Abstract
Numerous recent studies have illuminated global distributions of human cases of dengue and other mosquito-transmitted diseases, yet the potential distributions of key vector species have not been incorporated integrally into those mapping efforts. Projections onto future conditions to illuminate potential distributional shifts in coming decades are similarly lacking, at least outside Europe. This study examined the global potential distributions of Aedes aegypti and Aedes albopictus in relation to climatic variation worldwide to develop ecological niche models that, in turn, allowed anticipation of possible changes in distributional patterns into the future. Results indicated complex global rearrangements of potential distributional areas, which—given the impressive dispersal abilities of these two species—are likely to translate into actual distributional shifts. This exercise also signalled a crucial priority: digitization and sharing of existing distributional data so that models of this sort can be developed more rigorously, as present availability of such data is fragmentary and woefully incomplete.
Keywords: mosquitoes, dengue, chikungunya, potential distribution, climate change
1. Introduction
The global distributional potential of mosquito-borne viruses has seen considerable research attention in recent years, particularly as regards viruses transmitted by Aedes mosquitoes [1–5]. These diseases—e.g. dengue, yellow fever and chikungunya—present serious public health concerns, particularly in the light of recent spread events, in which each of the diseases has emerged either in new regions [6] or in new environments [7,8]. As a consequence, Aedes-transmitted viral diseases have been of considerable concern in recent years across much of the tropics and subtropics worldwide.
The situation for this suite of diseases is complicated still more by the global spread of two vector species, Aedes aegypti and Aedes albopictus [9–12]. These two species have spread essentially worldwide at lower and middle latitudes in recent decades, providing new or newly reinfested, particularly efficient vectors for transmission of ‘forest diseases' to humans, particularly in periurban settings [7]. Of particular interest are a series of intriguing results regarding interactions between the two species: Ae. albopictus appears to be the superior larval competitor [13–15], yet Ae. aegypti appears to be the vector species responsible for most or all massive outbreaks of dengue [16]. As a consequence, quite a bit is to be gained from a deep understanding of the present geographical distribution of these species, which has been the focus of several recent studies [9,10,16–18]. Hence, in light of already-occurring changes in climate and climate effects on potential distributions of the mosquitoes during their global invasions [19–21], a predictive view of their distributional potential in coming decades is quite useful.
In this paper, then, we use correlative ecological niche modelling approaches [22] to evaluate and assess the distributional potential of Ae. aegypti and Ae. albopictus at present and, most importantly, into the future. We use diverse models and scenarios for future-climate conditions, and constrain our results and interpretations carefully to avoid overinterpretation of patterns in the data. The chief result—that, with the exception of smaller regional shifts, distributional potential of these two species will likely be relatively stable in coming decades—can be understood as the consequence of broad climate tolerances in both species, such that changes in global climate over coming decades may not translate into major distributional shifts in these vector species. Model results also, however, point to the potential for reorganization of the distributions of the two species, each in response to its own particular ecological niche profile, in several areas, which may have implications for disease transmission.
2. Material and methods
We collected primary occurrence data (i.e. data documenting occurrences of individual animals at points in time and space) for the two focal species (Ae. aegypti and Ae. albopictus), and indeed records for the entire genus Aedes, accumulating digital accessible records from VectorMap (http://www.vectormap.org/), Atlas of Living Australia (http://www.ala.org.au/), speciesLink (http://www.splink.org.br/) and GBIF (http://www.gbif.org/; searches made 9 April 2013; individual totals of records from each data set not possible, as these data sources frequently overlap in their coverage of specific data records). The data for the two focal species were used to calibrate models, as is described in detail below; the data for the genus were used to characterize sampling effort across the Earth, which is quite uneven, focusing on the many species in the genus, such that collecting intensity and sampling techniques would be broadly representative of sampling that would yield records of the focal species; this step was necessitated by the almost-universal lack of reporting of negative data in biodiversity datasets (i.e. ‘searched for species X but did not find it’). Data were inspected to remove data records not referring to this genus of mosquitoes. Finally, to provide a qualitative independent test of model predictions, one of us (D.M.-L.) extracted an independent dataset from the broader scientific literature, grey literature and personal collections; these data were not used in model calibration, for lack of information on underlying sampling, but provided a useful check on how comprehensive our model predictions really are.
To summarize sampling effort globally, we created a ‘fishnet’ at a spatial resolution of 10′ that was coincident in position and orientation with the environmental data that would be used in model calibration (see below). Via this polygon shapefile, we counted individual records falling in each of the square polygons out of the 118 134 overall records of the genus—these sampling intensities varied from zero (covering approx. 99.5% of the terrestrial portion of the Earth's surface) to as many as 454 data records in a single 10′ pixel. Using these counts as values, we created a raster dataset that could be used to represent sampling intensity for this genus.
We characterized present-day climates (1950–2000) via data layers provided as part of the WorldClim climate data archive [23]. Specifically, we used the 19 ‘bioclimatic’ variables (10′ resolution) that are derived from monthly average maximum temperature, monthly average minimum temperature and monthly precipitation, all across the half-century time period. We obtained parallel data layers for general circulation model (GCM) projections to 2050 from the CMIP4 future-climate model projections [24]; these future-climate projections summarized four to eight different GCMs (i.e. distinct implementations of simulation models for global climate dynamics) for three scenarios of future-climate emissions (A2, B1 and A1B).
Ecological niche models were calibrated in Maxent v. 3.3.3 [25]. Initial exploratory runs served to detect a number of apparent artefacts in some of the climate datasets: bio 8–9, bio 18–19 were particularly notable in the present-day coverages, apparently as a consequence of their linking between temperature and precipitation variables, so we removed them from analysis at the outset; a few other variables presented odd artefacts upon visual inspection and also were removed. To reduce dimensionality, and to reduce collinearity among layers, we applied principal components analysis to the remaining bioclimatic layers, and used the component loadings in the present to transform the future-climate model projections in parallel. We used initial, exploratory Maxent runs with its jackknife functionality to explore the relative contributions of each of the principal components, and eliminated layers that showed low contribution to model predictions. As a consequence, we ran final models based on sets of six and eight principal components for each species.
To provide a general evaluation of the predictive ability of our models, and also to allow decisions about whether the six- or eight-component models were preferable for interpretation, we used partial receiver operating characteristic (ROC) analysis [26]. Given concerns about the appropriateness of traditional ROC applications [27], which plot omission and commission errors across a set of model thresholds, and compare the area under that curve to the area under a line of null expectations, the partial ROC approach rescales one axis of the ROC curve to reflect proportional area identified as suitable (instead of commission error), and focuses only on predictions that have acceptable levels of omission error (termed E; in this case, we used E = 5%). We divided spatially unique occurrence points randomly into equal subsets for model calibration and model evaluation. Partial ROC calculations were carried out via programs developed by N. Barve (http://hdl.handle.net/1808/10059). Probability values were determined by direct count of AUC ratios greater than 1.0, among 1000 replicate 50% bootstrap subsamplings.
In general, final models were calibrated in Maxent with its bootstrapping/replicated run functionality. In view of the apparently massive invasive potential of both mosquito species involved [9,28], we used a very broad hypothesis of the accessible area (M) for them, considering much of the world, and eliminating only the highest southern latitudes (i.e. above 60°S). We set aside 50% of input occurrence points as a ‘test percentage’ and conducted 10 replicate analyses to consider effects of particular calibration datasets on model outcomes. The sampling bias layer described above was included in the Maxent ‘bias layer’ function as a guide to sampling towards a characterization of the broader ‘background’ for model fitting, akin to pseudoabsence sampling in other niche modelling algorithms [29]; this layer is illustrated in the electronic supplementary materials and is available as a GeoTIFF dataset at http://hdl.handle.net/1808/15275. Models were calibrated for present-day conditions, and then transferred onto each of the future-climate scenarios and models that were available to us (see table 1 for a summary).
Table 1.
Summary of future-climate scenarios (B1, A1B and A2) and climate models assessed in this study.
| model | host country | A1B and A2 | B1 |
|---|---|---|---|
| BCCR-BCM 2.0 | Norway | X | X |
| CSIRO-MK 3.0 | Australia | X | |
| CSIRO-MK 3.5 | Australia | X | X |
| INM-CM 3.0 | Russia | X | X |
| MIROC medium resolution | Japan | X | X |
| NCAR-CCSM 3.0 | United States | X | X |
Once all models were calibrated and all future transfers developed, we used the median value for all replicate analyses for each combination of model, scenario and number of principal components. We calculated the median of medians for a given scenario and number of principal components, seeking a central tendency across all of the replicate models developed from different random resamplings from available occurrence data. We calculated differences between future and present in these logistic suitability scores; we also thresholded the data using an E = 5% training presence data criterion, in which we sought the highest suitability score that included 95% of the data used to calibrate the models, to convert raw suitability scores into binary estimates of potential presence and absence across regions, with the estimate of E derived from assessment of approximate error rates in input data [26]. Based on these binary outputs, we explored anticipated range expansions and contractions.
3. Results
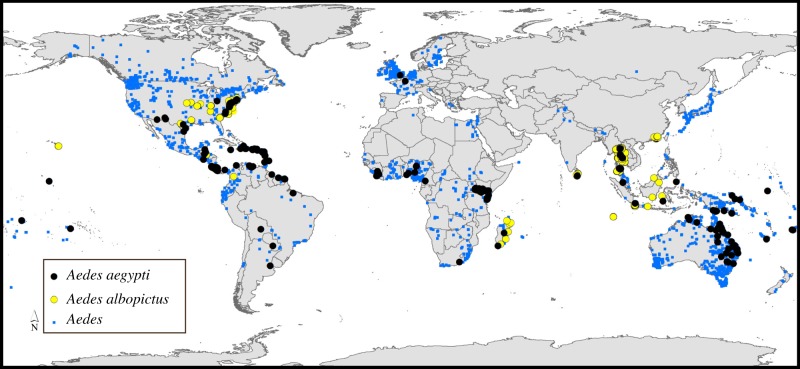
In all, we assembled 2108 and 8040 occurrence records for Ae. aegypti and Ae. albopictus, respectively, which distilled down to 338 and 350 spatially unique records at the resolution of our analysis. The overall picture of records of Aedes mosquitoes, however, indicated sampling of this genus as rather more concentrated in the temperate zone than are the populations of these two species (figure 1). In preliminary explorations, inclusion of this surface as a ‘bias layer’ in model calibration made substantial differences in model outputs, such that we emphasize the importance of considering sampling effort in any modelling exercises that are cast on an uneven landscape of sampling, even within the accessible area for a species.
Figure 1.
Summary of primary occurrence data available globally for Aedes mosquitoes in general (blue), and Ae. aegypti (black) and Ae. albopictus (yellow) in particular. (Online version in colour.)
Calibrating models across the entire accessible region for the species (i.e. most of the world) based on a subset of available occurrences resulted in model predictions that performed uniformly better than random expectations. That is, using partial ROC analysis on random 50% subsets of dataset aside from model calibration, all comparisons yielded AUC ratios that were significantly above null expectations (p < 0.001). Comparing among different numbers of principal components as descriptors of environmental spaces, AUC ratios for eight-component models were higher than those for six-component models by 1.72–2.57%; hence, we focused all subsequent explorations on models based on eight principal components (six-component results are presented in the electronic supplementary material and do not differ markedly from those for eight-component models).
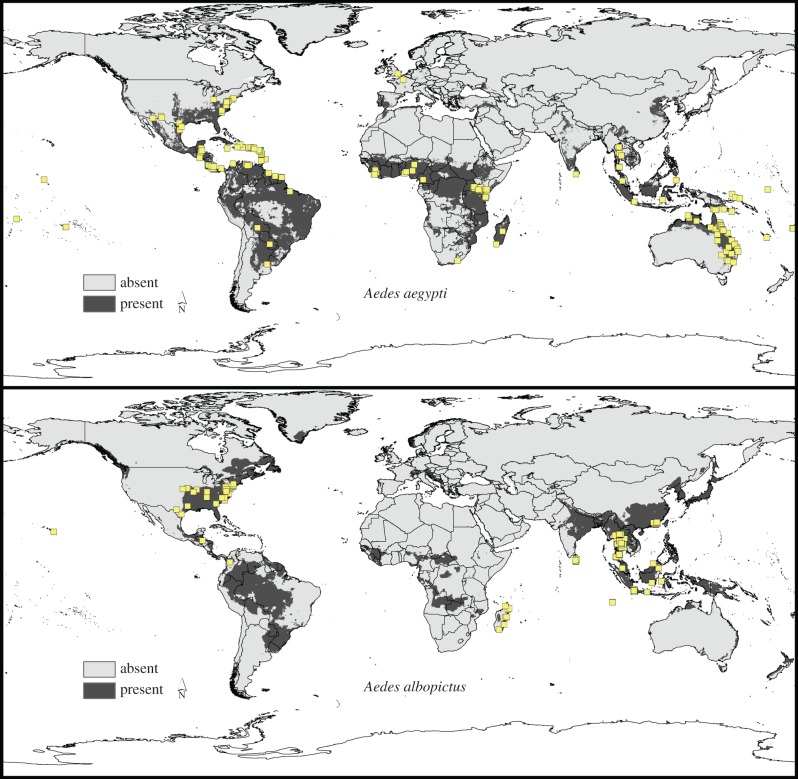
Model predictions for the present day reflected well the known global distributions of each of the two species (figure 2). Indeed, some of the more interesting features include an indication of markedly broader distributional potential for Ae. aegypti than for Ae. albopictus in Australia, and indeed perhaps overall broader distributional potential in tropical and subtropical regions for Ae. aegypti, but then, curiously, a broader overall potential distribution of Ae. albopictus in North America. These explorations also confirm that these two successful invasive species have indeed fulfilled much or all of their invasive potential globally—broad regions that appear to be suitable climatically, but from which no occurrence data were available to us included eastern China, Vietnam, The Philippines, Borneo and northeastern Brazil for Ae. aegypti, and South America, Africa, India, Japan and New Zealand for Ae. albopictus. Nonetheless, searches via Google Scholar quickly revealed known presences of these species in almost all of these areas [30–39], so the gap is one of availability of occurrence data, rather than absence of the species from these potential areas. The sole significant exception is that of New Zealand, which has been reached by Ae. albopictus, but apparently does not yet have established populations [20,21,40].
Figure 2.
Summary of modelled potential distributional patterns of Ae. aegypti and Ae. albopictus under present-day conditions based on analysis of eight principal components. (Online version in colour.)
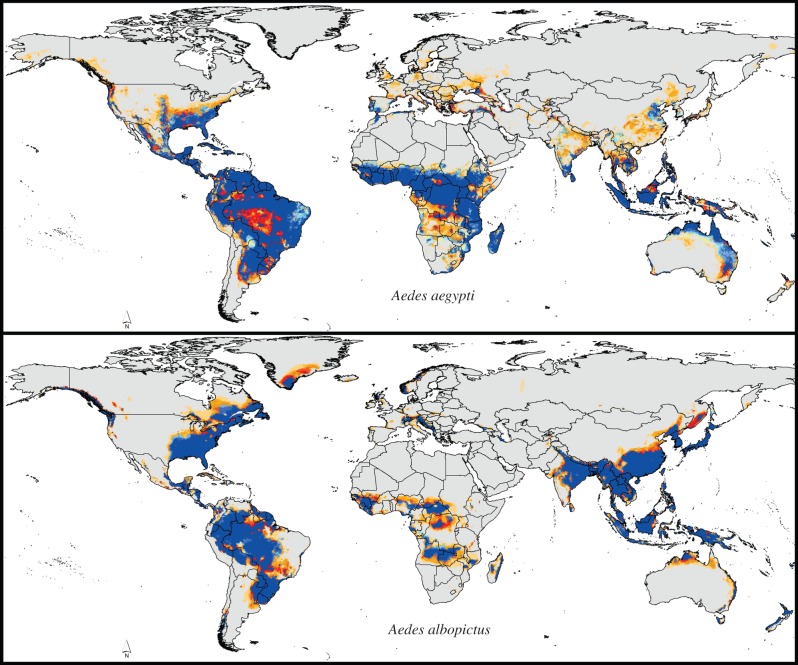
Model transfers to future conditions were generally similar to present-day patterns, at least in the broadest terms (A1B scenario shown in figure 3; other scenarios provided in the electronic supplementary material). For Ae. aegypti, model predictions indicated some potential for northward expansion in eastern North America, South Asia and East Asia, and southward in Africa and Australia; broadening distributional potential was indicated in interior South America and Central Africa. For Ae. albopictus, model predictions gave clearer indications of expanding distributional potential in eastern North America and East Asia, plus expanding potential across Africa and in eastern and southern South America; distributional potential in Australia was anticipated to expand rather markedly for this species.
Figure 3.
Summary of modelled potential distributional patterns of Ae. aegypti and Ae. albopictus under future conditions (A1B) based on analysis of eight principal components. Confidence in present-day and future distributional potential is based on agreement among six climate models (table 1): present-day-only distributional areas are shown in blue, with model agreement regarding stability of present-day distributional areas shown via the intensity of blue shading (light blue denotes low and dark blue denotes high model agreement); future distributional potential is shown as shades of orange (light orange denotes low and dark orange denotes high model agreement in predicting future suitability). (Online version in colour.)
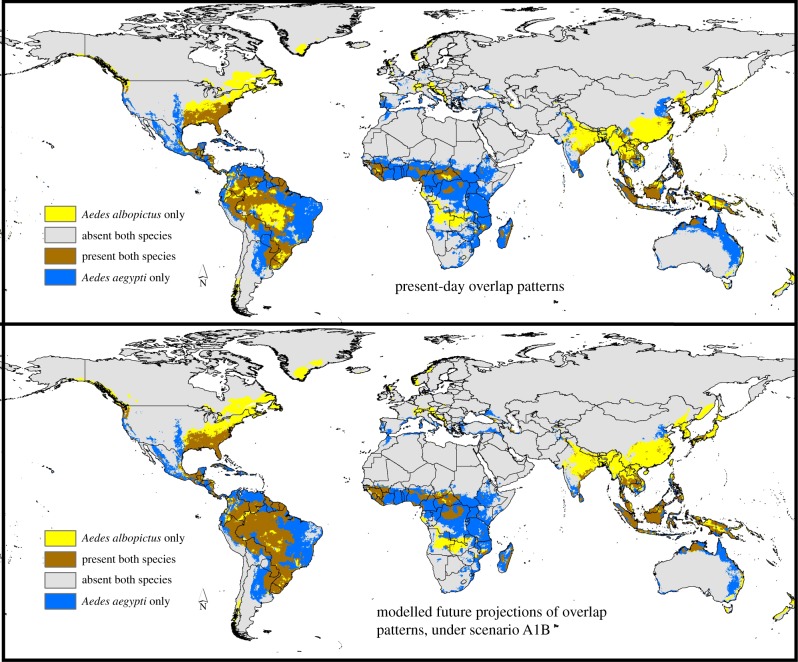
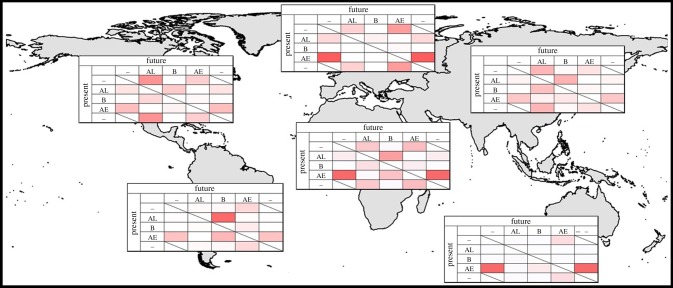
Assessing the distributional potential of the two species in tandem suggests a complex mosaic of the distributions of the two species around the world (figure 4): while Ae. albopictus has the broadest potential distribution in North America, Ae. aegypti appears to have a broader potential in Africa and Australia. Assessing how the distributional patterns of the two species will change, continent by continent, we see complex rearrangements (figure 5): Ae. aegypti appears to gain potential distributional area in South America, lose ground in Australia, and rearrange its distributional area in Europe, while Ae. albopictus may be able to expand its distributional area more than Ae. aegypti in North America; Asia shows particularly complicated shifts in dominance of the two species geographically.
Figure 4.
Summary of patterns of potential distributional overlap derived from ecological niche models of Ae. aegypti and Ae. albopictus worldwide, both under current conditions and under modelled future conditions (A1B scenario), based on eight principal components. (Online version in colour.)
Figure 5.
Summary of patterns of change in potential presence and distributional overlap of Ae. aegypti and Ae. albopictus worldwide under the A1B scenario, continent by continent. Patterns of shift under climate change scenario A1B are shown via transition matrices for each continent: rows indicate present-day situation, and columns indicate model projections of future potential. AL, Ae. albopictus; AE, Ae. aegypti; B, both species; –, neither species. (Online version in colour.)
4. Discussion
Mosquito distributions are highly dynamic in space and time, as their life cycles are short and heavily influenced by environmental variation [41]. Therefore their distributions respond fluidly to new opportunities, e.g. in terms of (i) overcoming dispersal barriers to colonize new areas (e.g. the global invasion of the two species under analysis herein) [9], (ii) ready expansion into new areas as conditions change and improve for their population biology [42] and (iii) the dynamic interactions and potential competitive exclusion between the two species [13–15]. As such, for example, the difference between the two species in terms of Australian distribution is intriguing—while Ae. aegypti is present and established, Ae. albopictus is not established there, in spite of having been introduced to at least four states [19]: based on our results, we view this lack of establishment as reflecting non-ideal conditions for the species in Australia, although Fischer et al. [43] presented a more optimistic and suitable picture for the species under present-day conditions in Australia, and certainly the Australian Quarantine Service has expended considerable effort in assuring its non-establishment. Similarly demonstrated colonization pressure by this species without successful establishment has occurred in New Zealand, though probably thanks to rapid and effective mitigation efforts [21]. More generally, in view of the impressive dispersal and colonization abilities of these two species, our models of potential distributional areas replicate quite well their actual present-day distributional areas.
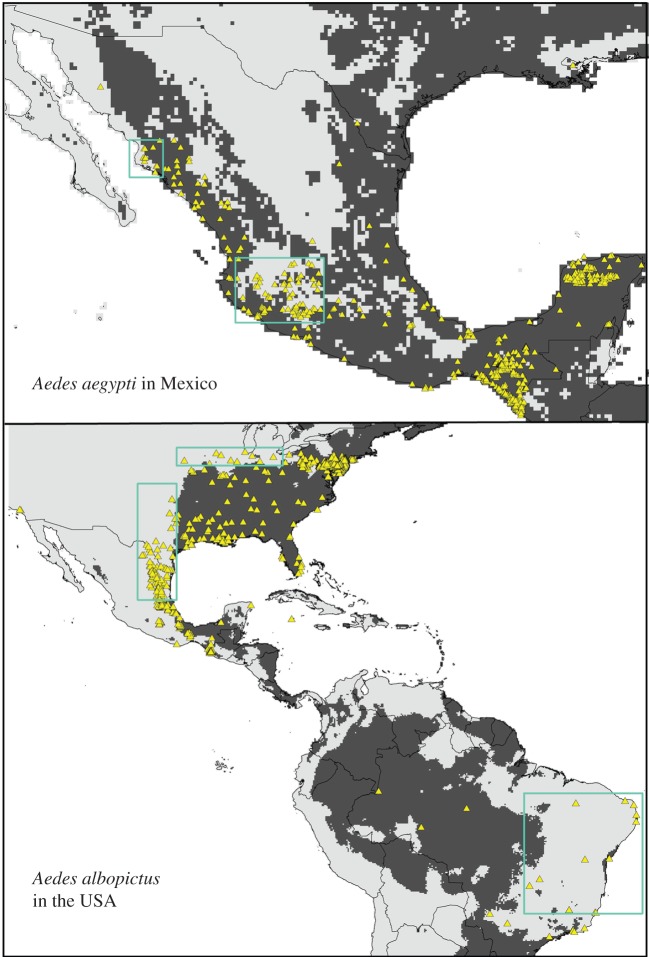
Our models, while largely coincident with known present-day distributions of the two species, were developed to explore climate change implications for these two species. Still, these models did fail in replicating some aspects of the species' known, present-day distributions, as can be appreciated from figure 6, with the overlay of independent occurrence data. For example, model predictions failed to identify suitable habitat for Ae. albopictus in northeastern Mexico and northeastern Brazil. This omission error was likely a consequence of incomplete sampling in our training dataset, resulting in under-representation or no representation of environmental combinations manifested in these regions. Although incomplete input data may account for some model failures, anthropogenic factors could also impact model results locally: for example, Ae. albopictus populations introduced into and established in Los Angeles, in southern California [44] may be able to survive there thanks to urban subsidy of moisture, but the details are not clear. In addition to omissions, model predictions appeared to extrapolate into several high-latitude regions (e.g. Greenland), reflecting model extrapolation beyond the set of environmental conditions associated with the calibration region. These inconsistencies may derive from peripherality of environmental characteristics of occurrences within the calibration region, leading to wild and inappropriate extrapolation [45].
Figure 6.
Overlay of independent occurrence dataset (yellow triangles) on model predictions for present-day potential distributions of Ae. aegypti and Ae. albopictus. Green boxes delimit areas in which model predictions failed to anticipate known occurrences. (Online version in colour.)
Our future-climate projections, however, indicate considerable potential for shifting establishment of these two vector species in several directions, as follows: (i) more broadly in eastern North America, (ii) farther south in southern South America (particularly in Ae. aegypti); (iii) locally northward into southern Europe, (iv) more broadly in Central Africa, (v) more broadly in East Asia and (vi) across northern and eastern Australia (Ae. albopictus). While these potential range shifts in response to climate are relatively subtle, they are the implications of environmental shifts that would transform areas from presenting conditions outside the modeled ecological niches of the vector species (e.g. no establishment in Australia despite repeated introductions) to presenting conditions that do permit maintenance of populations. Clearly, even within the present distributional areas of these species, changing conditions may cause changes in their abundance and dominance, although those shifts are not included in our present modeling efforts [46].
Regional-scale modeling efforts have shed considerable light on the distributional potential of Ae. albopictus, thanks to a series of detailed analyses [18,43]. These studies have provided a level of rigor that is unusual in the present literature on climate change effects on species' distributions, and offer considerable detail on likely distributional shifts of this species across Europe as climates change. Both the Fischer et al. [43] models and our own results indicate a curious middle-latitude focus of suitability for Ae. albopictus in Europe, which contrasts with the southern and southeastern suitability profiles for this species in North America.
The relative roles and importance of these two species in dengue transmission appear to be unequal. That is, a recent analysis indicated that Ae. albopictus does not appear to drive major dengue outbreaks [16], such that Ae. aegypti emerges as the chief driver of large-scale dengue transmission. Both the pattern of known occurrences and the maps of potential distributions for the two species, in large part, suggest that Ae. aegypti is more extensive distributionally than Ae. albopictus, with the exceptions of eastern North America and East Asia, neither of which has a long history of dengue transmission; both of these regions, however, appear now to have active transmission [47–49]. These imbalances, however, may not hold for other Aedes-transmitted diseases, such as chikungunya, which is readily transmitted by Ae. albopictus, at least in some cases [50], and which has shown recent major distributional expansion [51,52], with importation events presenting potential for further colonization [53].
The global distribution of Aedes-borne viruses, particularly dengue, has seen a lot of attention in the scientific literature in recent years (e.g. [1,17]). However, these efforts have not included deep contemplation and detailed mapping of distributions and distributional shifts in vector species: rather, they have focused in largest part on human infections. While clearly vector populations can exist without virus presence, such populations set the stage for easy disease introduction, as is evidenced by recent dengue emergence in the southern USA [47,48], particularly as both vector populations and human populations rearrange spatially in response to climate change (in the case of the vectors) and economic opportunity (humans). In this sense, we see the results of this study as offering a quantitative input that can be incorporated into future summaries of human disease distributions—to this end, we have placed GeoTIFF versions of our raster model outputs in a data repository at the University of Kansas (http://hdl.handle.net/1808/15275).
The models presented herein are far from definitive, however. While this study is novel in its global scope with consideration of multiple models and climate change scenarios, several ‘next steps' emerge as highly desirable. First, the availability of large quantities of occurrence data for mosquitoes globally is simultaneously an opportunity and a constraint: the opportunity is that as much data as we found were readily available for analysis; however, these data are still sparse and even lacking for some key regions (e.g. most of Asia, much of South America, parts of the USA). Clearly, more data exist, but are cloistered in national, institutional and even personal research databases, and are not available to the broader community.
Second, distributional complexities also need to be incorporated into these models. The competitive interactions between the two species when they co-occur present a first dimension of complexity. Another major trend in dengue transmission has been its urbanization in many parts of the world—this trend, and the unique opportunities offered to the mosquitoes by urban environments, is not reflected in these models, and yet is crucial to understanding present-day dengue transmission [28]. Multiscalar, nested models may represent a useful way forward for dealing with such effects.
Finally, a major factor in transmission of mosquito-borne diseases is the effect of temporal and spatio-temporal dynamics of conditions. For example, a recent analysis indicated the potential for chikungunya transmission across the eastern United States, but in very limited seasons at the northernmost site analysed (New York City), such that transmission would not be at all efficient [54]. A previous study by our research group [55] indicated clear predictivity of spatio-temporal dynamics of dengue vector mosquito populations across Mexico, but further exploration of this potential has been obstructed by lack of adequate and sufficiently dense (in time and space) occurrence data for the species. Overall, though, the indication is of a system that is highly predictable, and one in which climate change is likely to produce distributional shifts that, while not massive, will have significant public health implications worldwide.
Supplementary Material
Acknowledgements
We thank our colleagues Rick Wilkerson and Des Foley at WRAIR, for their efforts to enable broad and open sharing of data regarding mosquito distributions, and we thank the organizers of this collection of papers for their kind invitation to contribute.
References
- 1.Brady OJ, et al. 2012. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 6, e1760 ( 10.1371/journal.pntd.0001760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charrel RN, de Lamballerie X, Raoult D. 2007. Chikungunya outbreaks—the globalization of vectorborne diseases. New Engl. J. Med. 356, 769 ( 10.1056/NEJMp078013) [DOI] [PubMed] [Google Scholar]
- 3.Jentes ES, et al. 2011. The revised global yellow fever risk map and recommendations for vaccination, 2010: consensus of the Informal WHO Working Group on Geographic Risk for Yellow Fever. Lancet Infect. Dis. 11, 622–632. ( 10.1016/S1473-3099(11)70147-5) [DOI] [PubMed] [Google Scholar]
- 4.Rogers D, Wilson A, Hay S, Graham A. 2006. The global distribution of yellow fever and dengue. Adv. Parasitol. 62, 181–220. ( 10.1016/S0065-308X(05)62006-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Kleef E, Bambrick H, Hales S. 2010. The geographic distribution of dengue fever and the potential influence of global climate change. TropIKA.net. See http://journal.tropika.net/scielo.php?script=sci_arttext&pid=S2078-86062010005000001&lng=en. [Google Scholar]
- 6.Depoortere E, Salmaso S, Pompa M, Guglielmetti P, Coulombier D. 2008. Chikungunya in Europe. Lancet 371, 723 ( 10.1016/S0140-6736(08)60336-0) [DOI] [PubMed] [Google Scholar]
- 7.Massad E, Burattini MN, Coutinho FAB, Lopez LF. 2003. Dengue and the risk of urban yellow fever reintroduction in São Paulo state, Brazil. Revista de Saúde Pública 37, 477–484. ( 10.1590/S0034-89102003000400013) [DOI] [PubMed] [Google Scholar]
- 8.Vasconcelos PF, Rosa AP, Rodrigues SG, Rosa ES, Monteiro HA, Cruz AC, Barros VL, Souza MR, Rosa JF. 2001. Yellow fever in Para state, Amazon region of Brazil, 1998–1999: entomologic and epidemiologic findings. Emerg. Infect. Dis. 7, 565–569. ( 10.3201/eid0707.010738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benedict MQ, Levine RS, Hawley WA, Lounibos LP. 2007. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector-Borne Zoonotic Dis. 7, 76–85. ( 10.1089/vbz.2006.0562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caminade C, Medlock JM, Ducheyne E, McIntyre KM, Leach S, Baylis M, Morse AP. 2012. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J. R. Soc. Interface 9, 2708–2717. ( 10.1098/rsif.2012.0138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson RA, Hayhoe K, Presley SM, Allen LJS, Long KR, Cox SB. 2012. Potential impacts of climate change on the ecology of dengue and its mosquito vector the Asian tiger mosquito (Aedes albopictus). Environ. Res. Lett. 7, 034003 ( 10.1088/1748-9326/7/3/034003) [DOI] [Google Scholar]
- 12.Simard F, Nchoutpouen E, Toto JC, Fontenille D. 2005. Geographic distribution and breeding site preference of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) in Cameroon, Central Africa. J. Med. Entomol. 42, 726–731. ( 10.1603/0022-2585(2005)042[0726:GDABSP]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 13.Braks MAH, Honório N, Lounibos L, Lourenço-de-Oliveira R, Juliano S. 2004. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Ann. Entomol. Soc. Am. 97, 130–139. ( 10.1603/0013-8746(2004)097[0130:ICBTIS]2.0.CO;2) [DOI] [Google Scholar]
- 14.Juliano SA, Lounibos LP, O'Meara GF. 2004. A field test for competitive effects of Aedes albopictus on A. aegypti in South Florida: differences between sites of coexistence and exclusion? Oecologia 139, 583–593. ( 10.1007/s00442-004-1532-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lounibos L, Suárez S, Menéndez Z, Nishimura N, Escher R, O'Connell S, Rey J. 2002. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? J. Vector Ecol. 27, 86–95. [PubMed] [Google Scholar]
- 16.Lambrechts L, Scott TW, Gubler DJ. 2010. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl. Trop. Dis. 4, e646 ( 10.1371/journal.pntd.0000646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatt SE, et al. 2013. The global distribution and burden of dengue. Nature 496, 504–507. ( 10.1038/nature12060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer D, Thomas S, Neteler M, Tjaden N, Beierkuhnlein C. 2014. Climatic suitability of Aedes albopictus in Europe referring to climate change projections: comparison of mechanistic and correlative niche modelling approaches. Eurosurveillance 19, 20696 ( 10.2807/1560-7917.ES2014.19.6.20696) [DOI] [PubMed] [Google Scholar]
- 19.ALA 2014. Atlas of living Australia (http://www.ala.org.au/; accessed 2 July 2014) Canberra, Australia: Atlas of Living Australia. [Google Scholar]
- 20.Derraik JG. 2006. A scenario for invasion and dispersal of Aedes albopictus (Diptera: Culicidae) in New Zealand. J. Med. Entomol. 43, 1–8. ( 10.1603/0022-2585(2006)043[0001:ASFIAD]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 21.Holder P, George S, Disbury M, Singe M, Kean JM, McFadden A. 2010. A biosecurity response to Aedes albopictus (Diptera: Culicidae) in Auckland, New Zealand. J. Med. Entomol. 47, 600–609. ( 10.1603/ME09111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson AT, Soberón J, Pearson RG, Anderson RP, Martínez-Meyer E, Nakamura M, Araújo MB. 2011. Ecological niches and geographic distributions. Princeton, NJ: Princeton University Press. [Google Scholar]
- 23.Hijmans R, Cameron S, Parra J, Jones P, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 24.CI 2014. Globally downscaled climate projections for assessing the conservation impacts of climate change (http://futureclimates.conservation.org/; accessed 2 July 2014) Washington, DC: Conservation International. [DOI] [PubMed] [Google Scholar]
- 25.Phillips SJ, Anderson RP, Schapire RE. 2006. Maximum entropy modeling of species geographic distributions. Ecol. Model. 190, 231–259. ( 10.1016/j.ecolmodel.2005.03.026) [DOI] [Google Scholar]
- 26.Peterson AT, Papeş M, Soberón J. 2008. Rethinking receiver operating characteristic analysis applications in ecological niche modelling. Ecol. Model. 213, 63–72. ( 10.1016/j.ecolmodel.2007.11.008) [DOI] [Google Scholar]
- 27.Lobo JM, Jiménez-Valverde A, Real R. 2008. AUC: a misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 17, 145–151. ( 10.1111/j.1466-8238.2007.00358.x) [DOI] [Google Scholar]
- 28.Jansen CC, Beebe NW. 2010. The dengue vector Aedes aegypti: what comes next. Microbes Infect. 12, 272–279. ( 10.1016/j.micinf.2009.12.011) [DOI] [PubMed] [Google Scholar]
- 29.Phillips S, Dudik M, Elith J, Graham C, Lehman A, Leathwick J, Ferrier S. 2009. Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecol. Appl. 19, 181–197. ( 10.1890/07-2153.1) [DOI] [PubMed] [Google Scholar]
- 30.Dias J, Pedral-Sampaio DB, Jones TC. 1997. Aedes aegypti surveillance and correlation with the occurrence of dengue fever in Bahia, Brazil. Braz. J. Infect. Dis. 1, 36–41. [PubMed] [Google Scholar]
- 31.Honório NA, Silva WDC, Leite PJ, Gonçalves JM, Lounibos LP, Lourenço-de-Oliveira R. 2003. Dispersal of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in an urban endemic dengue area in the state of Rio de Janeiro, Brazil. Memórias do Instituto Oswaldo Cruz 98, 191–198. ( 10.1590/S0074-02762003000200005) [DOI] [PubMed] [Google Scholar]
- 32.Huber K, Le Loan L, Hoang TH, Tien TK, Rodhain F, Failloux A. 2002. Temporal genetic variation in Aedes aegypti populations in Ho Chi Minh City (Vietnam). Heredity 89, 7–14. ( 10.1038/sj.hdy.6800086) [DOI] [PubMed] [Google Scholar]
- 33.Kalra N, Kaul S, Rastogi R. 1997. Prevalence of Aedes aegypti and Aedes albopictus: vectors of dengue and dengue haemorrhagic fever in north, north-east and central India. Dengue Bull. 21, 84–92. [Google Scholar]
- 34.Kobayashi M, Nihei N, Kurihara T. 2002. Analysis of northern distribution of Aedes albopictus (Diptera: Culicidae) in Japan by geographical information system. J. Med. Entomol. 39, 4–11. ( 10.1603/0022-2585-39.1.4) [DOI] [PubMed] [Google Scholar]
- 35.Macdonald W, Rajapaksa N. 1972. A survey of the distribution and relative prevalence of Aedes aegypti in Sabah, Brunei, and Sarawak. Bull. World Health Organ. 46, 203–209. [PMC free article] [PubMed] [Google Scholar]
- 36.Pagès F, Peyrefitte CN, Mve MT, Jarjaval F, Brisse S, Iteman I, Gravier P, Nkoghe D, Grandadam M. 2009. Aedes albopictus mosquito: the main vector of the 2007 Chikungunya outbreak in Gabon. PLoS ONE 4, e4691 ( 10.1371/journal.pone.0004691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savage H, Ezike V, Nwankwo A, Spiegel R, Miller B. 1992. First record of breeding populations of Aedes albopictus in continental Africa: implications for arboviral transmission. J. Am. Mosquito Control Assoc. 8, 101–103. [PubMed] [Google Scholar]
- 38.Schultz G. 1993. Seasonal abundance of dengue vectors in Manila, Republic of the Philippines. Southeast Asian J. Trop. Med. Public Health 24, 369–375. [PubMed] [Google Scholar]
- 39.Tsuda Y, Takagi M, Wang S, Wang Z, Tang L. 2001. Movement of Aedes aegypti (Diptera: Culicidae) released in a small isolated village on Hainan Island, China. J. Med. Entomol. 38, 93–98. ( 10.1603/0022-2585-38.1.93) [DOI] [PubMed] [Google Scholar]
- 40.Laird M, Calder L, Thornton R, Syme R, Holder P, Mogi M. 1994. Japanese Aedes albopictus among four mosquito species reaching New Zealand in used tires. J. Am. Mosquito Control Assoc. 10, 14–23. [PubMed] [Google Scholar]
- 41.Crans WJ. 2004. A classification system for mosquito life cycles: life cycle types for mosquitoes of the northeastern United States. J. Vector Ecol. 29, 1–10. [PubMed] [Google Scholar]
- 42.Roiz D, Neteler M, Castellani C, Arnoldi D, Rizzoli A. 2011. Climatic factors driving invasion of the tiger mosquito (Aedes albopictus) into new areas of Trentino, northern Italy. PLoS ONE 6, e14800 ( 10.1371/journal.pone.0014800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer D, Thomas SM, Niemitz F, Reineking B, Beierkuhnlein C. 2011. Projection of climatic suitability for Aedes albopictus Skuse (Culicidae) in Europe under climate change conditions. Glob. Planet. Change 78, 54–64. ( 10.1016/j.gloplacha.2011.05.008) [DOI] [Google Scholar]
- 44.Zhong D, et al. 2013. Genetic analysis of invasive Aedes albopictus populations in Los Angeles County, California and its potential public health impact. PLoS ONE 8, e68586 ( 10.1371/journal.pone.0068586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owens HL, et al. 2013. Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol. Model. 263, 10–18. ( 10.1016/j.ecolmodel.2013.04.011) [DOI] [Google Scholar]
- 46.Martínez-Meyer E, Díaz-Porras D, Peterson AT, Yáñez-Arenas C. 2012. Ecological niche structure determines rangewide abundance patterns of species. Biol. Lett. 9, 20120637 ( 10.1098/rsbl.2012.0637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radke EG, et al. 2012. Dengue outbreak in Key West, Florida, USA, 2009. Emerg. Infect. Dis. 18, 135–137. ( 10.3201/eid1801.110130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramos MM, et al. The Dengue Serosurvey Working Group 2008. Epidemic dengue and dengue hemorrhagic fever at the Texas-Mexico border: Results of a household-based seroepidemiologic survey, December 2005. Am. J. Trop. Med. Hyg. 78, 364–369. [PubMed] [Google Scholar]
- 49.Wu J-Y, Lun Z-R, James AA, Chen X-G. 2010. Dengue fever in mainland China. Am. J. Trop. Med. Hyg. 83, 664–671. ( 10.4269/ajtmh.2010.09-0755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsetsarkin KA, Chen R, Sherman MB, Weaver SC. 2011. Chikungunya virus: evolution and genetic determinants of emergence. Curr. Opin. Virol. 1, 310–317. ( 10.1016/j.coviro.2011.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fischer D, Thomas SM, Suk JE, Sudre B, Hess A, Tjaden NB, Beierkuhnlein C, Semenza JC. 2013. Climate change effects on Chikungunya transmission in Europe: geospatial analysis of vector's climatic suitability and virus’ temperature requirements. Int. J. Health Geogr. 12, 51 ( 10.1186/1476-072X-12-51) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Bortel W, et al. 2014. Chikungunya outbreak in the Caribbean region, December 2013 to March 2014, and the significance for the European Union. Euro Surveill. 19, 20759. [DOI] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention 2006. Chikungunya fever diagnosed among international travelers--United States, 2005–2006. Morb. Mortality Wkly Rep. 55, 1040–1042. [PubMed] [Google Scholar]
- 54.Ruiz-Moreno D, Vargas IS, Olson KE, Harrington LC. 2012. Modeling dynamic introduction of chikungunya virus in the United States. PLoS Negl. Trop. Dis. 6, e1918 ( 10.1371/journal.pntd.0001918) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peterson AT, Martínez-Campos C, Nakazawa Y, Martínez-Meyer E. 2005. Time-specific ecological niche modeling predicts spatial dynamics of vector insects and human dengue cases. Trans. R. Soc. Trop. Med. Hyg. 99, 647–655. ( 10.1016/j.trstmh.2005.02.004) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.