Abstract
Vector-borne diseases continue to contribute significantly to the global burden of disease, and cause epidemics that disrupt health security and cause wider socioeconomic impacts around the world. All are sensitive in different ways to weather and climate conditions, so that the ongoing trends of increasing temperature and more variable weather threaten to undermine recent global progress against these diseases. Here, we review the current state of the global public health effort to address this challenge, and outline related initiatives by the World Health Organization (WHO) and its partners. Much of the debate to date has centred on attribution of past changes in disease rates to climate change, and the use of scenario-based models to project future changes in risk for specific diseases. While these can give useful indications, the unavoidable uncertainty in such analyses, and contingency on other socioeconomic and public health determinants in the past or future, limit their utility as decision-support tools. For operational health agencies, the most pressing need is the strengthening of current disease control efforts to bring down current disease rates and manage short-term climate risks, which will, in turn, increase resilience to long-term climate change. The WHO and partner agencies are working through a range of programmes to (i) ensure political support and financial investment in preventive and curative interventions to bring down current disease burdens; (ii) promote a comprehensive approach to climate risk management; (iii) support applied research, through definition of global and regional research agendas, and targeted research initiatives on priority diseases and population groups.
Keywords: climate change, vector-borne disease, health policy, research
1. Importance of climate-sensitive vector-borne diseases
The WHO estimates that one-sixth of the illness and disability suffered worldwide is owing to vector-borne diseases, with more than half of the world's population currently at risk. Every year, more than one billion people are infected, and more than one million people die from vector-borne diseases, including malaria, dengue, schistosomiasis, leishmaniasis, Chagas disease and African trypanosomiasis [1,2]. In addition to deaths, many vector-borne diseases, such as lymphatic filariasis and onchocerciasis, also cause significant debilitation and suffering, contributing to a much larger overall burden of disease [1,3].
Vector-borne diseases also have wider socioeconomic impacts, increasing health inequities, and acting as a brake on socioeconomic development. The burden of climate-sensitive diseases is greatest for the poorest populations. For example, the per capita mortality rate from vector-borne diseases is almost 300 times greater in developing nations than in developed regions [1], both because vector-borne diseases are more common in the tropical climates of many developing countries, and also because of low levels of socioeconomic development and coverage of health services in these areas. In addition, vector-borne disease risks are typically much greater for poor individuals within any population owing to poorer environmental and social conditions (e.g. lower-quality housing situated closer to vector-breeding sites), and lack of access to preventive and curative health interventions and services [4].
Even for diseases that are less strictly correlated with poverty, and populations that are comparatively better protected, vector-borne diseases nonetheless have important impacts on individuals, households and on health systems. For example, an estimated 500 000 people with severe dengue require hospitalization each year, a large proportion of whom are children. Estimates based on studies from across eight countries indicate that an average dengue episode represents 14.8 lost days for ambulatory patients (at an average cost of US$ 514) and 18.9 days for non-fatal hospitalized patients (at an average cost of US$ 1491) [5]. Epidemics of vector-borne disease have the capacity to overwhelm health systems, and impact on other sectors, such as tourism.
Important progress has been made against vector-borne diseases, through a combination of poverty alleviation and socioeconomic development, increased access to health services, larger scale and more coordinated control programmes, and the development and deployment of more effective interventions. As a result of these successes, the proportional contribution of vector-borne diseases to global mortality has fallen in recent years [2]. Nonetheless, important challenges remain. Not all vector-borne diseases are decreasing in incidence globally, and some diseases such as malaria, which are decreasing at the global scale, are stable or increasing in specific locations. In addition, the sustainability of the gains that have been made in combatting vector-borne disease is at risk from factors such as insecticide and drug resistance, the difficulties in maintaining political will and resources for disease control programmes as incidence is driven to low levels, and the potential for spread or re-emergence of diseases, with potentially much greater health impacts in populations that have lost immunity (e.g. for malaria, see [6]).
In summary, vector-borne diseases constitute an important cause of death, disease burden and health inequity, a brake on socioeconomic development, and a strain on health services. Continued progress in controlling these diseases is therefore an important contribution to global health, development and security.
Against this context, health agencies engaged in control of vector-borne disease need to consider (i) the scale and nature of the risks that climate change may present to vector-borne disease control, by disease and location; (ii) whether these may either undermine or overwhelm the effect of control programmes; and (iii) effective measures to increase the resilience of health—and health programmes—to long-term climate change, while also reinforcing current disease control efforts.
2. Climate as one of many interacting determinants of vector-borne disease
Vector-borne diseases are among the most well studied of the diseases associated with climate change, owing to their large disease burden, widespread occurrence and high sensitivity to climatic factors. In contrast to some other climate-sensitive health risks, such as heat-stress, or exposure to storms and floods, the influence of meteorological factors are less direct, and more diverse, both within and between individual diseases [7]. The simplest connections are through temperature, affecting the biting, survival and reproductive rates of the vectors, and the survival and development rates of the pathogens that they carry. Precipitation also exerts a very strong influence, most obviously in the case of diseases transmitted by vectors that have aquatic developmental stages (such as mosquitoes), but also, via humidity, on diseases transmitted by vectors without such stages, such as ticks or sandflies.
Climate and weather conditions also exert a range of more indirect effects, through wider effects on the natural environment and on human systems, for example as drought may affect water-storage, land-use and irrigation practices, and population movement, in turn, affecting vector ecology, and human exposure to infection (e.g. see reviews in references [7–11]). For example, a recent WHO report summarizing the importance of vector-borne diseases states that previously relatively stable geographical distributions are now changing owing to a range of factors, ‘including climate change, intensive farming, dams, irrigation, deforestation, population movements, rapid unplanned urbanization, and phenomenal increases in international travel and trade’ [12, p. 6]. These environmental and social factors may either reinforce climate effects (for example where dams and water transport projects combine with increasingly suitable temperature conditions for schistosomiasis transmission [13]), or counteract them (e.g. as urbanization reduces the relative importance of more rural vector-borne diseases, such as malaria [14]).
Given the strength and range of these connections, it is not surprising that there is abundant observational evidence of the effects of meteorological factors, from seasonal and interannual patterns of disease incidence in specific locations, to the strong explanatory power of climate variables in accounting for the geographical distribution of most, if not all, vector-borne diseases [7]. Long-term anthropogenic climate change interacts with natural variability, influencing vector-borne disease transmission from shorter (e.g. annual) to longer (e.g. decadal) time scales, with variable effects at different times and in different locations. These influences may reinforce each other, for example in locations where the temperature increases associated with El Niño events are superimposed on long-term increase in temperature, or may oppose each other, for example as changes in global temperature over the past decade or so appear to have currently damped the longer-term upward temperature trends [15].
The complexity of these interactions means that the effect of climate change, and the nature and extent of interaction with non-climate factors, varies markedly by diseases and by location. The effects of climate on disease transmission may be obscured, for example where the vectors are relatively buffered against weather and climate owing to living entirely inside houses (such as the triatomine bugs, which transmit Chagas disease), or where the pathogens have long development periods in the host (such as the nematode worms that cause filariasis). Even in diseases with superficially similar transmission cycles can be affected very differently by climate change. The difference can be illustrated by consideration of malaria and dengue, two of the most important and well-studied vector-borne diseases, both anthroponoses transmitted by mosquitoes.
The WHO estimates that in 2012 there were about 207 million cases and 627 000 deaths attributable to malaria [6,12]. The climate changes that have occurred over the previous century have significantly altered the areas climatically suitable for transmission. For example, in Africa, areas that have become unsuitable for transmission, mainly through drying, have approximately equalled those that have become suitable areas owing to increased temperatures and greater precipitation [16] (although the latter tend to be more densely populated). At the same time, the strong protective effect of improving socioeconomic conditions, and increased coverage of the range of effective preventive and curative interventions for malaria, have led to a decreasing global distribution of transmission over the past century [17] and a decreasing aggregate disease burden in the past decade or so [6].
The relationships between meteorological factors and either the components of the transmission cycle (e.g. parasite development rates, vector biting and survival rates) or the observed geographical distribution of disease have been used to generate predictive models. These link projections of future scenarios of climate change, with more recent models also including other determinants including GDP (as a measure of socioeconomic and technological development), and, in some cases, urbanization. The outputs of most of the models produced to date are necessarily highly approximate as they are affected both by uncertainties in climate projections and future development trends and by the confounding effect of natural climate variability over short to medium timescales (i.e. years to one to two decades). They can therefore give indications of broad trends for periods averaged over several decades (e.g. the proportional effect of climate changes in population at risk at the global or continental scale for the 2030s or the 2050s), rather than at local level for specific years.
The indications from the large-scale modelling work to date are that climate change is expected to make malaria burdens higher than they would otherwise have been [14,18,19]. However, providing that it is possible to maintain poverty alleviation and investment in overall health sector and disease-specific interventions, and to manage risks such as insecticide and drug resistance, then it should be possible to continue to drive down global malaria rates in the coming decades. Climate change may still, however, have important effects in specific locations, including at the margins of distributions, and in any locations where the protective factors above are not maintained [20,21].
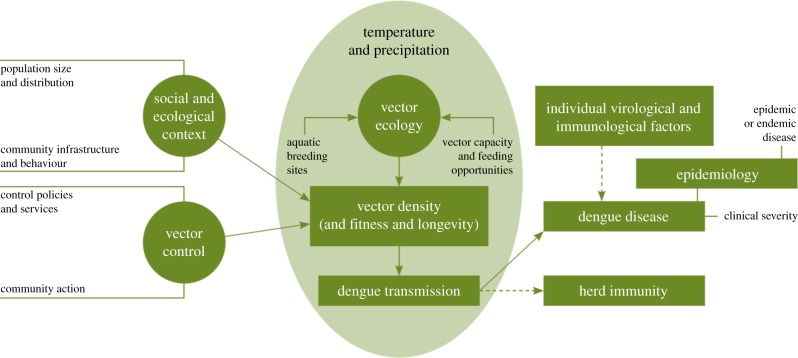
The evidence of past trends and scenarios of future effects for dengue is very different. It is estimated that there are approximately 100 million dengue infections worldwide each year [6,12]. As for malaria, there is very strong laboratory and field evidence for sensitivity to meteorological variables, and for the modifying effect of non-climatic factors. Modelling studies also suggest that climate change has favoured, and will continue to favour dengue transmission [22,23]. By contrast, the evidence of the protective effect of either general socioeconomic development, or specific disease control measures, is much weaker than for malaria. Consequently, dengue incidence is high in many regions where malaria has been effectively controlled or eradicated, including highly developed economies [24]. In addition, dengue transmission tends to be higher in urban areas, particularly those with poor-quality housing, provision of water and sanitation and waste management [25]. The increasing movement of people and of goods has facilitated the international spread of strains of dengue virus, and of dengue vectors [25]. Dengue infection gives rise to a complex immunological response, which is affected by age and possibly pre-exposure to other strains; there is currently no effective vaccine and comparatively limited evidence for the effectiveness of vector-control interventions [26–28]. In the case of dengue, therefore, trends in non-climatic factors such as increases in urbanization (particularly slums), and international trade and travel, can be expected to amplify, rather than oppose, the effects of climate change (figure 1).
Figure 1.
Interaction of meteorological and other determinants of dengue transmission cycles and clinical disease [29]. (Online version in colour.)
In summary, climate is an important influence on vector-borne disease transmission, and there is evidence that ongoing climate change is affecting, and will continue to affect the distributions and burdens of these infections. The interactions are complex, however, and to move beyond these broad generalizations to inform health policy requires assessment of individual diseases with respect to specific disease control decisions.
3. The relevance of different kinds of evidence for health policy
There has been a marked increase in research output on climate change and health in recent years, with vector-borne disease among the better-represented subject areas [30]. As the field develops further, there is a need to consider carefully what kinds of research will be most useful in supporting the societal response to climate change. At the World Health Assembly in 2008, the 192 Member States of the WHO passed a resolution to strengthen action on health and climate change [31], which included specification of the areas of research and pilot projects that should be supported (box 1). This has been further elaborated through consultation with a range of stakeholders [32]. The emphasis in the resolution and the associated workplan [33] is therefore on applied research with a relatively direct connection to policy decisions, particularly within the health sector.
Box 1. Priorities for climate change and health research and pilot studies, as defined by WHO Member States.
World Health Assembly Resolution 61.19, on climate change and health [31, p. 2], requests the WHO ‘to continue close cooperation with appropriate United Nations organizations, other agencies and funding bodies, and Member States, to develop capacity to assess the risks from climate change for human health and to implement effective response measures, by promoting further research and pilot projects in this area, including work on
— health vulnerability to climate change and the scale and nature thereof;
— health protection strategies and measures relating to climate change and their effectiveness, including cost-effectiveness;
— the health impacts of potential adaptation and mitigation measures in other sectors such as water resources, land use and transport, in particular where these could have positive benefits for health protection;
— decision-support and other tools, such as surveillance and monitoring, for assessing vulnerability and health impacts and targeting measures appropriately; and
— assessment of the likely financial costs and other resources necessary for health protection from climate change’.
(a). Detection and attribution studies
A significant proportion of studies in this field aim to detect changes in vector-borne disease, and assess whether these can be attributed wholly or in part to long-term anthropogenic climate change. Such direct empirical tests are difficult, as they require very long time series of data on disease, relevant meteorological variables and potential confounding factors such as socioeconomic determinants, coverage of control programmes and resistance to insecticides or treatment [34]. There has consequently been considerable controversy over the attribution of some changes to climate change (e.g. [35–37] for malaria in the East African highlands). Despite these challenges, there are now studies with relatively robust evidence of long-term (i.e. decadal) climate change that have contributed, at least in part, to increases in disease incidence or distribution in specific locations [38–40].
Isolating the effect of climate change on specific changes in disease incidence contributes to the evidence base on climate change impacts, and supports the overall case for mitigation and adaptation. Such studies are also of local relevance, to the extent that they can demonstrate that climate change has impacted disease incidence in the past, and by inference may do so in the future, as well as allowing quantitative assessment of the sensitivity of the disease in question to meteorological variables. However, such studies in isolation are insufficient guidance for health programmes, because sufficient high-quality and long time series of data are not routinely available to carry out such analyses for most diseases in most locations, and because evaluations of past effects of climate change are not automatically predictive of the future. More fundamentally, the main justification for epidemiological attribution studies is to isolate and assess the effects of individual risk factors that may be addressed by interventions. Attribution is of limited relevance to managers of disease control programmes, who do not have at their disposal specific interventions to reduce climate change.
(b). Scenario modelling
There is now a substantial body of work applying scenarios of future climate change to models based either on the known effects of meteorological variables on the various processes within the disease transmission cycle, or the observed statistical relationships between climate and the spatial or temporal distribution of vector-borne disease [18]. These approaches have been used to generate projections of the potential effects of future climate change on trends in disease. In recent years, there have been improvements in both the degree to which such models are validated against observed distributions and incidence in the past [20,41], and the extent to which they incorporate both the independent and interactive effects of non-climatic factors, such as changes in population size and distribution (e.g. urbanization rates), and economic development [23]. Such studies can therefore give broad indications of potential future effects of climate change, of the relative importance of climate versus other determinants, and of different diseases, and indicate areas that are likely to become more or less suitable for transmission in the future.
As for attribution studies, however, there are important limitations on the application of such approaches to inform current decisions to protect populations from vector-borne diseases. First, the initial interest in scenario modelling was mainly to examine the effect of different scenarios of human-induced climate change, and the degree to which these may be affected by different trajectories of greenhouse gas emissions, so as to inform global discussions on mitigation. As it is only possible to differentiate the effect of anthropogenic climate change from natural variation over several decades [15,42], these projections are necessarily long term (e.g. proportional changes in population at risk averaged for the 2030s or the 2050s), and usually at global or continental level. The majority of scenario studies therefore do not match closely to the shorter time-frames and narrower geographical and sectoral focus that characterize most disease control decisions.
Second, modelling approaches remain limited in the scope of mechanisms that they cover. For example, much of the early work in this area has focused more on temperature rather than precipitation changes, because these are comparatively easier to represent both in global climate models, and in terms of their effects on disease transmission. Precipitation is at least as important for vector-borne disease transmission, but projections from climate models are considerably less consistent between global climate models, or robust at regional or smaller scales [43]. This is even more of a challenge for likely, but more complex, interactions such as the effect of climate change on land-use, irrigation or water-storage practices, and in turn, on vector-borne disease.
Third, they are also subjected to the limitations of compounding uncertainties in the various stages of the modelling process, and of the fact that the realized outcome in terms of disease burdens is contingent on decisions over several decades, affecting components throughout the causal chain, from emissions of greenhouse gases, to rates and patterns of economic growth, to the development and deployment of control interventions [7,42].
Further development of approaches to scenario analysis would therefore help to make them more relevant to national level disease control decisions. This would require more explicit definition of the kinds of control decisions that may be informed by climate scenarios, and their associated timescales (e.g. weeks or months for the deployment of health personnel, years or decades for the design of disease eradication programmes, or the siting of health facilities) [44]: (i) more integrated assessment of projected changes in climate alongside other determinants, such as population distribution and economic development; (ii) use of climate and disease transmission models with higher spatial and temporal resolution, for example, through the use of regional climate models; and (iii) a more holistic approach to understanding the combined effects of both natural- and human-induced climate change.
(c). Research on resilience and risk management approaches
The research priorities identified by national governments (box 1) provide useful guidance to us in making more direct connections with implementation. This includes calls not only for improved assessments of the scale and nature of climate risks (which can be considered to include detection and attribution and scenario modelling studies), but also for more applied research questions, covering intervention effectiveness, decision-support systems, assessment of policy decisions and resource implications [45].
These requests from senior health policy-makers are currently not well matched by research output. A systematic scoping review of the peer-reviewed literature on climate change and health, including vector-borne diseases, identified a number of research studies corresponding to the various priorities outlined by the WHO member states. However, there was a notable absence of studies identified on effective adaptation options as well as a general under-representation of research from poorer regions. The review notes that this partly represents a failure to connect the very large body of research on effective interventions to control climate-sensitive diseases (e.g. on vector-control methods) to the longer-term challenge of climate change [30].
The mismatch between the expressed requirements of policy-makers and the coverage of peer-reviewed studies argues for focusing less explicitly on climate change per se, and instead taking a broader approach to increasing resilience to climate, alongside other risks. This approach starts from the premise that the ultimate objective is not to address the specific health risks that are uniquely attributable to climate change (which are difficult to isolate from other determinants, either analytically or within control programmes), but instead to ensure sustained progress in decreasing vector-borne disease into the future.
This approach avoids the unhelpful characterization of control of vector-borne disease, and addressing long-term climate change (including its health risks), as opposing and competing interests. Instead, it recognizes that one of the most effective ways to protect health against climate change impacts in the long term is to drive down disease rates in the present. It validates increased coverage of basic public health interventions, and disease-specific control measures, not only as beneficial interventions in their own right but also as effective measures to increase protection from climate change.
Applying the research priorities identified by national governments to vector-borne disease suggests a programme of applied research that would include (i) assessments of the risks, including quantitative detection and attribution and scenario studies as above, but also more qualitative vulnerability and adaptation assessment that can explore a wider range of mechanisms [46]; (ii) evaluation of the effectiveness of individual interventions, or control programmes, including the degree to which climate variability and change may influence their effectiveness [47–49]; (iii) health impact assessment for climate adaptation and mitigation decisions that may affect vector-borne disease, such as irrigation schemes or changes in water-storage practice providing breeding sites for vectors; (iv) surveillance, monitoring and associated decision-support tools, including the use of climate information as a resource to provide earlier warning of infectious disease epidemics and improve spatial targeting, for example [50], and connection to standard operating procedures, such as the International Health Regulations, to address public health emergencies of international concern [51]; and (v) assessment of financial and other resource requirements, such as the costs that would be necessary to extend vector-borne disease surveillance or control interventions to newly suitable locations or seasons.
4. Priorities for WHO support to operational programmes
Increased research effort on identifying ‘no regrets' measures to increase climate resilience would facilitate the link to operational programmes. In recent years, the field of climate change and health has developed from awareness raising and academic research, to a much more direct connection with core health policy and implementation programmes. For example, since 2008, the WHO has implemented large-scale pilot projects on health adaptation to climate change in 17 countries, across all of its regions [52]. Despite this rapid growth, the scale of this investment is only a fraction of the estimated costs of damage to health, or of investment in adaptation in non-health sectors. There is therefore widespread acceptance of the need for more resources to be devoted to this area.
As investments are hopefully scaled up, it will become increasingly important to have a clear and structured framework for directing and monitoring their effectiveness. One of the most important considerations is to ensure that new resources strengthen, rather than distract from or compete with, existing health structures and priorities. In order to do this, the WHO is building on the initial work of the African workplan for health adaptation to climate change, to develop an operational framework that identifies the key health systems components that need to be strengthened to increase resilience to climate change, and which should be applicable across climate-sensitive health risks, from vector-borne disease to extreme weather events.
To support coherence and efficiency with existing health programmes, it links directly with the six core building blocks of health systems [53]: (i) governance and policy (i.e. intersectoral environment and health governance); (ii) capacity development (e.g. training on climate and health linkages); (iii) information (vulnerability and adaptation assessments, climate informed disease surveillance and research); (iv) service delivery (management of environmental health risks, climate-informed health programmes, disaster risk reduction); (v) essential products and technologies (for increasing climate resilience and reducing the environmental footprint of health services); and (vi) financing (to cover the additional costs required to enhance protection against climate risks). Applied research is a critical individual component within this framework, and is also relevant to the evaluation of all of the others (box 2).
Box 2. Linking policy, programme and research initiatives to vector-borne disease: focus on Africa.
Policy frameworks. Building on the World Health Assembly Resolution on climate change and health in 2008 [31], the African Regional Health and Environment Ministerial process has identified strengthening health protection from climate change as a top priority for the region. In their 2008 and 2010 conferences, health and environment ministers from across the region expressed their concern that Africa is already experiencing the effects of climate change, which are likely to become more severe, exacerbate environmental risk factors to human health, and undermine Africa's progress towards the Millennium Development Goals [54,55]. They consequently committed to work across sectors to manage environmental and health risks related to climate variability and change.
Connecting climate and health programmes. In order to support implementation of the commitments made by governments, the WHO is supporting countries to include health as a priority within their multisectoral National Adaptation Plans for climate change under the UN Framework Convention on Climate Change [56]. The WHO Regional Office for Africa has led the development of an essential public health package to enhance climate change resilience, closely tied to core public health programmes and functions, which should therefore form the basis of the health adaptation plan. The African Health and Environment Ministers endorsed a regional workplan to undertake: (i) baseline risk and capacity assessments, (ii) capacity building, (iii) integrated environment and health surveillance, (iv) awareness raising and social mobilization, (v) management of environmental health risks, (vi) scale-up of existing public health interventions for vector-borne disease, (vii) strengthening and operationalizing the health components of disaster risk reduction plans, (viii) promotion of research on climate change impacts and adaptation, and (ix) strengthening partnerships and intersectoral collaboration [57].
The model has been slightly adapted for different purposes, and is now being used as a common structure for developing a portfolio of pilot projects on health adaptation to climate change, now totalling over US$30 million globally [52].
Thematic approaches. In order to move climate effectively into mainstream health programming, there is a need for further development and piloting on specific issues, whether on individual diseases, or components of the overall response. For example, there is widespread interest in making better use of climate and weather information to improve disease surveillance and response [58]. The World Meteorological Organization has led the development of a Global Framework on Climate Services (GFCS) to provide climate information and support that is more relevant and accessible to end-user communities, and has established a joint office with the WHO to support the connection to health policy-makers [59]. The Organizations are now working with a range of external partners to pilot the implementation of the GFCS approach in Tanzania and Malawi, including application to malaria control, alongside nutritional and disaster risks [52].
Applied research. The identification of research as an important component within the wider policy and programmatic response to climate change enhances legitimacy and coherence. Research agendas and initiatives that respond to these policy mandates are more likely to be taken up into operations by health and related sectors. For example, the Special Programme for Research and Training in Tropical Diseases (TDR), with support from the International Development Research Centre (IDRC), is implementing a US$6.8 million research initiative to understand the impact of climate change in sub-Saharan Africa on people's vulnerability to vector-borne diseases, including malaria, schistosomiasis, African trypanosomiasis and Rift Valley fever. It does not attempt to isolate climate change as a single risk factor, instead placing it within the context of other social and environmental changes. It further focuses on those populations that are most vulnerable to risks arising from the combination of these factors, with an explicit aim to develop tools and strategies for adaptation to climate change. The initiative also connects directly to the political mandate from the Health and Environment Ministerial process, and to implementation mechanisms for adaptation to climate change, such as the National Adaptation Plans.
5. Conclusion
Applied research is essential to ensure continued progress in reducing the burden of vector-borne diseases in the face of the additional challenges caused by anthropogenic climate change, along with rapid changes in other environmental and social determinants. Current research output in this area is only weakly matched to the demands of health policy-makers. To increase relevance to current health programming, there is a need to complement current work on detection and attribution of health effects to climate change, and modelling of future scenarios, with a more directly applied approach to assessing and managing climate-related risks in the present.
Rapid progress has been made in recent years in developing policy mandates, operational frameworks and pilot initiatives on health adaptation to climate change, including vector-borne disease as a particular priority. These present an excellent opportunity for a stronger and more coherent connection of applied research and public health policy.
References
- 1.WHO. 2008. The global burden of disease: 2004 update. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2.Lozano R, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 380, 2095–2128. ( 10.1016/S0140-6736(12)61728-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJ, et al. 2012. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 380, 2197–2223. ( 10.1016/S0140-6736(12)61689-4) [DOI] [PubMed] [Google Scholar]
- 4.Ottesen EA, Duke BO, Karam M, Behbehani K. 1997. Strategies and tools for the control/elimination of lymphatic filariasis. Bull. World Health Organ. 75, 491–503. [PMC free article] [PubMed] [Google Scholar]
- 5.Suaya JA, et al. 2009. Cost of dengue cases in eight countries in the Americas and Asia: a prospective study. Am. J. Trop. Med. Hyg. 80, 846–855. [PubMed] [Google Scholar]
- 6.WHO. 2013. World malaria report 2013. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 7.Smith KR, et al. 2014. Human health; impacts adaptation and co-benefits. In Part A: global and sectoral aspects contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change (eds Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE.), pp. 709–754. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.Kovats RS, Campbell-Lendrum DH, McMichael AJ, Woodward A, Cox JS. 2001. Early effects of climate change: do they include changes in vector-borne disease? Phil. Trans. R. Soc. Lond. B 356, 1057–1068. ( 10.1098/rstb.2001.0894) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers DJ, Randolph SE. 2006. Climate change and vector-borne diseases. Adv. Parasitol. 62, 345–381. ( 10.1016/S0065-308X(05)62010-6) [DOI] [PubMed] [Google Scholar]
- 10.Lafferty KD. 2009. The ecology of climate change and infectious diseases. Ecology 90, 888–900. ( 10.1890/08-0079.1) [DOI] [PubMed] [Google Scholar]
- 11.Mills JN, Gage KL, Khan AS. 2010. Potential influence of climate change on vector-borne and zoonotic diseases: a review and proposed research plan. Environ. Health Perspect. 118, 1507–1514. ( 10.1289/ehp.0901389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. 2014. A global brief on vector-borne diseases. Geneva, Switzerland: World Health Organization; Contract no: WHO/DCO/WHD/2014.1. [Google Scholar]
- 13.Zhou XN, et al. 2008. Potential impact of climate change on schistosomiasis transmission in China. Am. J. Trop. Med. Hyg. 78, 188–194. [PubMed] [Google Scholar]
- 14.Hay SI, Tatem AJ, Guerra CA, Snow RW. 2006. Foresight on population at malaria risk in Africa: 2005, 2015 and 2030. Scenario review paper prepared for the Detection and Identification of Infectious Diseases Project (DIID), Foresight Project, Office of Science and Innovation. London, UK. [Google Scholar]
- 15.IPCC. 2013. Summary for policymakers. In Climate change 2013: The physical science basis contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change (eds Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM.), pp. 1–29. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 16.Small J, Goetz SJ, Hay SI. 2003. Climatic suitability for malaria transmission in Africa, 1911–1995. Proc. Natl Acad. Sci. USA 100, 15 341–15 345. ( 10.1073/pnas.2236969100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gething PW, Smith DL, Patil AP, Tatem AJ, Snow RW, Hay SI. 2010. Climate change and the global malaria recession. Nature 465, 342–345. ( 10.1038/nature09098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caminade C, Kovats S, Rocklov J, Tompkins AM, Morse AP, Colon-Gonzalez FJ, Stenlund H, Martens P, Lloyd SJ. 2014. Impact of climate change on global malaria distribution. Proc. Natl Acad. Sci. USA 111, 3286–3291. ( 10.1073/pnas.1302089111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. 2014. Quantitative risk assessment of the effects of climate change on selected causes of death, 2030s and 2050s (eds Hales S, Kovats RS, Lloyd S, Campbell-Lendrum D.). Geneva, Switzerland: World Health Organization. [Google Scholar]
- 20.Ermert V, Fink AH, Morse AP, Paeth H. 2012. The impact of regional climate change on malaria risk due to greenhouse forcing and land-use changes in tropical Africa. Environ. Health Perspect. 120, 77–84. ( 10.1289/ehp.1103681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siraj AS, Santos-Vega M, Bouma MJ, Yadeta D, Ruiz Carrascal D, Pascual M. 2014. Altitudinal changes in malaria incidence in highlands of Ethiopia and Colombia. Science 343, 1154–1158. ( 10.1126/science.1244325) [DOI] [PubMed] [Google Scholar]
- 22.Hales S, de Wet N, Maindonald J, Woodward A. 2002. Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet 360, 830–834. ( 10.1016/S0140-6736(02)09964-6) [DOI] [PubMed] [Google Scholar]
- 23.Astrom C, Rocklov J, Hales S, Beguin A, Louis V, Sauerborn R. 2012. Potential distribution of dengue fever under scenarios of climate change and economic development. Ecohealth 9, 448–454. ( 10.1007/s10393-012-0808-0) [DOI] [PubMed] [Google Scholar]
- 24.Ooi EE, Wilder-Smith A, Ng LC, Gubler DJ. 2010. The 2007 dengue outbreak in Singapore. Epidemiol. Infect. 138, 958–959; author reply 9–61 ( 10.1017/S0950268810000026) [DOI] [PubMed] [Google Scholar]
- 25.Gubler DJ. 2004. The changing epidemiology of yellow fever and dengue, 1900 to 2003: full circle? Comp. Immunol. Microbiol. Infect. Dis. 27, 319–330. ( 10.1016/j.cimid.2004.03.013) [DOI] [PubMed] [Google Scholar]
- 26.Erlanger TE, Keiser J, Utzinger J. 2008. Effect of dengue vector control interventions on entomological parameters in developing countries: a systematic review and meta-analysis. Med. Vet. Entomol. 22, 203–221. ( 10.1111/j.1365-2915.2008.00740.x) [DOI] [PubMed] [Google Scholar]
- 27.WHO. 2009. Dengue: guidelines for diagnosis, treatment, prevention and control: new edition. Geneva, Switzerland: World Health Organization; Contract no. WHO/HTM/NTD/DEN/2009.1. [PubMed] [Google Scholar]
- 28.Simmons CP, Farrar JJ, Nguyen V, Wills B. 2012. Dengue. N. Engl. J. Med. 366, 1423–1432. ( 10.1056/NEJMra1110265) [DOI] [PubMed] [Google Scholar]
- 29.WMO/WHO. 2012. Atlas of health and climate. Geneva, Switzerland: World Meteorological Organization. [Google Scholar]
- 30.Hosking J, Campbell-Lendrum D. 2012. How well does climate change and human health research match the demands of policymakers? A scoping review. Environ. Health Perspect. 120, 1076–1082. ( 10.1289/ehp.1104093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO. 2008. Climate change and health: resolution of the 61st world health assembly. Geneva, Switzerland: World Health Organization; See http://www.who.int/gb/ebwha/pdf_files/A61/A61_R19-en.pdf. [Google Scholar]
- 32.WHO. 2009. Protecting health from climate change: global research priorities. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 33.WHO. 2009. Climate change and health: report by the secretariat for the 62nd world health assembly. Geneva, Switzerland: World Health Organization; See http://www.who.int/globalchange/A62_11_en.pdf. [Google Scholar]
- 34.Wilkinson P, Campbell-Lendrum DH, Bartlett CL. 2003. Monitoring the health effects of climate change. In Climate change and human health: risks and responses (eds McMichael A, et al.), p. 322 Geneva, Switzerland: World Health Organization. [Google Scholar]
- 35.Hay SI, Cox J, Rogers DJ, Randolph SE, Stern DI, Shanks GD, Myers MF, Snow RW. 2002. Climate change and the resurgence of malaria in the East African highlands. Nature 415, 905–909. ( 10.1038/415905a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patz JA, et al. 2002. Climate change: regional warming and malaria resurgence. Nature 420, 627–628; discussion 8 ( 10.1038/420627a) [DOI] [PubMed] [Google Scholar]
- 37.Pascual M, Ahumada JA, Chaves LF, Rodo X, Bouma M. 2006. Malaria resurgence in the East African highlands: temperature trends revisited. Proc. Natl Acad. Sci. USA 103, 5829–5834. ( 10.1073/pnas.0508929103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purse BV, Mellor PS, Rogers DJ, Samuel AR, Mertens PP, Baylis M. 2005. Climate change and the recent emergence of bluetongue in Europe. Nat. Rev. Microbiol. 3, 171–181. ( 10.1038/nrmicro1090) [DOI] [PubMed] [Google Scholar]
- 39.Omumbo JA, Lyon B, Waweru SM, Connor SJ, Thomson MC. 2011. Raised temperatures over the Kericho tea estates: revisiting the climate in the East African highlands malaria debate. Malar. J. 10, 12 ( 10.1186/1475-2875-10-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alonso D, Bouma MJ, Pascual M. 2011. Epidemic malaria and warmer temperatures in recent decades in an East African highland. Proc. R. Soc. B 278, 1661–1669. ( 10.1098/rspb.2010.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tompkins AM, Ermert V. 2013. A regional-scale, high resolution dynamical malaria model that accounts for population density, climate and surface hydrology. Malar. J. 12, 65 ( 10.1186/1475-2875-12-65) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.IPCC. 2014. Summary for policymakers. In Climate change 2014: impacts, adaptation, and vulnerability part A: global and sectoral aspects contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change (eds Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE.), pp. 1–32. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 43.Mehran A, AghaKouchak A, Phillips TJ. 2010. Evaluation of CMIP5 continental precipitation simulations relative to satellite-based gauge- adjusted observations. J. Geophys. Res. Atmos. 119, 1695–1707. ( 10.1002/2013JD021152) [DOI] [Google Scholar]
- 44.Cane MA. 2010. Climate science: decadal predictions in demand. Nat. Geosci. 3, 231–232. ( 10.1038/ngeo823) [DOI] [Google Scholar]
- 45.Campbell-Lendrum D, Bertollini R, Neira M, Ebi K, McMichael A. 2009. Health and climate change: a roadmap for applied research. Lancet 373, 1663–1665. ( 10.1016/S0140-6736(09)60926-0) [DOI] [PubMed] [Google Scholar]
- 46.WHO. 2013. Protecting health from climate change: vulnerability and adaptation assessment. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 47.WHO/DFID. 2009. Vision 2030: the resilience of water supply and sanitation in the face of climate change summary and policy implications. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 48.Graves PM, et al. 2008. Effectiveness of malaria control during changing climate conditions in Eritrea, 1998–2003. Trop. Med. Int. Health 13, 218–228. ( 10.1111/j.1365-3156.2007.01993.x) [DOI] [PubMed] [Google Scholar]
- 49.Baeza A, Bouma MJ, Dhiman R, Pascual M. 2014. Malaria control under unstable dynamics: reactive vs. climate-based strategies. Acta Trop. 129, 42–51. ( 10.1016/j.actatropica.2013.04.001) [DOI] [PubMed] [Google Scholar]
- 50.Thomson MC, Doblas-Reyes FJ, Mason SJ, Hagedorn R, Connor SJ, Phindela T, Morse AP, Palmer TN. 2006. Malaria early warnings based on seasonal climate forecasts from multi-model ensembles. Nature 439, 576–579. ( 10.1038/nature04503) [DOI] [PubMed] [Google Scholar]
- 51.WHO. 2005. International health regulations, 2nd edition Geneva, Switzerland: World Health Organization. [Google Scholar]
- 52.WHO. 2014. Climate Change and Health Projects. Geneva, Switzerland: World Health Organization. (cited 3 November 2014). See www.who.int/globalchange/projects. [Google Scholar]
- 53.WHO. 2010. Monitoring the building blocks of health systems: a handbook of indicators and their measurement strategies. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 54.WHO/AFRO. 2008. Libreville declaration on health and environment in Africa. Gabon, Libreville: WHO Regional Office for Africa. [Google Scholar]
- 55.WHO/AFRO. 2010. African Ministers of Health and Environment joint statement on climate change and health. Luanda, Angola: WHO Regional Office for Africa. [Google Scholar]
- 56.WHO. 2014. WHO guidance to protect health from climate change through health adaptation planning. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 57.WHO/AFRO. 2011. Adaptation to climate change in Africa: Plan of action for the health sector 2012–2016. Brazzaville, Gabon: WHO Regional Office for Africa. [Google Scholar]
- 58.Jancloes M, Thomson M, Costa MM, Hewitt C, Corvalan C, Dinku T, Lowe R, Hayden M. 2014. Climate services to improve public health. Int. J. Environ. Res. Public Health 11, 4555–4559. ( 10.3390/ijerph110504555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guillemot J. 2014. Health exemplar: annex to the implementation plan for the global framework for climate services. Geneva, Switzerland: World Meteorological Organization. [Google Scholar]