Abstract
AIM: To report the incidence and potential risk factors of small-volume chylous ascites (SVCA) following laparoscopic radical gastrectomy (LAG).
METHODS: A total of 1366 consecutive gastric cancer patients who underwent LAG from January 2008 to June 2011 were enrolled in this study. We analyzed the patients based on the presence or absence of SVCA.
RESULTS: SVCA was detected in 57 (4.17%) patients, as determined by the small-volume drainage (range, 30-100 mL/24 h) of triglyceride-rich fluid. Both univariate and multivariate analyses revealed that the total number of resected lymph nodes (LNs), No. 8 or No. 9 LN metastasis and N stage were independent risk factors for SVCA following LAG (P < 0.05). Regarding hospital stay, there was a significant difference between the groups with and without SVCA (P < 0.001). The 3-year disease-free and overall survival rates of the patients with SVCA were 47.4% and 56.1%, respectively, which were similar to those of the patients without SVCA (P > 0.05).
CONCLUSION: SVCA following LAG developed significantly more frequently in the patients with ≥ 32 harvested LNs, ≥ 3 metastatic LNs, or No. 8 or No. 9 LN metastasis. SVCA, which was successfully treated with conservative management, was associated with a prolonged hospital stay but was not associated with the prognosis.
Keywords: Gastric cancer, Laparoscopic gastrectomy, Small volume, Chylous ascites
Core tip: Postoperative chylous ascites (CA) following abdominal surgery is uncommon. Laparoscopic radical gastrectomy (LAG) has been increasingly used as a promising approach to manage gastric cancer. However, in the field of LAG surgery, no large studies have been conducted focusing on postoperative CA. Therefore, we conducted the current study to evaluate the incidence, management, and predisposing factors of CA, as well as the impact of CA on outcomes after LAG. This is the first study to evaluate SVCA after LAG in a large population with the aim of identifying the potential risk factors for its onset and determining the appropriate treatment.
INTRODUCTION
Chylous ascites (CA), which was first reported by Morton in 1694[1], is a rare form of ascites characterized by a milky fluid that contains high levels of triglycerides. In a cohort of 1103 patients undergoing abdominal surgery, the incidence of postoperative CA was only 1.1%[2]. In addition to oncologic abdominal surgery[2],CA can also occur following abdominal aortic surgery[3] and donor nephrectomy[4]. Following retroperitoneal lymphadenectomy for testicular cancer, the incidence was reported to be as high as 7%[5]. Because chyle consists of lymphatic fluid enriched with triglycerides, lymphocytes and immunoglobulins, CA presents challenges for treatment and can have metabolic consequences; massive and prolonged chyle leaks may induce infection, malnutrition and immunodeficiency[6].
Gastric cancer is one of the most common malignant tumors worldwide, particularly in Asia, including China[7]. Since 1991[8], laparoscopic radical gastrectomy (LAG) has been increasingly used as a promising approach to manage gastric cancer because of its minimal invasiveness and its potential to successfully treat patients with lymph node (LN) metastasis[9,10]. Although LAG has recently been accepted as a safe and effective surgical treatment for gastric cancer, no report has focused on CA after LAG. The aim of this study was to determine the incidence, risk factors and management of CA following LAG. The lack of data is a result of the low incidence of CA, as well as the lack of a clear definition of CA following LAG. To date, little is known about the incidence, clinical symptoms or risk factors of atraumatic CA following this particular type of surgical intervention.
We observed that most cases of abdominal chylous leakage following LAG ranged from 30 to 100 mL/d. Thus, for the first time, we defined these cases as “small-volume chylous ascites (SVCA)”. The occurrence of postoperative SVCA can cause fever, abdominal pain, abdominal distension, abnormal white blood cell (WBC) count and delay in the withdrawal of the abdominal drainage tube. Even more serious, improper treatment will lead to celiac infection and abdominal bleeding, which results in prolonged hospitalization and increased costs. Therefore, we conducted the current study to evaluate SVCA following LAG in a large population, with the aim of detecting its incidence and identifying the potential risk factors for its onset and determining the appropriate treatment.
MATERIALS AND METHODS
Patients
One group of surgeons performed 1366 LAG procedures at the Fujian Medical University Union Hospital between January 2008 and June 2011. All data were prospectively collected in a database and retrospectively analyzed. The pathological tumor depth, nodal status, and curability of surgery were assigned according to the American Joint Committee on Cancer (AJCC) classification system[11]. The histological type was classified according to the Japanese Gastric Cancer Association (JGCA) classification system[12], in which tubular adenocarcinoma and papillary adenocarcinoma are defined as differentiated adenocarcinoma, and poorly differentiated adenocarcinoma, signet-ring cell carcinoma, and mucinous adenocarcinoma are defined as undifferentiated adenocarcinoma[13]. The median number of resected LNs was 32 per patient.
The patient characteristics and the pathological and surgical findings were collected from our database records and individual patient electronic medical records. Data collection and analysis were approved by the Institutional Review Board of the Fujian Medical University Union Hospital. The clinical and pathological data of the SVCA and non-SVCA groups are summarized in Table 1.
Table 1.
Clinical manifestations of small-volume chylous ascites following laparoscopic radical gastrectomy
| Symptom | n (%) |
| Abnormal blood leukocyte increase | 54 (94.7) |
| Abdominal pain | 40 (70.1) |
| Fever | 20 (35.1) |
| Abdominal distension | 19 (33.3) |
| Abdominal bleeding | 2 (3.51) |
Operation
All patients voluntarily chose laparoscopic surgery and underwent a D2 lymphadenectomy, as described by the JGCA[12]. All operations were completed by the same experienced group of surgeons (i.e., they have successfully performed more than 500 LAG procedures).
Definition
CA is defined as the extravasation of milky or creamy, triglyceride-rich peritoneal fluid from the thoracic or intestinal lymphatic vessels in the abdominal cavity[6,14]. There has been no unified standard for CA following gastrectomy to date[15-17]. In this study, we additionally defined SVCA as follows:(1) a drain output with milky appearance that is concurrent with the initiation of enteral feeding, with a long-chain triglyceride (TG) concentration > 1.2 mmol/L[18]; (2) a range of CA drainage equal to 30-100 mL/d; and (3) pancreatic and anastomotic leakages were excluded. The time to onset was the interval between the completion of the operation and the appearance of SVCA, as confirmed by clinical detection. SVCA was determined to have resolved when a patient no longer required active treatment for CA and tolerated a regular diet without abdominal distention or pain related to abdominal distension, and the abdominal drainage was removed.
Follow-up
The patients were followed from the date of surgery until June 30, 2014 or until death, with the exception of 132 patients who had their final follow-ups between September 31, 2008 and July 30, 2013 and were censored on the last day of the follow-up. The follow-up period for survivors ranged from 1 to 80 mo, with a median of 41 mo.
Statistical analysis
All data were analyzed using χ2 and Student’s t-tests. All statistically significant variables identified in the univariate analysis were included in the multivariate survival analysis using a Logistic hazards model. The 3-year overall survival rate, 3-year disease-free survival rate and 95% confidence intervals (CIs) were calculated using the Kaplan-Meier method. Deaths from any other causes and the patients’ lost to follow-up were treated as censored data for the survival analysis. The log-rank test was used to detect the differences in the survival curves of the different subgroups. For all analyses, the P values were two-sided, and only P < 0.05 was considered to be significant. All statistical analyses were performed using the SPSS software (the Statistical Package for the Social Sciences, Version 17.0, SPSS Inc., Chicago, IL, United States).
RESULTS
Incidence
During the study period, 1366 patients underwent LAG, and 57 patients developed SVCA (4.17%). In the SVCA group, 43 patients (75.4%) were male, and 14 patients (24.6%) were female. The average age of the SVCA patients was 63.60 ± 9.344 years (range, 12-80 years). SVCA was diagnosed at a median of 5 d (range, 3-9 d). The median volume of drain output of CA was 60 mL/d (range, 30 -100 mL/d).
Clinical symptoms and treatments
An abnormal blood leukocyte increase and abdominal pain were the most common clinical manifestations, as shown in Table 1. The treatment of SVCA is primarily conservative: (1) an unobstructed abdominal drainage tube should be maintained. If the drainage tube has been removed prior to the occurrence of SVCA, the tube should be repositioned; if the SVCA originates from the abdominal compartment, CT-guided paracentesis and tube insertion should be performed; (2) a high-protein, low-fat diet containing medium-chain triglycerides is recommended; (3) total parenteral nutrition may be appropriate if the patient is unable to tolerate the diet; and (4) somatostatin is another useful form of conservative management. The average hospital stay of the SVCA patients was 25.14 ± 15.69 d (range, 13-40). The cure rate was 100%. There were no perioperative deaths (algorithm shown in Figure 1).
Figure 1.
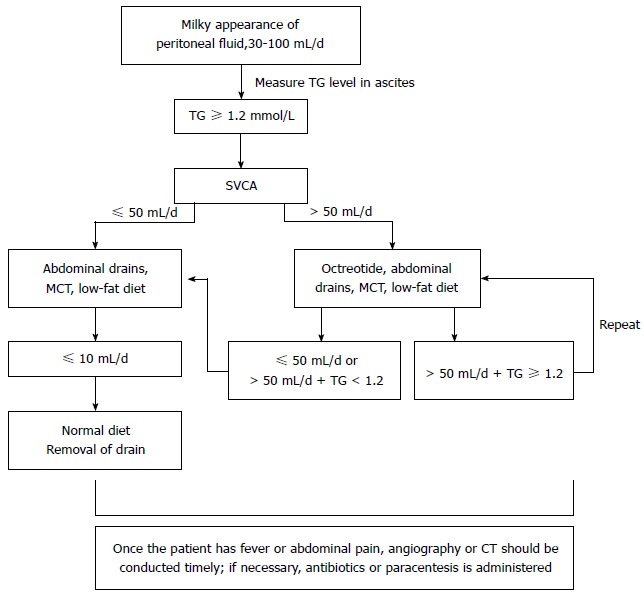
Algorithm for the treatment of small-volume chylous ascites after laparoscopic radical gastrectomy. TG: Triglycerides; MCT: Medium-chain triglyceride; SVCA: Small-volume chylous ascites; LAG: Laparoscopic radical gastrectomy.
Univariate analysis of risk factors for SVCA
As shown in Table 2, the univariate analysis revealed that preoperative anemia, preoperative hypoalbuminemia, N stage and the number of resected LNs were associated with the development of SVCA following LAG. However, the baseline characteristics (age, gender), tumor size, tumor location, depth of tumor invasion, histological classification, type of surgery and operative time were not significantly different between the groups (P > 0.05).
Table 2.
Comparison of the variables of patients with and without small-volume chylous ascites, according to a univariate analysis
| Characteristic | SVCA (n = 57) | No SVCA (n = 1309) | P value |
| Gender | 0.963 | ||
| Male | 43 | 991 | |
| Female | 14 | 318 | |
| Age (yr) | 63.60 ± 9.344 | 61.18 ± 11.346 | 0.091 |
| Preoperative anemia | 0.013 | ||
| Present | 26 | 395 | |
| Absent | 31 | 914 | |
| Preoperative hypoproteinemia | 0.012 | ||
| Present | 22 | 314 | |
| Absent | 35 | 995 | |
| Tumor size (cm) | 0.735 | ||
| ≥ 5 | 26 | 627 | |
| < 5 | 31 | 682 | |
| Tumor location | 0.179 | ||
| Upper | 12 | 303 | |
| Middle | 18 | 283 | |
| Lower | 20 | 609 | |
| Diffuse | 7 | 114 | |
| Histology | 0.911 | ||
| Differentiated | 24 | 599 | |
| Undifferentiated | 33 | 710 | |
| Tumor depth | 0.690 | ||
| T1 | 15 | 319 | |
| T2 | 6 | 147 | |
| T3 | 13 | 388 | |
| T4 | 23 | 455 | |
| Surgical management | 0.481 | ||
| Distal gastrectomy | 28 | 603 | |
| Total gastrectomy | 29 | 706 | |
| Lymph node status | 0.004 | ||
| N0 | 12 | 480 | |
| N1 | 9 | 195 | |
| N2 | 16 | 201 | |
| N3 | 20 | 433 | |
| Resected LNs (n) | 35.12 ± 13.691 | 31.35 ± 11.494 | 0.045 |
| Operative time (min) | 201.12 ± 66.076 | 192.24 ± 84.062 | 0.430 |
SVCA: Small-volume chylous ascites.
Relationship between SVCA and the status of regional LNs
The No. 8 and No. 9 LN metastasis rates of the SVCA group were significantly higher compared with the non-SVCA group, regardless of the surgical style (P < 0.05). However, no significant differences were observed in the rates of No. 1, No. 2, No. 3, No. 4, No. 5, No. 6, No. 7, No. 10, No. 11 or No. 12a LN metastasis in the SVCA and non-SVCA groups (P > 0.05) (Table 3).
Table 3.
Correlation between small-volume chylous ascites and perigastric lymph nodes metastasis (n)
|
LADG |
P value |
LATG |
P value | |||
| SVCA | No SVCA | SVCA | No SVCA | |||
| No. 1 | 0.969 | 0.314 | ||||
| + | 1 | 31 | 5 | 138 | ||
| - | 18 | 581 | 33 | 559 | ||
| No. 2 | / | / | / | 0.896 | ||
| + | / | / | 8 | 153 | ||
| - | 30 | 544 | ||||
| No. 3 | 0.948 | 0.115 | ||||
| + | 7 | 221 | 18 | 420 | ||
| - | 12 | 391 | 20 | 277 | ||
| No. 4 | 0.692 | 0.161 | ||||
| + | 6 | 168 | 15 | 201 | ||
| - | 13 | 444 | 23 | 496 | ||
| No. 5 | 0.458 | 0.093 | ||||
| + | 4 | 91 | 1 | 79 | ||
| - | 15 | 521 | 37 | 618 | ||
| No. 6 | 0.353 | 0.837 | ||||
| + | 4 | 190 | 6 | 119 | ||
| - | 15 | 422 | 32 | 578 | ||
| No. 7 | 0.431 | 0.505 | ||||
| + | 1 | 67 | 9 | 200 | ||
| - | 18 | 545 | 29 | 497 | ||
| No. 8 | 0.014 | 0.035 | ||||
| + | 7 | 96 | 9 | 285 | ||
| - | 12 | 516 | 29 | 412 | ||
| No. 9 | 0.017 | 0.000 | ||||
| + | 6 | 78 | 32 | 240 | ||
| - | 13 | 534 | 6 | 457 | ||
| No. 10 | / | 0.126 | ||||
| + | / | / | 5 | 167 | ||
| - | / | / | 33 | 530 | ||
| No. 11 | 0.898 | 0.134 | ||||
| + | 2 | 59 | 5 | 165 | ||
| - | 17 | 553 | 33 | 532 | ||
| No. 12a | 0.579 | 0.182 | ||||
| + | 15 | 450 | 21 | 459 | ||
| - | 4 | 162 | 17 | 238 | ||
LADG: Laparoscopic-assisted distal gastrectomy; SVCA: Small-volume chylous ascites; +: Positive; -: Negative.
Multivariable analysis of risk factors for SVCA
Multivariable regression analysis demonstrated that No. 8 and No. 9 LN metastasis, N stage and the number of resected LNs were independently associated with SVCA following LAG (P < 0.05, Table 4).
Table 4.
Multivariable analysis of the factors associated with the development of small-volume chylous ascites
| Factor | P value | OR | 95%CI | |
| Resected LNs (n) | 0.010 | 2.069 | 1.187 | 3.605 |
| (≥ 32 vs < 32) | ||||
| No. 8 metastasis | 0.039 | 2.102 | 1.037 | 4.261 |
| (positive vs negative) | ||||
| No. 9 metastasis | 0.000 | 4.019 | 2.011 | 8.030 |
| (positive vs negative) | ||||
| Preoperative anemia | 0.486 | 1.247 | 0.67 | 2.321 |
| (present vs absent) | ||||
| Preoperative hypoproteinemia | 0.076 | 0.566 | 0.302 | 1.062 |
| (present vs absent) | ||||
| N stage | 0.000 | |||
| N1 vs N0 | 0.053 | 1.519 | 0.688 | 7.426 |
| N2 vs N0 | 0.000 | 5.194 | 2.244 | 12.021 |
| N3 vs N0 | 0.000 | 7.342 | 2.774 | 19.431 |
LNs: Lymph nodes.
Postoperative recovery
There were no significant differences in the postoperative anal exhaust time, postoperative first feeding time, or white blood cell (WBC) count on the first postoperative day and discharge day in the patients with SVCA compared with the patients without SVCA (P > 0.05). Overall, the hospital stay was significantly longer for the patients with SVCA (P < 0.05). In addition, the WBC counts on postoperative days 3, 5, and 7 in the SVCA group were significantly higher compared with the non-SVCA group (P < 0.05) (Table 5).
Table 5.
Comparison of postoperative recovery between the small-volume chylous ascites and non-small-volume chylous ascites groups
| Factor | SVCA (n = 57) | No SVCA (n = 1309) | P value |
| Postoperative anal exhaust (d) | 3.54 ± 1.283 | 3.54 ± 1.343 | 0.963 |
| Postoperative food intake (d) | 4.19 ± 2.326 | 4.62 ± 1.772 | 0.078 |
| Postoperative hospital stay (d) | 25.14 ± 15.693 | 13.11 ± 5.894 | < 0.001 |
| WBC count (POD1, × 109/L) | 12.5 ± 3.8 | 13.1 ± 4.8 | 0.376 |
| WBC count (POD3, × 109/L) | 15.2 ± 2.4 | 12.2 ± 3.6 | 0.023 |
| WBC count (POD5, × 109/L) | 17.6 ± 4.7 | 11.6 ± 2.2 | 0.018 |
| WBC count (POD7, × 109/L) | 16.2 ± 5.8 | 10.9 ± 3.1 | 0.013 |
| WBC count (discharge) | 8.1 ± 1.5 | 7.3 ± 2.2 | 0.541 |
POD: Postoperative day; SVCA: Small-volume chylous ascites.
Prognostic analysis
Of the 1366 patients, 1234 (90.3%) were followed. The median follow-up period was 41 mo (range, 1-80 mo). The 3-year overall survival rates for the patients with or without SVCA were 56.1% and 60.8%, respectively (P > 0.05, Figure 2A). The 3-year disease-free survival rates were 47.4% and 51.4% in the SVCA and no-SVCA patients, respectively, and this difference was also not significant (P > 0.05, Figure 2B).
Figure 2.
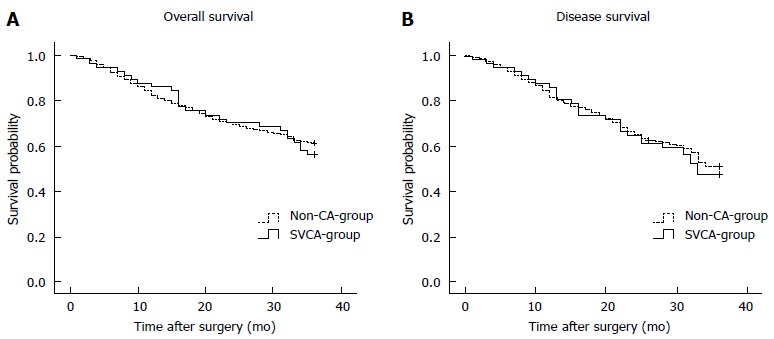
Overall (A) and disease-free (B) survival curves for patients with and without small-volume chylous ascites. There was no difference in overall survival between the groups (log-rank test, A: P = 0.601; B: P = 0.646).
DISCUSSION
CA is an infrequent postoperative complication that has been described in reports of abdominal and retroperitoneal surgeries. The incidence of CA following abdominal surgery ranges from 0.17% to 1.1%[19,20]. The reported incidence of CA following liver transplantation varies from 0.6% to 4.7%[19,20]. Following retroperitoneal, esophageal, or cytoreductive surgery, the postoperative incidence is even higher (7%-7.4%)[5,19,21]. Unfortunately, it is difficult to determine the true incidence of CA following radical surgery for gastric cancer secondary to other gastrectomy procedures because the research groups at most centers have reported only isolated cases[16,17]. Thus, the diagnostic criteria, epidemiology, onset features, treatment strategies and prognostic impact of CA following LAG remain unclear. To the best of our knowledge, this study is the first to examine the risk factors for CA in gastric cancer following LAG, and it provides the first indication of the incidence of this condition. Our data indicated that the volume of CA following LAG ranged from 30 to 100 mL per day, which was defined as SVCA. In this study, the incidence of SVCA was 4.17%.
In most patients, the symptoms of postoperative CA develop a few days to 1 mo following surgery but most commonly after approximately 1 wk[9]. The clinical presentation and findings from the physical examination of CA are frequently nonspecific and include abdominal distention, indigestion, nausea, and vomiting[22,23]. However, other authors have suggested that CA can lead to life-threatening conditions, such as sepsis, severe respiratory distress and death[24]. In our patients, SVCA developed on day 1 or 2 following the initiation of oral food intake; these symptoms were mild and may have resolved without surgical treatment. The most common clinical manifestations of SVCA include an abnormal WBC count and abdominal pain and can also include serious postoperative complications, such as abdominal bleeding; these findings are inconsistent with previous reports of traditional CA, as shown in Table 2. Thus, although the amount of SVCA is small and may not be severe in most patients, the accumulation of ascites fluid may cause gastrointestinal symptoms[25]; early diagnosis and management aid in the postoperative recovery.
In our study, SVCA was resolved successfully with conservative management, which included dietary restriction with a high-protein, low-fat diet that contains medium-chain triglycerides, somatostatin therapy, paracentesis, or continuous drainage. The aim is to decrease intestinal lymph flow and triglyceride transport and, thus, to prevent lymph accumulation. Fatty meal ingestion may increase chyle flow from a fasting baseline < 1 mL/min to > 200 mL/min, whereas bowel rest and a low-fat diet decrease lymph flow[5,15]. Dietary restriction with a high-protein, low-fat diet that contains medium-chain triglycerides can reduce lymph flow. Somatostatin reduces the output of lymphatic leakage in 24 to 72 h[26]. In addition, SVCA induced by LAG may require more serious forms of medical management, such as a policies on the use of abdominal drains and paracentesis. In most reported series[27,28], the use of a surgical drain was not specifically described; however, without the use of a drain, SVCA may be misdiagnosed because only a minority of patients will develop the signs and symptoms that prompt specific investigations. We acknowledge that closed-suction drains, which may reveal cases of subclinical SVCA, can also prevent abdominal infection and abdominal bleeding caused by SVCA. Thus, an abdominal drainage tube can be a simple and effective approach and should be considered in treating SVCA. At our center, we typically leave a drain following gastric LN dissection, which is useful for diagnosing postoperative chylous fistulas and monitoring their resolution. For patients with effusion in the peritoneal compartment, paracentesis becomes paramount not only to reach a diagnosis and to relieve abdominal distension[23] but also to reduce the risk of infection, which can cause abdominal bleeding. Computed tomography, radiography and antibiotic treatment are often performed if the patient has a fever or abdominal pain. From our experience with these 57 cases and a review of patient management in other reports, we devised the treatment algorithm shown in Figure 1.
There are multiple causes of CA. Some researchers believe that[29] the occurrence of postoperative CA should meet two criteria: (1) the destruction or interruption of the lymphatic circulation pathway; and (2) lymphatic fluid pressure greater than the pressure of the body cavity or tissue fluid. Kuboki et al[30] determined that the manipulation of the para-aortic area, retroperitoneal invasion and early enteral feeding following operation were independent risk factors associated with CA. Assumpcao et al[31] analyzed 3532 cases of patients who underwent pancreatic surgery and determined that as the number of resected LNs increased, the incidence of postoperative CA also increased. Therefore, the number of resected LNs is an independent risk factor for CA following pancreatic surgery. This study demonstrated that preoperative anemia, preoperative hypoalbuminemia, N stage, total number of harvested LNs, and No. 8 or No. 9 LN metastasis are closely associated with SVCA following LAG. In our patients, we suggest four potential types of lymphatic injury that may cause SVCA. First, SVCA may be related to coagulation of the major lymphatic vessels by ultrasonically activated shears during laparoscopic lymphadenectomy. LN metastasis leads to flow obstruction, thus further increasing the pressure within the lymph duct stump, which does not completely close with an ultrasonic scalpel, and resulting in chylous leakage. Second, the gastric peripheral lymphoid pathway is intricate. Radical LN dissection for gastric cancer in retroperitoneal tissue is one of the most densely clustered areas of the lymphatic trunk and its major tributaries; thus, it is conceivable that removing the No. 8 or No. 9 LNs during laparoscopy could cause a tear in the lymphatic channels. Third, injured lymphatic channels can result in self-healing closure with solidified lymphatic fluid or wound inflammation[32]. However, these SVCA patients have an increased incidence of preoperative anemia or hypoalbuminemia, which indicates poor nutritional status and a poor lymphatic repair ability. Fourth, the greater the number of LNs harvested is, the greater the probability of lymphatic channel injury.
Despite evidence that most SVCA can be cured by non-surgical treatment, it can lead to fever, abdominal pain, abdominal distention, an abnormal WBC count, prolonged drain maintenance, and even abdominal bleeding, thereby prolonging the hospitalization time and increasing the treatment costs. Therefore, we should take measures to reduce the incidence of SVCA following LAG. Our data demonstrated that the total number of harvested LNs, the total number of metastatic LNs, and No. 8 or No. 9 LN metastasis were independent risk factors for postoperative SVCA. Thus, if during the operation we determine that the LNs around the celiac artery are large, we should be aware of the possible occurrence of postoperative SVCA. To prospectively prevent this complication, the surgeon should be familiar with the gastric vessels and anatomy of the fascia and be able to differentiate the lymphoid tissues in the correct anatomical plane to ensure the complete resection of LNs. Furthermore, surgeons addressing the retroperitoneum should have a firm understanding of the lymphatic network in this region, and they should control the major lymphatics by suture ligation or using hemoclips during surgery. Meticulous and extensive clipping remains the safest approach to securing the lymphatic channels within the dissection area, particularly around the celiac artery. At the end of the LN dissection, the surgeon should carefully determine whether exudate or a coarse non-vascular tissue cord stump is present in the operative area.
In addition, the results of this study indicate that SVCA alone is not an unfavorable prognostic factor for gastric cancer. However, SVCA patients are more likely to present with advanced N stage and poor prognosis. Further studies of larger series are needed to clarify our findings.
In conclusion, SVCA is an important complication following LAG. Conservative management may be a reasonable treatment option because many cases may resolve with nonsurgical intervention. However, it is also our opinion that more controlled studies are needed to verify the efficacy and propriety of the recommended treatment.
ACKNOWLEDGMENTS
The authors are thankful to the medical staff who contributed to the success of the patient management.
COMMENTS
Background
Chylous ascites (CA) as a postoperative complication of abdominal surgery presents a challenge for treatment and can have serious metabolic consequences. Since its introduction by Kitano and colleagues in 1994, the number of patients undergoing laparoscopic radical gastrectomy (LAG) for gastric cancer (GC) has been increasing rapidly in Japan, South Korea, and China, where there is a high incidence of GC. Compared with open gastrectomy, LAG has many advantages including less postoperative pain and earlier return to work. Unfortunately, these benefits are sometimes negated by postoperative complications. Among these, CA is a rare but potential serious problem. Up to date, little is known about the incidence, etiology, and clinical treatments for atraumatic CA in GC in the era of minimally invasive surgery.
Research frontiers
In spite of a few case reports and small series, studies on CA in large series of GC patients who underwent LG are lacking.
Innovations and breakthroughs
A total of 1366 consecutive gastric cancer patients who underwent LAG were enrolled in this study. The results of the current study demonstrate that the incidence of postoperative small-volume chylous ascites (SVCA) was 4.17%. Multivariable analysis revealed that the total number of resected lymph nodes (LNs), No. 8 or No. 9 LN metastasis and N stage were independent risk factors for SVCA following LAG. Moreover, this study specifically demonstrated the short- and long-term outcomes of SVCA.
Applications
SVCA as an important complication following LAG that occurred significantly more frequently in patients with ≥ 32 harvested LNs, ≥ 3 metastatic LNs, or No. 8 or No. 9 LN metastasis. The results of this study indicate that SVCA alone is not an unfavorable prognostic factor for gastric cancer. However, SVCA patients are more likely to present with advanced N stage and poor prognosis.
Terminology
Different from previous reports, we observed that most cases of abdominal chylous leakage following LAG ranged from 30-100 mL/d. Thus, for the first time, we defined these cases as “small-volume chylous ascites (SVCA)”.
Peer-review
This is a good work in which the authors reveal the characters of chyle leakage following LAG, the prevention-treatment strategies and the short- and long-term clinical outcomes of SVCA.
Footnotes
Supported by Grants from National Key Clinical Specialty Discipline Construction Program of China, No. [2012]649.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 13, 2014
First decision: September 27, 2014
Article in press: October 21, 2014
P- Reviewer: Maldonado-Bernal C, Tepes B S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Tulunay G, Ureyen I, Turan T, Karalok A, Kavak D, Ozgul N, Ocalan R, Tapisiz OL, Boran N, Kose MF. Chylous ascites: analysis of 24 patients. Gynecol Oncol. 2012;127:191–197. doi: 10.1016/j.ygyno.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 2.Kaas R, Rustman LD, Zoetmulder FA. Chylous ascites after oncological abdominal surgery: incidence and treatment. Eur J Surg Oncol. 2001;27:187–189. doi: 10.1053/ejso.2000.1088. [DOI] [PubMed] [Google Scholar]
- 3.Olthof E, Blankensteijn JD, Akkersdijk GJ. Chyloperitoneum following abdominal aortic surgery. Vascular. 2008;16:258–262. doi: 10.2310/6670.2008.00035. [DOI] [PubMed] [Google Scholar]
- 4.Aerts J, Matas A, Sutherland D, Kandaswamy R. Chylous ascites requiring surgical intervention after donor nephrectomy: case series and single center experience. Am J Transplant. 2010;10:124–128. doi: 10.1111/j.1600-6143.2009.02883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans JG, Spiess PE, Kamat AM, Wood CG, Hernandez M, Pettaway CA, Dinney CP, Pisters LL. Chylous ascites after post-chemotherapy retroperitoneal lymph node dissection: review of the M. D. Anderson experience. J Urol. 2006;176:1463–1467. doi: 10.1016/j.juro.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Aalami OO, Allen DB, Organ CH. Chylous ascites: a collective review. Surgery. 2000;128:761–778. doi: 10.1067/msy.2000.109502. [DOI] [PubMed] [Google Scholar]
- 7.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 8.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 9.Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial) Ann Surg. 2010;251:417–420. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5. doi: 10.1007/s101200200000. [DOI] [PubMed] [Google Scholar]
- 11.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 12.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 13.Tokunaga M, Sugisawa N, Tanizawa Y, Bando E, Kawamura T, Terashima M. The impact of preoperative lymph node size on long-term outcome following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1598–1603. doi: 10.1245/s10434-012-2699-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Hu W, Hou X, Zhou Q. Chylous ascites after laparoscopic lymph node dissection in gynecologic malignancies. J Minim Invasive Gynecol. 2014;21:90–96. doi: 10.1016/j.jmig.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Du JJ, Li JP, Ding ZR, Zheng JY, Ji G, Gao ZQ, Wang WZ, Dou KF. [Management of chylous leakage after radical operation of gastric cancer] Zhonghua Yi Xue Zazhi. 2007;87:1414–1416. [PubMed] [Google Scholar]
- 16.Rajasekar A, Ravi NR, Diggory RT. Chylous ascites: a rare complication of radical gastrectomy. Int J Clin Pract. 2000;54:201–203. [PubMed] [Google Scholar]
- 17.Halkic N, Abdelmoumene A, Suardet L, Mosimann F. Postoperative chylous ascites after radical gastrectomy. A case report. Minerva Chir. 2003;58:389–391. [PubMed] [Google Scholar]
- 18.van der Gaag NA, Verhaar AC, Haverkort EB, Busch OR, van Gulik TM, Gouma DJ. Chylous ascites after pancreaticoduodenectomy: introduction of a grading system. J Am Coll Surg. 2008;207:751–757. doi: 10.1016/j.jamcollsurg.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Yilmaz M, Akbulut S, Isik B, Ara C, Ozdemir F, Aydin C, Kayaalp C, Yilmaz S. Chylous ascites after liver transplantation: incidence and risk factors. Liver Transpl. 2012;18:1046–1052. doi: 10.1002/lt.23476. [DOI] [PubMed] [Google Scholar]
- 20.Ijichi H, Soejima Y, Taketomi A, Yoshizumi T, Uchiyama H, Harada N, Yonemura Y, Maehara Y. Successful management of chylous ascites after living donor liver transplantation with somatostatin. Liver Int. 2008;28:143–145. doi: 10.1111/j.1478-3231.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 21.Steinemann DC, Dindo D, Clavien PA, Nocito A. Atraumatic chylous ascites: systematic review on symptoms and causes. J Am Coll Surg. 2011;212:899–905.e1-4. doi: 10.1016/j.jamcollsurg.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Vettoretto N, Odeh M, Romessis M, Pettinato G, Taglietti L, Giovanetti M. Acute abdomen from chylous peritonitis: a surgical diagnosis. Case report and literature review. Eur Surg Res. 2008;41:54–57. doi: 10.1159/000129599. [DOI] [PubMed] [Google Scholar]
- 23.Capocasale E, Iaria M, Vistoli F, Signori S, Mazzoni MP, Dalla Valle R, De Lio N, Perrone V, Amorese G, Mosca F, et al. Incidence, diagnosis, and treatment of chylous leakage after laparoscopic live donor nephrectomy. Transplantation. 2012;93:82–86. doi: 10.1097/TP.0b013e31823b2d8e. [DOI] [PubMed] [Google Scholar]
- 24.Chen FP, Lo TS, Soong YK. Management of chylous ascites following laparoscopic presacral neurectomy. Hum Reprod. 1998;13:880–883. doi: 10.1093/humrep/13.4.880. [DOI] [PubMed] [Google Scholar]
- 25.Kim BS, Yoo ES, Kim TH, Kwon TG. Chylous ascites as a complication of laparoscopic nephrectomy. J Urol. 2010;184:570–574. doi: 10.1016/j.juro.2010.03.128. [DOI] [PubMed] [Google Scholar]
- 26.Chan KY, Teoh CM, Sukumar N. Chylous ascites after anterior resection for rectal carcinoma: a rare but significant incident. Asian J Surg. 2006;29:46–48. doi: 10.1016/S1015-9584(09)60295-5. [DOI] [PubMed] [Google Scholar]
- 27.Var T, Güngor T, Tonguc E, Ozdener T, Mollamahmutoğlu L. The conservative treatment of postoperative chylous ascites in gynecologic cancers: four case reports. Arch Gynecol Obstet. 2012;285:849–851. doi: 10.1007/s00404-011-2050-3. [DOI] [PubMed] [Google Scholar]
- 28.Williams C, Petignat P, Alobaid A, Provencher D, Gauthier P. Chylous ascites after pelvic lymph node dissection for gynecologic cancer. Eur J Surg Oncol. 2007;33:399–400. doi: 10.1016/j.ejso.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 29.Crumley RL, Smith JD. Postoperative chylous fistula prevention and management. Laryngoscope. 1976;86:804–813. doi: 10.1288/00005537-197606000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Kuboki S, Shimizu H, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Furukawa K, Miyazaki M. Chylous ascites after hepatopancreatobiliary surgery. Br J Surg. 2013;100:522–527. doi: 10.1002/bjs.9013. [DOI] [PubMed] [Google Scholar]
- 31.Assumpcao L, Cameron JL, Wolfgang CL, Edil B, Choti MA, Herman JM, Geschwind JF, Hong K, Georgiades C, Schulick RD, et al. Incidence and management of chyle leaks following pancreatic resection: a high volume single-center institutional experience. J Gastrointest Surg. 2008;12:1915–1923. doi: 10.1007/s11605-008-0619-3. [DOI] [PubMed] [Google Scholar]
- 32.Leibovitch I, Mor Y, Golomb J, Ramon J. The diagnosis and management of postoperative chylous ascites. J Urol. 2002;167:449–457. doi: 10.1016/S0022-5347(01)69064-5. [DOI] [PubMed] [Google Scholar]


