in the transmitter function of dopamine and indicates, furthermore, that it is directly involved in dopamine receptor supersensitivity in vivo produced by denervation.
Keywords: dopamine receptor, nigro-striatal pathway, Parkinson's disease, cyclic AMP
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brimijoin S., Pluchino S., Trendelenburg U. On the mechanism of supersensitivity to norepinephrine in the denervated cat spleen. J Pharmacol Exp Ther. 1970 Nov;175(2):503–513. [PubMed] [Google Scholar]
- Brown J. H., Makman M. H. Influence of neuroleptic drugs and apomorphine on dopamine-sensitive adenylate cyclase of retina. J Neurochem. 1973 Aug;21(2):477–479. doi: 10.1111/j.1471-4159.1973.tb04268.x. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Makman M. H. Stimulation by dopamine of adenylate cyclase in retinal homogenates and of adenosine-3':5'-cyclic monophosphate formation in intact retina. Proc Natl Acad Sci U S A. 1972 Mar;69(3):539–543. doi: 10.1073/pnas.69.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasin M., Rivkin I., Mamrak F., Samaniego S. G., Hess S. M. Alpha- and beta-adrenergic receptors as mediators of accumulation of cyclic adenosine 3',5'-monophosphate in specific areas of guinea pig brain. J Biol Chem. 1971 May 10;246(9):3037–3041. [PubMed] [Google Scholar]
- Clement-Cormier Y. C., Kebabian J. W., Petzold G. L., Greengard P. Dopamine-sensitive adenylate cyclase in mammalian brain: a possible site of action of antipsychotic drugs. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1113–1117. doi: 10.1073/pnas.71.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P., Kebabian J. W. Role of cyclic AMP in synaptic transmission in the mammalian peripheral nervous system. Fed Proc. 1974 Apr;33(4):1059–1067. [PubMed] [Google Scholar]
- Hoffer B. J., Siggins G. R., Oliver A. P., Bloom F. E. Cyclic AMP-mediated adrenergic synapses to cerebellar Purkinje cells. Adv Cyclic Nucleotide Res. 1972;1:411–423. [PubMed] [Google Scholar]
- Horn A. S., Cuello A. C., Miller R. J. Dopamine in the mesolimbic system of the rat brain: endogenous levels and the effects of drugs on the uptake mechanism and stimulation of adenylate cyclase activity. J Neurochem. 1974 Feb;22(2):265–270. doi: 10.1111/j.1471-4159.1974.tb11589.x. [DOI] [PubMed] [Google Scholar]
- Kalisker A., Rutledge C. O., Perkins J. P. Effect of nerve degeneration by 6-hydroxydopamine on catecholamine-stimulated adenosine 3',5'-monophosphate formation in rat cerebral cortex. Mol Pharmacol. 1973 Sep;9(5):619–629. [PubMed] [Google Scholar]
- Kebabian J. W., Petzold G. L., Greengard P. Dopamine-sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the "dopamine receptor". Proc Natl Acad Sci U S A. 1972 Aug;69(8):2145–2149. doi: 10.1073/pnas.69.8.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaman M. W., Weinreich D., McCaman R. E. The determination of picomole levels of 5-hydroxytryptamine and dopamine in Aplysia, Tritonia and leech nervous tissues. Brain Res. 1973 Apr 13;53(1):129–137. doi: 10.1016/0006-8993(73)90772-5. [DOI] [PubMed] [Google Scholar]
- Miller R. J., Iversen L. L. Stimulation of a dopamine-sensitive adenylate cyclase in homogenates of rat striatum by a metabolite of piribedil (ET 495). Naunyn Schmiedebergs Arch Pharmacol. 1974;282(2):213–216. doi: 10.1007/BF00499035. [DOI] [PubMed] [Google Scholar]
- POIRIER L. J., SOURKES T. L. INFLUENCE OF THE SUBSTANTIA NIGRA ON THE CATECHOLAMINE CONTENT OF THE STRIATUM. Brain. 1965 Mar;88:181–192. doi: 10.1093/brain/88.1.181. [DOI] [PubMed] [Google Scholar]
- Perkins J. P., Moore M. M. Characterization of the adrenergic receptors mediating a rise in cyclic 3'-5'-adenosine monophosphate in rat cerebral cortex. J Pharmacol Exp Ther. 1973 May;185(2):371–378. [PubMed] [Google Scholar]
- Poirier L. J., Singh P., Boucher R., Bouvier G., Olivier A., Larochelle P. Effect of brain lesions on striatal monoamines in the cat. Arch Neurol. 1967 Dec;17(6):601–608. doi: 10.1001/archneur.1967.00470300043008. [DOI] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Adenyl cyclase as an adrenergic receptor. Ann N Y Acad Sci. 1967 Feb 10;139(3):703–723. doi: 10.1111/j.1749-6632.1967.tb41239.x. [DOI] [PubMed] [Google Scholar]
- Trendelenburg U., Maxwell R. A., Pluchino S. Methoxamine as a tool to assess the importance of intraneuronal uptake of l-norepinephrine in the cat's nictitating membrane. J Pharmacol Exp Ther. 1970 Mar;172(1):91–99. [PubMed] [Google Scholar]
- Von Voigtlander P. F., Boukma S. J., Johnson G. A. Dopaminergic denervation supersensitivity and dopamine stimulated adenyl cyclase activity. Neuropharmacology. 1973 Nov;12(11):1081–1086. doi: 10.1016/0028-3908(73)90052-x. [DOI] [PubMed] [Google Scholar]
- Weiss B. Effects of environmental lighting and chronic denervation on the activation of adenyl cyclase of rat pineal gland by norepinephrine and sodium fluoride. J Pharmacol Exp Ther. 1969 Jul;168(1):146–152. [PubMed] [Google Scholar]
- Weiss B., Strada S. J. Neuroendocrine control of the cyclic AMP system of brain and pineal gland. Adv Cyclic Nucleotide Res. 1972;1:357–374. [PubMed] [Google Scholar]
- von Hungen D., Roberts S. Adenylate-cyclase receptors for adrenergic neurotransmitters in rat cerebral cortex. Eur J Biochem. 1973 Jul 16;36(2):391–401. doi: 10.1111/j.1432-1033.1973.tb02924.x. [DOI] [PubMed] [Google Scholar]



