Abstract
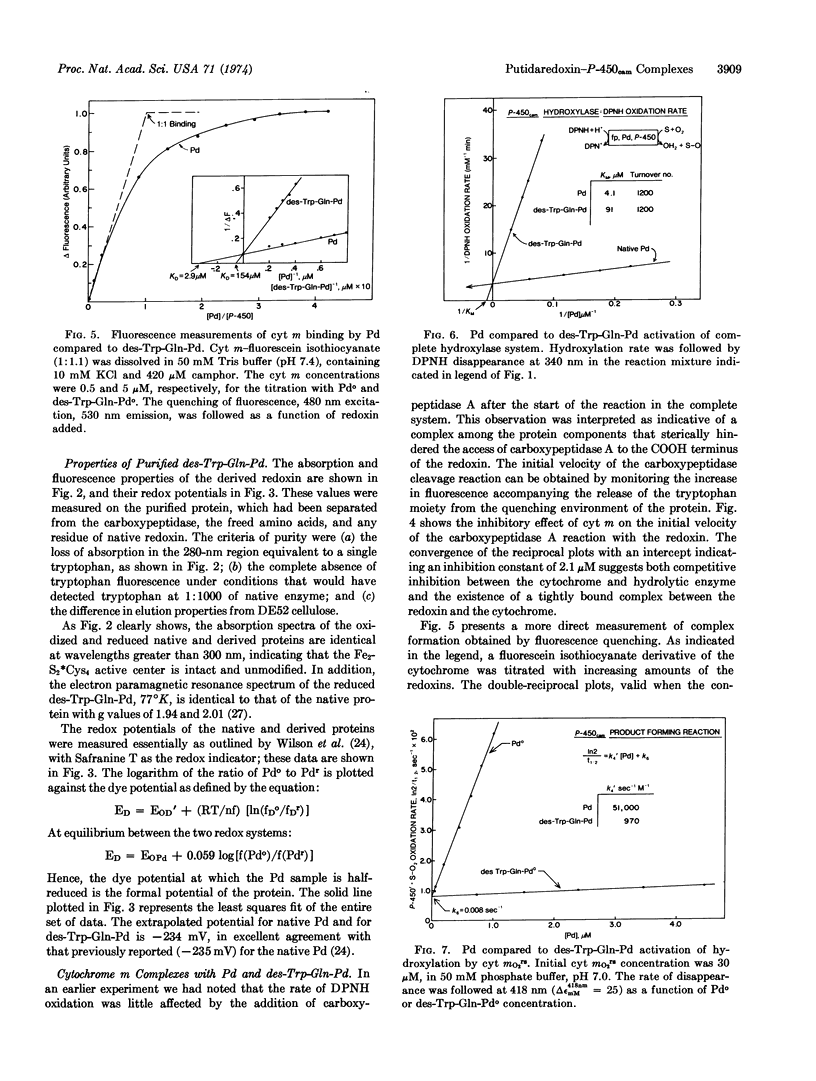
Methylene hydroxylation by cytochrome P-450cam (cytochrome m) can be resolved into four distinct steps: substrate addition, mo → mos; reduction, mos → mrs; dioxygen addition, mrs → mO2rs; followed by a second putidaredoxin (Pseudomonas putida ferredoxin)-mediated reduction and product formation. The isolated ferrous oxy-substrate complex exhibits first-order decay kinetics with the relatively slow rate constant of k [unk] 0.01 sec-1, at 25°, without product release. Putidaredoxin addition accelerates the decomposition with second-order kinetics, k [unk] 51,000 M-1 sec-1, and initiation of product formation. Cytochrome m forms a complex with putidaredoxin with dissociation constant of KD = 3 μM. In the complete three-protein hydroxylase system, consisting of cytochrome m, putidaredoxin, and the reductase (a DPNH-specific flavo-protein), camphor hydroxylation occurs with a stoichiometry of 1 mole each of DPNH and O2 used per mole of product formed; the KM for putidaredoxin is about 4.2 μM.
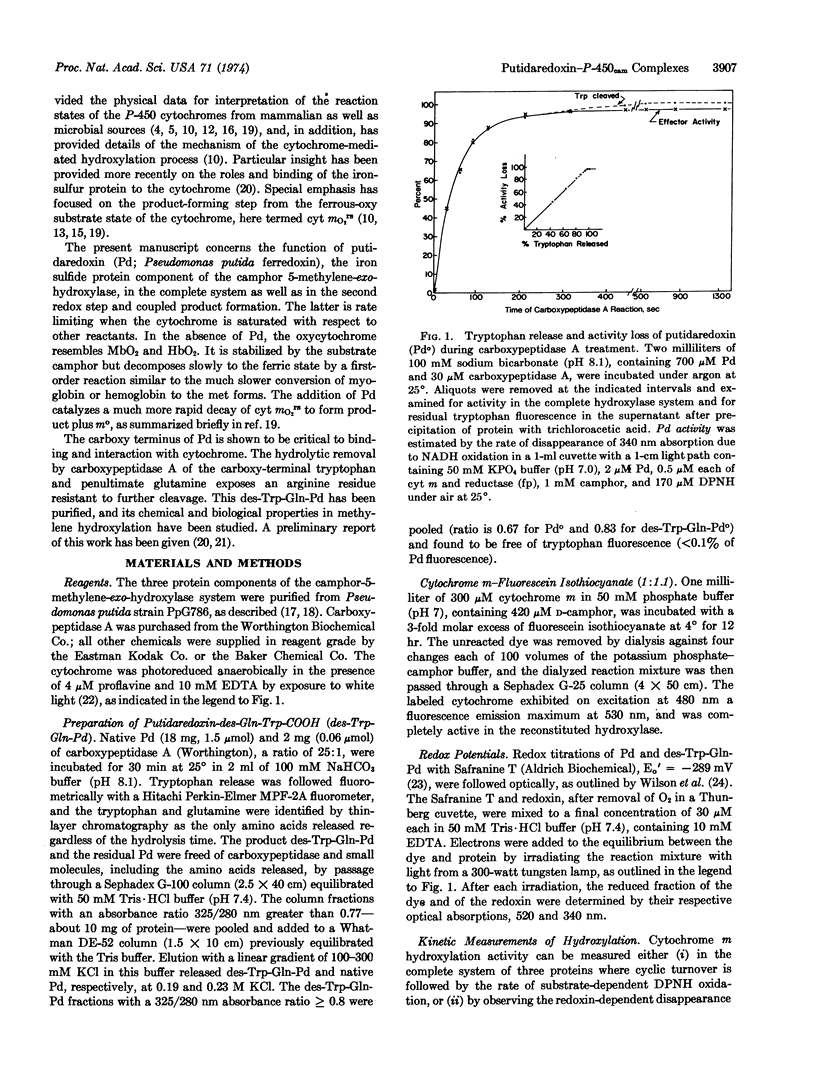
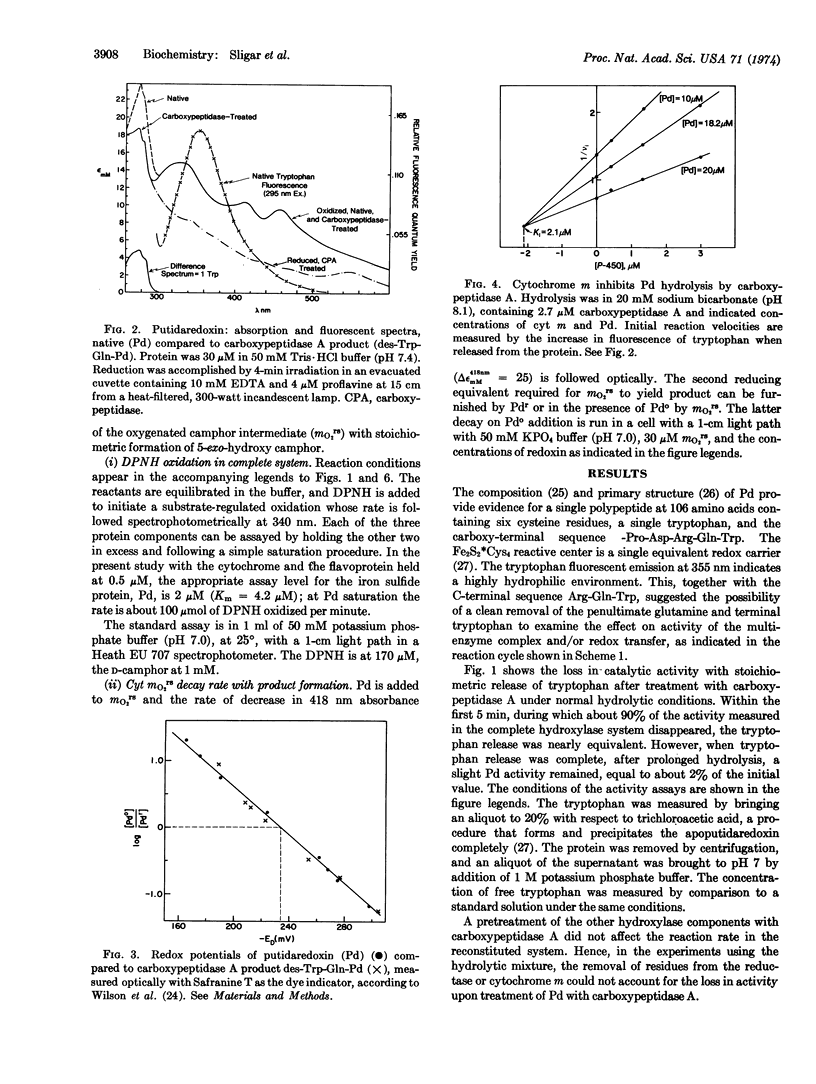
Putidaredoxin, on treatment with carboxypeptidase A, loses one molecule each of tryptophan and glutamine sequentially from the carboxy terminus to expose a terminal arginine. The tryptophan-free product has been separated from native putidaredoxin and other impurities, and retains the visible and electron paramagnetic resonance spectra and the redox potential of the active center of native putidaredoxin. This modified redoxin binds less tightly to cytochrome m, KD [unk] 150 μM, and is 50 times less effective in stimulation of the mO2rs decay rate. A similar decrease in specific activity is observed in the complete hydroxylase system.
Keywords: monooxygenase activity, protein modification, iron-sulfur protein-cytochrome complex, fluorescence, cytochrome P-450
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby A. C. A soluble haemoprotein P 450 from nitrogen-fixing Rhizobium bacteroids. Biochim Biophys Acta. 1967 Oct 23;147(2):399–402. doi: 10.1016/0005-2795(67)90423-0. [DOI] [PubMed] [Google Scholar]
- Coon M. J., Strobel H. W., Autor A. P., Heidema J., Duppel W. Functional components and mechanism of action of the resolved liver microsomal enzyme system catalyzing fatty acid, hydrocarbon and drug hydroxylation. Biochem Soc Symp. 1972;34:45–54. [PubMed] [Google Scholar]
- Estabrook R. W., Baron J., Peterson J., Ishimura Y. Oxygenated cytochrome P-450 as an intermediate in hydroxylation reactions. Biochem Soc Symp. 1972;34:159–185. [PubMed] [Google Scholar]
- Greenbaum E., Austin R. H., Frauenfelder H., Gunsalus I. C. Photoreduction of NADP + sensitized by synthetic pigment systems. Proc Natl Acad Sci U S A. 1972 May;69(5):1273–1276. doi: 10.1073/pnas.69.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus I. C., Lipscomb J. D., Marshall V., Frauenfelder H., Greenbaum E., Münck E. Structure and reactions of oxygenase active centers: cytochrome P-450 and iron sulfur proteins. Biochem Soc Symp. 1972;34:135–157. [PubMed] [Google Scholar]
- Herskovitz T., Averill B. A., Holm R. H., Ibers J. A., Phillips W. D., Weiher J. F. Structure and properties of a synthetic analogue of bacterial iron--sulfur proteins. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2437–2441. doi: 10.1073/pnas.69.9.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri M., Ganguli B. N., Gunsalus I. C. A soluble cytochrome P-450 functional in methylene hydroxylation. J Biol Chem. 1968 Jun 25;243(12):3543–3546. [PubMed] [Google Scholar]
- OMURA T., SATO R. A new cytochrome in liver microsomes. J Biol Chem. 1962 Apr;237:1375–1376. [PubMed] [Google Scholar]
- Omura T., Sato R., Cooper D. Y., Rosenthal O., Estabrook R. W. Function of cytochrome P-450 of microsomes. Fed Proc. 1965 Sep-Oct;24(5):1181–1189. [PubMed] [Google Scholar]
- SUZUKI K., KIMURA T. AN IRON PROTEIN AS A COMPONENT OF STEROID 11-BETA-HYDROXYLASE COMPLEX. Biochem Biophys Res Commun. 1965 Apr 23;19:340–345. doi: 10.1016/0006-291x(65)90465-1. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Yasunobu K. T. The amino acid sequence of putidaredoxin, an iron-sulfur protein from Pseudomonas putida. J Biol Chem. 1974 Jun 25;249(12):3689–3701. [PubMed] [Google Scholar]
- Tsai R. L., Gunsalus I. C., Dus K. Composition and structure of camphor hydroxylase components and homology between putidaredoxin and adrenodoxin. Biochem Biophys Res Commun. 1971 Dec 3;45(5):1300–1306. doi: 10.1016/0006-291x(71)90160-4. [DOI] [PubMed] [Google Scholar]
- Tsibris J. C., Tsai R. L., Gunsalus I. C., Orme-Johnson W. H., Hansen R. E., Beinert H. The number of iron atoms in the paramagnetic center (G =1.94) of reduced putidaredoxin, a nonheme iron protein. Proc Natl Acad Sci U S A. 1968 Mar;59(3):959–965. doi: 10.1073/pnas.59.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. S., Tsibris J. C., Gunsalus I. C. Electrochemical studies of putidaredoxin and its selenium analog. J Biol Chem. 1973 Sep 10;248(17):6059–6061. [PubMed] [Google Scholar]
- Yu C., Gunsalus I. C. Crystalline cytochrome P-450cam. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1431–1436. doi: 10.1016/0006-291x(70)90027-6. [DOI] [PubMed] [Google Scholar]
- Yu C., Gunsalus I. C., Katagiri M., Suhara K., Takemori S. Cytochrome P-450cam. I. Crystallization and properties. J Biol Chem. 1974 Jan 10;249(1):94–101. [PubMed] [Google Scholar]


