Abstract
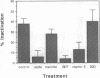
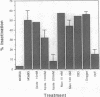
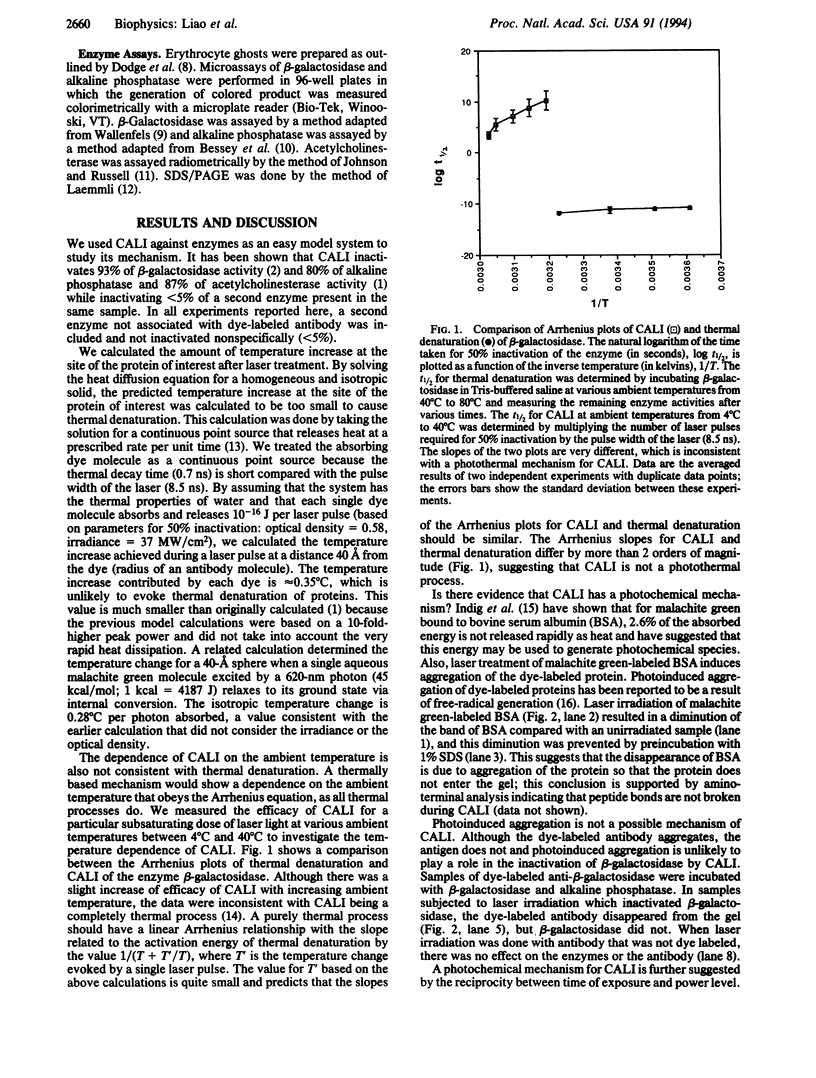
Chromophore-assisted laser inactivation (CALI) is a technique that selectively inactivates proteins of interest to elucidate their in vivo functions. This method has application to a wide array of biological questions and an understanding of its mechanism is required for its judicious application. We report here that CALI is not mediated by photoinduced thermal denaturation but by photogenerated free radicals. Thermal diffusion calculations suggest that the temperature changes resulting from CALI are too small to cause thermal denaturation, and Arrhenius plots of CALI are inconsistent with a photothermal mechanism. CALI shows an energy dose reciprocity above a threshold and can be inhibited by free-radical quenchers, thus demonstrating a photochemical mechanism of protein inactivation. The type of quenchers that are effective in inhibiting CALI indicates that the active species is a hydrogen abstractor which is not derived from molecular oxygen. We suggest that the active free-radical species is the hydroxyl radical and its very short lifetime explains the spatial specificity of CALI such that half-maximal damage is effected within 15 A from the dye moiety and no significant damage occurs at 34 A. The data are consistent with free-radical formation resulting from a sequential two-photon process.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. R., Parrish J. A. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983 Apr 29;220(4596):524–527. doi: 10.1126/science.6836297. [DOI] [PubMed] [Google Scholar]
- Czapski G. Reaction of .OH. Methods Enzymol. 1984;105:209–215. doi: 10.1016/s0076-6879(84)05027-8. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Diamond P., Mallavarapu A., Schnipper J., Booth J., Park L., O'Connor T. P., Jay D. G. Fasciclin I and II have distinct roles in the development of grasshopper pioneer neurons. Neuron. 1993 Sep;11(3):409–421. doi: 10.1016/0896-6273(93)90146-i. [DOI] [PubMed] [Google Scholar]
- Gorman A. A., Rodgers M. A. Current perspectives of singlet oxygen detection in biological environments. J Photochem Photobiol B. 1992 Jul 15;14(3):159–176. doi: 10.1016/1011-1344(92)85095-c. [DOI] [PubMed] [Google Scholar]
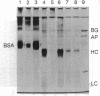
- Indig G. L., Jay D. G., Grabowski J. J. The efficiency of malachite green, free and protein bound, as a photon-to-heat converter. Biophys J. 1992 Mar;61(3):631–638. doi: 10.1016/S0006-3495(92)81868-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay D. G., Keshishian H. Laser inactivation of fasciclin I disrupts axon adhesion of grasshopper pioneer neurons. Nature. 1990 Dec 6;348(6301):548–550. doi: 10.1038/348548a0. [DOI] [PubMed] [Google Scholar]
- Jay D. G. Selective destruction of protein function by chromophore-assisted laser inactivation. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5454–5458. doi: 10.1073/pnas.85.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. D., Russell R. L. A rapid, simple radiometric assay for cholinesterase, suitable for multiple determinations. Anal Biochem. 1975 Mar;64(1):229–238. doi: 10.1016/0003-2697(75)90423-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lepock J. R., Thompson J. E., Kruuv J. Photoinduced crosslinking of membrane proteins by fluorescein isothiocyanate. Biochem Biophys Res Commun. 1978 Nov 14;85(1):344–350. doi: 10.1016/s0006-291x(78)80048-5. [DOI] [PubMed] [Google Scholar]
- Linden K. G., Liao J. C., Jay D. G. Spatial specificity of chromophore assisted laser inactivation of protein function. Biophys J. 1992 Apr;61(4):956–962. doi: 10.1016/S0006-3495(92)81902-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peak M. J., Peak J. G. Hydroxyl radical quenching agents protect against DNA breakage caused by both 365-nm UVA and by gamma radiation. Photochem Photobiol. 1990 Jun;51(6):649–652. [PubMed] [Google Scholar]
- Redpath J. L. The use of selective free radical probes to study active sites in enzymes and viruses. Methods Enzymol. 1984;105:491–501. doi: 10.1016/s0076-6879(84)05068-0. [DOI] [PubMed] [Google Scholar]
- Schmucker D., Su A. L., Beermann A., Jäckle H., Jay D. G. Chromophore-assisted laser inactivation of patched protein switches cell fate in the larval visual system of Drosophila. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2664–2668. doi: 10.1073/pnas.91.7.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]