Abstract
AIM: To investigate mortality reduction from gastric cancer based on the results of endoscopic screening.
METHODS: The study population consisted of participants of gastric cancer screening by endoscopy, regular radiography, and photofluorography at Niigata city in 2005. The observed numbers of cumulative deaths from gastric cancers and other cancers were accumulated by linkage with the Niigata Prefectural Cancer Registry. The standardized mortality ratio (SMR) of gastric cancer and other cancer deaths in each screening group was calculated by applying the mortality rate of the reference population.
RESULTS: Based on the results calculated from the mortality rate of the population of Niigata city, the SMRs of gastric cancer death were 0.43 (95%CI: 0.30-0.57) for the endoscopic screening group, 0.68 (95%CI: 0.55-0.79) for the regular radiographic screening group, and 0.85 (95%CI: 0.71-0.94) for the photofluorography screening group. The mortality reduction from gastric cancer was higher in the endoscopic screening group than in the regular radiographic screening group despite the nearly equal mortality rates of all cancers except gastric cancer.
CONCLUSION: The 57% mortality reduction from gastric cancer might indicate the effectiveness of endoscopic screening for gastric cancer. Further studies and prudent interpretation of results are needed.
Keywords: Gastric cancer screening, Mortality, Upper gastrointestinal endoscopy, Upper gastrointestinal radiography, Standardized mortality ratio
Core tip: We investigated mortality reduction from gastric cancer on the basis of results of endoscopic screening. The standardized mortality ratio (SMR) of gastric cancer and other cancer deaths in each screening group was calculated by applying the mortality rate of the reference population. Based on the results calculated from the mortality rate of the population of Niigata city, the SMRs of gastric cancer death were 0.43 (95%CI: 0.30-0.57) for the endoscopic screening group. The 57% mortality reduction from gastric cancer might indicate the effectiveness of endoscopic screening for gastric cancer.
INTRODUCTION
Despite the recent decline in the incidence and mortality of gastric cancer worldwide, gastric cancer remains to be the third leading cause of cancer mortality worldwide[1]. Moreover, the burden of gastric cancer still remains in Asia and East European countries. In most countries, gastric cancer screening has not been commonly carried out. However, in some Asian countries, gastric cancer screening using endoscopy has been performed as opportunistic screening[2]. Endoscopy, which is commonly used in clinical practice, is anticipated to be a promising screening method for gastric cancer. Although gastric cancer screening has been actively performed in South Korea and Japan[2-4], endoscopic screening has been carried out as a national program only in Korea.
Gastric cancer screening using the upper gastrointestinal series (UGI) (i.e., radiographic screening) is recommended in the Japanese guidelines for gastric cancer screening[5]. In particular, radiographic screening for gastric cancer was initiated in Japan in the 1960s[6]. The UGI with double-contrast study was originally adopted for radiographic screening. Photofluorography is one of the radiographic methods and it can be performed on board a vehicle because the equipment used is small compared with the regular radiographic equipment. In Japan, photofluorography was originally performed on a mobile car and has been used in communities. Regular radiographic screening has also been performed in clinical settings.
On the other hand, endoscopic examination has been widely used in clinical settings, but rarely for population-based screening programs. Since endoscopy can detect the early stage of gastric cancer, its introduction into communities for gastric cancer screening has been highly anticipated. To effectively introduce new techniques for population-based screening, mortality reduction should be evaluated. Except for radiographic screening, other methods have not been evaluated in terms of reduction of mortality from gastric cancer. Various screening methods for gastric cancer have been developed. In particular, the evaluation method used for radiographic screening was not randomized controlled studies but was limited to observational studies. Although several studies have reported the possibility of reducing mortality by endoscopic screening[7-10], definitive evidence remains to be established.
Serologic testing, including serum pepsinogen and Helicobacter pylori antibody testing, has also been used for targeting the high-risk group for gastric cancer; however, the effectiveness of these screening methods has not been fully clarified[2,4]. In this study, we investigated mortality reduction from gastric cancer on the basis of the results of gastric cancer screening by endoscopy and radiography in Niigata city, Japan.
MATERIALS AND METHODS
Ethics
This study was approved by the Institutional Review Board of National Cancer Center, Japan.
Screening program
Gastric cancer screening has been conducted and supported by the Health Service Law for the Aged since 1983 and it has been offered by local governments. Since 2003, endoscopic examination has been added to the screening programs for gastric cancer in Niigata city[10]. Both photofluorography and regular radiographic screening for the UGI have also been continued. Photofluorography has been performed as a mass screening program using mobile cars mainly in local areas. On the other hand, as endoscopic and regular radiographic screenings have been performed in clinical settings, individuals who visited regularly for any disease treatment were often recommended to undergo cancer screening by their own primary care physicians. The target populations of these screening programs vary as follows: individuals aged 40, 45, and 50 years or over can undergo endoscopic and regular radiographic screenings; individuals aged more than 40 years can undergo photofluorography. Individuals could choose any screening method based on their own preference. There is no upper age limit and the screening interval is every year for all screening methods. Although the participation rate in gastric cancer screening has increased since the introduction of endoscopic screening, the screening rate has remained at approximately 25%[10].
Physicians who perform endoscopic screening for gastric cancer in Niigata city have been approved by the local committee for gastric cancer screening based on certain requirements[10]. Although these endoscopic screenings have been performed in clinical settings, the results have been evaluated on the basis of a monitor screen review by the local committee, including experienced endoscopists.
Study population
The study population consisted of 54264 participants of gastric cancer screening by endoscopy, regular radiography, and photofluorography at Niigata city in 2005. The target population of this study was defined as individuals whose age was from 40 years to 79 years at the screening date in 2005. Individuals belonging to the age group of 80 years and over were excluded from the study. A flowchart showing the selection process for the study populations is shown in Figure 1. After the selection process, the total numbers of subjects for each screening group were 16373 for the endoscopic screening, 18221 for the regular radiographic screening, and 15927 for the photofluorography screening.
Figure 1.
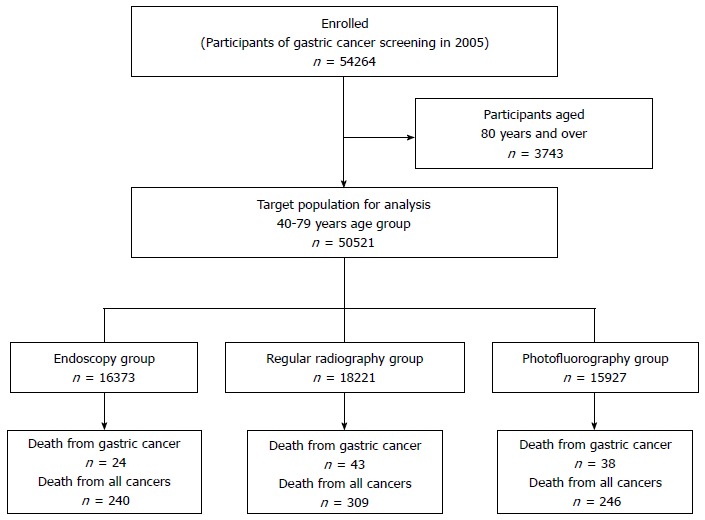
Flowchart for the selection of the target population. The target population consisted of participants of gastric cancer screening by endoscopy, regular radiography, and photofluorography at Niigata city, in 2005. The total number of participants of the gastric cancer screening in 2005 was 54264. The target population of this study was defined as individuals whose ages ranged from 40 years to 79 years; individuals belonging to the age group of 80 years and over were excluded from the study. The final numbers of subjects for the defined age group were 16373 for the endoscopic screening, 18221 for the regular radiographic screening, and 15927 for the photofluorography screening.
Since we could not obtain data regarding immigration rates and deceased cases except for cases of cancers, the number of all-cause mortality was estimated from the population data of Niigata city[11]. The immigration rates were calculated from the National Population Survey in 2010[12]. The all-causes mortality and immigration rates of the screening group were assumed to be equal to those of the whole population of Niigata city. Based on a 5-year immigration rate in the National Population Survey, our calculated annual immigration rates per 1000 individuals from Niigata city were as follows; for men: 19.2 (aged 40-44 years), 16.4 (45-49 years), 13.3 (50-54 years), 9.2 (55-59 years), 5.0 (60-64 years), 2.7 (65-69 years), 1.6 (70-74 years), and 1.5 (75-79 years); for women: 13.3 (aged 40-44 years), 6.8 (45-49 years), 4.5 (50-54 years), 3.2 (55-59 years), 2.6 (60-64 years), 2.0 (65-69 years), 1.4 (70-74 years), and 1.7 (75-79 years). The immigration rates and all-causes mortality were adopted for calculating the number of the study population of the 3 different screening groups within 5 years of follow-up (Table 1).
Table 1.
Target population of the follow-up study
| Screening method | Sex | Participants in 2005 (n) |
Follow-up |
||||
| 2006 | 2007 | 2008 | 2009 | 2010 | |||
| Endoscopy | Men | 6476 | 6314 | 6170 | 6029 | 5897 | 5771 |
| Women | 9897 | 9797 | 9707 | 9615 | 9525 | 9442 | |
| Regular radiography | Men | 7019 | 6841 | 6683 | 6548 | 6384 | 6246 |
| Women | 11202 | 11087 | 10985 | 10879 | 10775 | 10682 | |
| Photofluorography | Men | 5188 | 5056 | 4939 | 4824 | 4717 | 4614 |
| Women | 10739 | 10638 | 10545 | 10450 | 10357 | 10270 | |
Statistical analysis
The follow-up period was defined as 5 years from the index date of the screening in 2005. The observed numbers of cumulative death cases from gastric cancers and other cancers in each screening group were ascertained by linkage with the Niigata Prefectural Cancer Registry.
The expected numbers of gastric cancer death for the 5-year follow-up were calculated on the basis of a 5-year age group interval from 40 years to 79 years in both men and women by applying the mortality rate of the population of Niigata city[11], Niigata prefecture[12], and Japan[13,14]. The standardized mortality ratios (SMRs) and 95%CIs were also determined. The SMRs of gastric cancer death were the ratios in which the numerator represented the number of observed cancer and the denominator indicated the number of expected cancer in a reference population. The SMRs of all cancer deaths except gastric cancer deaths were also calculated using the same methods. Statistical analyses were carried out using STATA 11.0 (STATA, College Station, TX, United States).
RESULTS
The study population was divided into 3 screening groups on the basis of their participation in the screening programs in 2005. The total number of the study population was 50521 individuals and the number for each program was as follows: 16373 for the endoscopic screening, 18221 for the regular radiographic screening and 15927 for the photofluorography screening. Table 2 shows the basic characteristics of the 3 different screening groups. The participants in the photofluorography screening were younger than those in the endoscopic screening and regular radiographic screening. The number of female participants was higher in all the 3 screening groups. The cancer detection rate was higher in the endoscopic screening group than in the regular radiographic and photofluorography screening groups (Table 3). The total numbers of death from gastric cancer for 5 years after the index date of the screening in 2005 were 24 for the endoscopic screening group, 43 for the regular radiography group, and 38 for the photofluorography screening group.
Table 2.
Characteristics of the screening groups n (%)
| Endoscopy | Regular radiography | Photofluorography | |
| Age group | |||
| 40-49 yr | 229 (1.40) | 216 (1.19) | 1836 (11.53) |
| 50-59 yr | 2033 (12.42) | 2087 (11.45) | 3521 (22.11) |
| 60-69 yr | 7880 (48.13) | 8568 (47.02) | 5830 (36.60) |
| 70-79 yr | 6231 (38.06) | 7350 (40.34) | 4740 (29.76) |
| Sex | |||
| Men | 6476 (39.55) | 7019 (38.52) | 5188 (32.57) |
| Women | 9897 (60.45) | 11202 (61.48) | 10739 (67.43) |
Table 3.
Results of gastric cancer screening
| Sex | Total number of participants | Cancer detection rate (/1000) | Detected cancers (n) | Deaths from gastric cancer (n) | Deaths from all cancers except gastric cancer (n) | Screening group |
| All | 16373 | 6.29 | 103 | 24 | 216 | Endoscopy |
| Men | 6476 | 11.12 | 72 | 18 | 154 | |
| Women | 9897 | 3.13 | 31 | 6 | 62 | |
| All | 18221 | 4.28 | 78 | 43 | 266 | Regular radiography |
| Men | 7019 | 6.70 | 47 | 29 | 173 | |
| Women | 11202 | 2.77 | 31 | 14 | 93 | |
| All | 15927 | 0.75 | 12 | 38 | 208 | Photofluorography |
| Men | 5188 | 1.93 | 10 | 31 | 130 | |
| Women | 10739 | 0.19 | 2 | 7 | 78 |
The SMRs were calculated on the basis of the death rates of the 3 different reference populations (Table 4). Based on the results calculated for the population of Niigata city, the SMRs of gastric cancer death were 0.43 (95%CI: 0.30-0.57) for the endoscopic screening group, 0.68 (95%CI: 0.55-0.79) for the regular radiographic screening group and 0.85 (95%CI: 0.71-0.94) for the photofluorography screening group. The SMRs of all cancer deaths except gastric cancer deaths were 0.62 (95%CI: 0.57-0.67) for the endoscopic screening group, 0.68 (95%CI: 0.63-0.73) for the regular radiographic screening group, and 0.74 (95%CI: 0.68-0.79) for the photofluorography screening group. The mortality reduction from gastric cancer was higher in the endoscopic screening group than in the regular radiographic screening group despite the nearly equal mortality rates of all cancers except gastric cancer. The same results were obtained even if the reference population were changed to the population of Niigata prefecture and Japan. The SMRs for the endoscopic screening was 0.41 (95%CI: 0.29-0.55) in reference to the population of Niigata prefecture and 0.45 (95%CI: 0.31-0.59) in reference to the population of Japan. The same results for each screening group were obtained in both men and women. These results suggested mortality reduction from gastric cancer by endoscopic screening.
Table 4.
Comparison of standardized mortality ratio among the screening groups
| Reference population | Group |
Gastric cancer deaths |
All cancer deaths except gastric cancer deaths |
|||||
| Observed number | Expected number | SMR (95%CI) | Observed number | Expected number | SMR (95%CI) | |||
| Niigata city | Endoscopy | Total | 24 | 56 | 0.43 (0.30-0.57) | 216 | 349 | 0.62 (0.57-0.67) |
| Men | 18 | 37 | 0.49 (0.32-0.66) | 154 | 220 | 0.7 (0.64-0.76) | ||
| Women | 6 | 20 | 0.31 (0.12-0.54) | 62 | 129 | 0.48 (0.39-0.57) | ||
| Regular radiography | Total | 43 | 63 | 0.68 (0.55-0.79) | 266 | 393 | 0.68 (0.63-0.73) | |
| Men | 29 | 40 | 0.72 (0.56-0.85) | 173 | 244 | 0.71 (0.65-0.77) | ||
| Women | 14 | 23 | 0.62 (0.39-0.80) | 93 | 149 | 0.62 (0.53-0.70) | ||
| Photofluorography | Total | 38 | 45 | 0.85 (0.71-0.94) | 208 | 281 | 0.74 (0.68-0.79) | |
| Men | 31 | 27 | 1.13 (1.04-1.43) | 130 | 169 | 0.77 (0.70-0.83) | ||
| Women | 7 | 17 | 0.41 (0.18-0.67) | 78 | 112 | 0.69 (0.59-0.77) | ||
| Niigata prefecture | Endoscopy | Total | 24 | 58 | 0.41 (0.29-0.55) | 216 | 329 | 0.66 (0.61-0.71) |
| Men | 18 | 39 | 0.47 (0.30-0.63) | 154 | 204 | 0.75(0.68-0.81) | ||
| Women | 6 | 20 | 0.3 (0.12-0.54) | 62 | 125 | 0.5 (0.41-0.59) | ||
| Regular radiography | Total | 43 | 66 | 0.66 (0.52-0.76) | 266 | 371 | 0.72 (0.67-0.76) | |
| Men | 29 | 43 | 0.68 (0.44-0.75) | 173 | 227 | 0.76 (0.70-0.82) | ||
| Women | 14 | 23 | 0.61 (0.39-0.80) | 93 | 144 | 0.64 (0.56-0.72) | ||
| Photofluorography | Total | 38 | 46 | 0.83 (0.69-0.92) | 208 | 264 | 0.79 (0.74-0.84) | |
| Men | 31 | 29 | 1.08 (1.01-1.27) | 130 | 157 | 0.83 (0.76-0.88) | ||
| Women | 7 | 17 | 0.41 (0.18-0.67) | 78 | 108 | 0.72 (0.63-0.80) | ||
| Japan | Endoscopy | Total | 24 | 54 | 0.45 (0.31-0.59) | 216 | 357 | 0.6 (0.55-0.65) |
| Men | 18 | 36 | 0.5 (0.33-0.67) | 154 | 214 | 0.72 (0.65-0.78) | ||
| Women | 6 | 18 | 0.34 (0.13-0.59) | 62 | 143 | 0.43 (0.34-0.51) | ||
| Regular radiography | Total | 43 | 60 | 0.71 (0.59-0.83) | 266 | 403 | 0.66 (0.61-0.71) | |
| Men | 29 | 40 | 0.73 (0.56-0.85) | 173 | 238 | 0.73 (0.67-0.79) | ||
| Women | 14 | 21 | 0.68 (0.43-0.85) | 93 | 165 | 0.56 (0.48-0.63) | ||
| Photofluorography | Total | 38 | 42 | 0.9 (0.77-0.97) | 208 | 287 | 0.73 (0.68-0.78) | |
| Men | 31 | 27 | 1.15 (1.04-1.43) | 130 | 164 | 0.79 (0.72-0.85) | ||
| Women | 7 | 15 | 0.46 (0.21-0.73) | 78 | 123 | 0.64 (0.53-0.71) | ||
SMR: Standardized mortality ratio.
DISCUSSION
We assessed the SMRs of gastric cancer deaths in endoscopic screening and 2 radiographic screening procedures at the community level. The risk of gastric cancer death for the participants in the endoscopic screening was reduced by 57% compared with the risk for the reference population of Niigata city, Niigata prefecture, and Japan. Even if the reference population was changed, the SMRs of the endoscopic screening group were similar. The SMRs of all cancer deaths except gastric cancer deaths were nearly equal among the endoscopic screening group and the regular radiographic screening group, suggesting that the participants in the screening groups had a similar risk for gastric cancer. Even if the participants were a healthy population, morality reduction from gastric cancer was consistently higher in the endoscopic screening group than in the regular radiographic screening group.
Several studies have reported the possibility of reducing mortality from gastric cancer by endoscopic screening[7-10]. In particular, Matsumoto et al[8] showed that the SMRs of gastric cancer death decreased after the introduction of endoscopic screening in a small island as follows: 0.71 (95%CI: 0.33-1.10) for men and 0.62 (95%CI: 0.19-1.05) for women. However, an immediate decrease might be dependent on the long-term effects of radiographic screening. Since these previous reports were all observational studies and that their qualities were insufficient, the effectiveness of endoscopic screening for gastric cancer has remained unclear. Recently, 2 case-control studies have shown mortality reduction from gastric cancer by endoscopic screening[15,16]. A larger case-control study has suggested a 30% mortality reduction from gastric cancer by endoscopic screening compared with no screening, but a significant mortality reduction could not be obtained by radiographic screening[16]. Compared with previous studies, the present study showed the huge impact of endoscopic screening on mortality reduction from gastric cancer.
SMRs are commonly used for evaluating the effectiveness of cancer screening[8,17-20]. The resulting SMRs readily demonstrate the impact of cancer screening in communities. However, it is also possible to overestimate the impact of cancer screening on mortality reduction from cancers. Since the reference population included patients who could not participate in cancer screening, the mortality rate was higher than the healthy general population. Death cases from the general population included individuals whose diagnosis was made before the index date of the screening in 2005. Although the obtained impact of endoscopic screening on mortality reduction from gastric cancer in this study was considerably high at approximately 57%, careful interpretation of this result is also needed.
This study has several limitations which may result in an overestimation of the impact of gastric cancer screening on mortality reduction. First, there is possible self-selection bias in the screening groups. The participants in the screening groups were healthier than the general population and they could continue undergoing the screening. The backgrounds of the screening groups were not similar to those of the general population. Since details of the background information, including the smoking and family history, were not obtained, no adjustments could be made for the background differences. Fukao et al[21] reported differences in the smoking and family history between the participants and non-participants of gastric cancer screening.
Second, there were background differences even in the screening groups. Individuals can choose any screening method based on their preference. The age distribution of the participants was also different among the 3 screening groups. Since most of the older people have their own primary care doctor, screening could be offered easily at their clinic. The participants of the photofluorography screening program were younger than those of the other screening programs. This was because photofluorography screening was mainly provided as a mass screening program which was often participated in by individuals who had no primary care doctor.
Third, the screening history before the index date of the screening in 2005 was ignored. Radiographic screening was performed before the introduction of endoscopic screening. Some participants changed their subsequent screening program from radiographic screening to endoscopic screening. The rate of participants who had no screening history within 2 years from the index date of the screening in 2005 was 15.5% for the endoscopic screening and 5.7% for the regular radiographic screening.
Fourth, the sample size was small because of the low participation rate in gastric cancer screening. Although the participation rate in gastric cancer screening has increased since the introduction of endoscopic screening, the screening rate has remained at approximately 25%[10].
Finally, the follow-up period was limited to 5 years. Thus, the full impact of the screening program may not have been realized as the screening effect cannot be expected within a short period of time but within several years after the introduction of a new screening program[22]. Since more early-stage cancer was detected by endoscopic screening than by radiographic screening, there may be a difference in the preclinical phase between endoscopic screening and radiographic screening. A longer preclinical phase should be assessed, because most cancers detected by endoscopy were early-stage and slow-growing cancers. A longer follow-up is needed to comprehensively evaluate the effectiveness of endoscopic screening.
In conclusion, the present findings suggest that endoscopic screening might maximally reduce mortality from gastric cancer by 57%. Although such reduction rate suggests the effectiveness of endoscopic screening for gastric cancer, prudent interpretation of this result is needed considering the above-mentioned limitations of the present study. Additional evidence supporting mortality reduction from gastric cancer by endoscopic screening is desired to realize the introduction of endoscopic screening in communities.
ACKNOWLEDGMENTS
We thank the cooperation of the Niigata Prefecture Cancer Registry, Niigata Medical Association and Niigata City Public Health Center. We appreciate the helpful comments of Dr. Tomio Nakayama. We are also grateful to Dr. Edward F Barroga, Associate Professor and Senior Medical Editor of Tokyo Medical University for reviewing and editing of the manuscript.
COMMENTS
Background
The burden of gastric cancer still remains in Asia and East European countries. Endoscopy, which is commonly used in clinical practice, is anticipated to be a promising screening method for gastric cancer. Although several studies have reported the possibility of reducing mortality by endoscopic screening, definitive evidence remains to be established.
Research frontiers
Authors investigated mortality reduction from gastric cancer on the basis of the results of endoscopic screening. The standardized mortality ratio (SMR) of gastric cancer and other cancer deaths in each screening group was calculated by applying the mortality rate of the reference population.
Innovations and breakthroughs
The 57% mortality reduction from gastric cancer might indicate the effectiveness of endoscopic screening for gastric cancer. The mortality reduction from gastric cancer was higher in the endoscopic screening group than in the regular radiographic screening group despite the nearly equal mortality rates of all cancers except gastric cancer.
Applications
The results suggest mortality reduction from gastric cancer by endoscopic screening. This can serve as supporting evidence regarding the effectiveness of this screening method for gastric cancer and its possible introduction in communities.
Terminology
The SMRs of cancer death were the ratios in which the numerator represented the number of observed cancer and the denominator indicated the number of expected cancer in a reference population.
Peer-review
The authors investigated the effectiveness of endoscopic screening by calculating the mortality reduction from gastric cancer. Although many endoscopists believe that endoscopic screening is the most effective method for gastric cancer screening, there have been scarce data on the mortality reduction effect by endoscopic screening, thus radiographic screening for gastric cancer is presently recommended for the public in Japan. Therefore, this study is very valuable as it provides supporting evidence regarding the effectiveness of endoscopic screening in reducing mortality from gastric cancer.
Footnotes
Supported by Grant-in-Aid for H22-Third Term Comprehensive Control Research for Cancer 022 from the Japanese Ministry of Health, Labour and Welfare.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 29, 2014
First decision: August 15, 2014
Article in press: November 11, 2014
P- Reviewer: Bilir C, Tsuji Y S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
References
- 1.International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available from: http://globocan.iarc.fr/
- 2.Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–287. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute. Stomach (Gastric) Cancer Screening (PDQ®) Available from: http://www.cancer.gov/cancertopics/pdq/screening/gastric/HealthProfessional/page2.
- 4.Kim Y, Jun JK, Choi KS, Lee HY, Park EC. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev. 2011;12:725–730. [PubMed] [Google Scholar]
- 5.Hamashima C, Shibuya D, Yamazaki H, Inoue K, Fukao A, Saito H, Sobue T. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38:259–267. doi: 10.1093/jjco/hyn017. [DOI] [PubMed] [Google Scholar]
- 6.Oshima A. A critical review of cancer screening programs in Japan. Int J Technol Assess Health Care. 1994;10:346–358. doi: 10.1017/s0266462300006590. [DOI] [PubMed] [Google Scholar]
- 7.Riecken B, Pfeiffer R, Ma JL, Jin ML, Li JY, Liu WD, Zhang L, Chang YS, Gail MH, You WC. No impact of repeated endoscopic screens on gastric cancer mortality in a prospectively followed Chinese population at high risk. Prev Med. 2002;34:22–28. doi: 10.1006/pmed.2001.0925. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto S, Yamasaki K, Tsuji K, Shirahama S. Results of mass endoscopic examination for gastric cancer in Kamigoto Hospital, Nagasaki Prefecture. World J Gastroenterol. 2007;13:4316–4320. doi: 10.3748/wjg.v13.i32.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosokawa O, Miyanaga T, Kaizaki Y, Hattori M, Dohden K, Ohta K, Itou Y, Aoyagi H. Decreased death from gastric cancer by endoscopic screening: association with a population-based cancer registry. Scand J Gastroenterol. 2008;43:1112–1115. doi: 10.1080/00365520802085395. [DOI] [PubMed] [Google Scholar]
- 10.Ogoshi K, Narisawa R, Kato T, Fujita K, Sano M. Endoscopic screening for gastric cancer in Niigata city. Jpn J Endoscopic Forum Digestive Disease. 2010;26:5–16. [Google Scholar]
- 11.Niigata City Government. Annual report of health and welfare 2007-2013. Available from: http://www.city.niigata.lg.jp/shisei/toukei/hoken/shiryo.html.
- 12.Annual report of health and welfare 2007-2013. Available: from: URL: http://www.pref.niigata.lg.jp/fukushihoken/1197476210319.html
- 13.Ministry of Health, Welfare and Labour. National Population Survey 2010. Available from: http://www.e-stat.go.jp/estat/html/kokusei/NewList-000001039448.html.
- 14.National Cancer Center. Center for Cancer Control and Information Services. Available from: http://ganjoho.ncc.go.jp/professional/statistics/index.html.
- 15.Matsumoto S, Yoshida Y. Efficacy of endoscopic screening in an isolated island: a case-control study. Indian J Gastroenterol. 2014;33:46–49. doi: 10.1007/s12664-013-0378-2. [DOI] [PubMed] [Google Scholar]
- 16.Hamashima C, Ogoshi K, Okamoto M, Shabana M, Kishimoto T, Fukao A. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS One. 2013;8:e79088. doi: 10.1371/journal.pone.0079088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hakama M, Pukkala E, Söderman B, Day N. Implementation of screening as a public health policy: issues in design and evaluation. J Med Screen. 1999;6:209–216. doi: 10.1136/jms.6.4.209. [DOI] [PubMed] [Google Scholar]
- 18.Dominioni L, Poli A, Mantovani W, Pisani S, Rotolo N, Paolucci M, Sessa F, Conti V, D’Ambrosio V, Paddeu A, et al. Assessment of lung cancer mortality reduction after chest X-ray screening in smokers: a population-based cohort study in Varese, Italy. Lung Cancer. 2013;80:50–54. doi: 10.1016/j.lungcan.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Burnell M, Gentry-Maharaj A, Ryan A, Apostolidou S, Habib M, Kalsi J, Skates S, Parmar M, Seif MW, Amso NN, et al. Impact on mortality and cancer incidence rates of using random invitation from population registers for recruitment to trials. Trials. 2011;12:61. doi: 10.1186/1745-6215-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nawa T, Nakagawa T, Mizoue T, Kusano S, Chonan T, Hayashihara K, Suito T, Endo K. A decrease in lung cancer mortality following the introduction of low-dose chest CT screening in Hitachi, Japan. Lung Cancer. 2012;78:225–228. doi: 10.1016/j.lungcan.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Fukao A, Hisamichi S, Komatsu S, Shimizu H, Satoh H, Nakatsuka H, Watanabe T, Fujisaku S, Ichinowatari Y, Kuroda S. Comparison of characteristics between frequent participants and non-participants in screening program for stomach cancer. Tohoku J Exp Med. 1992;166:459–469. doi: 10.1620/tjem.166.459. [DOI] [PubMed] [Google Scholar]
- 22.Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441. doi: 10.1136/bmj.e8441. [DOI] [PMC free article] [PubMed] [Google Scholar]


