Abstract
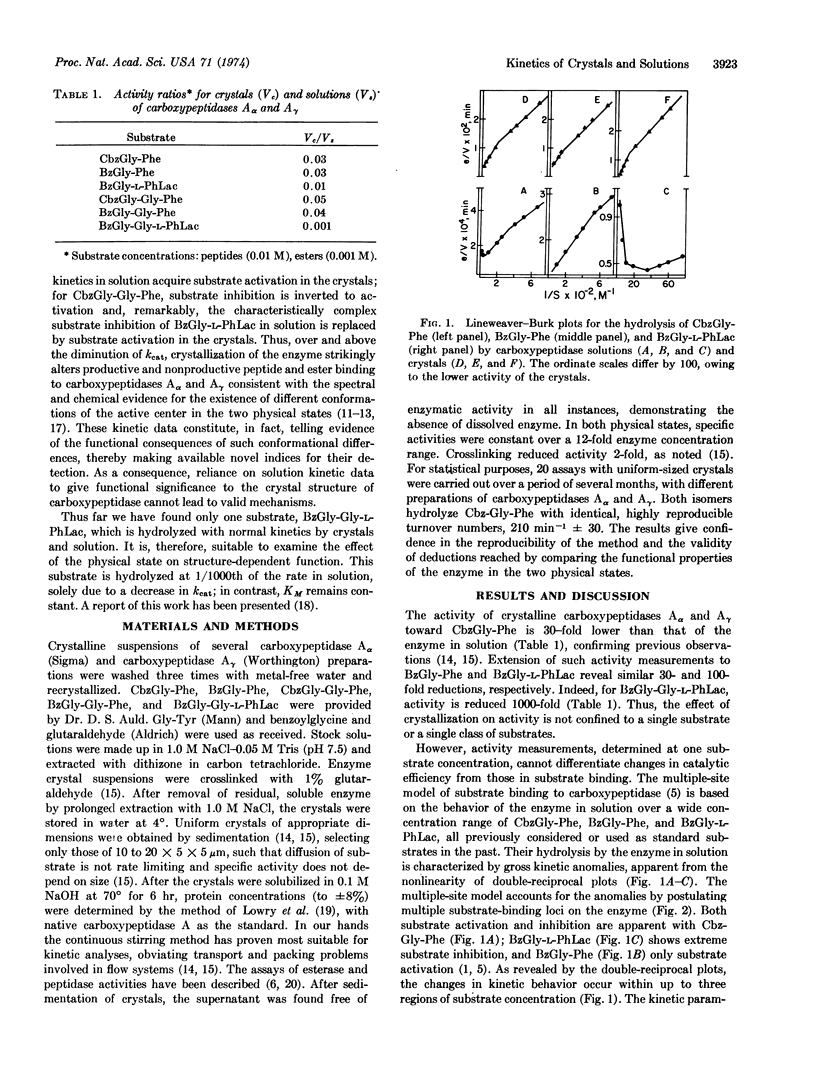
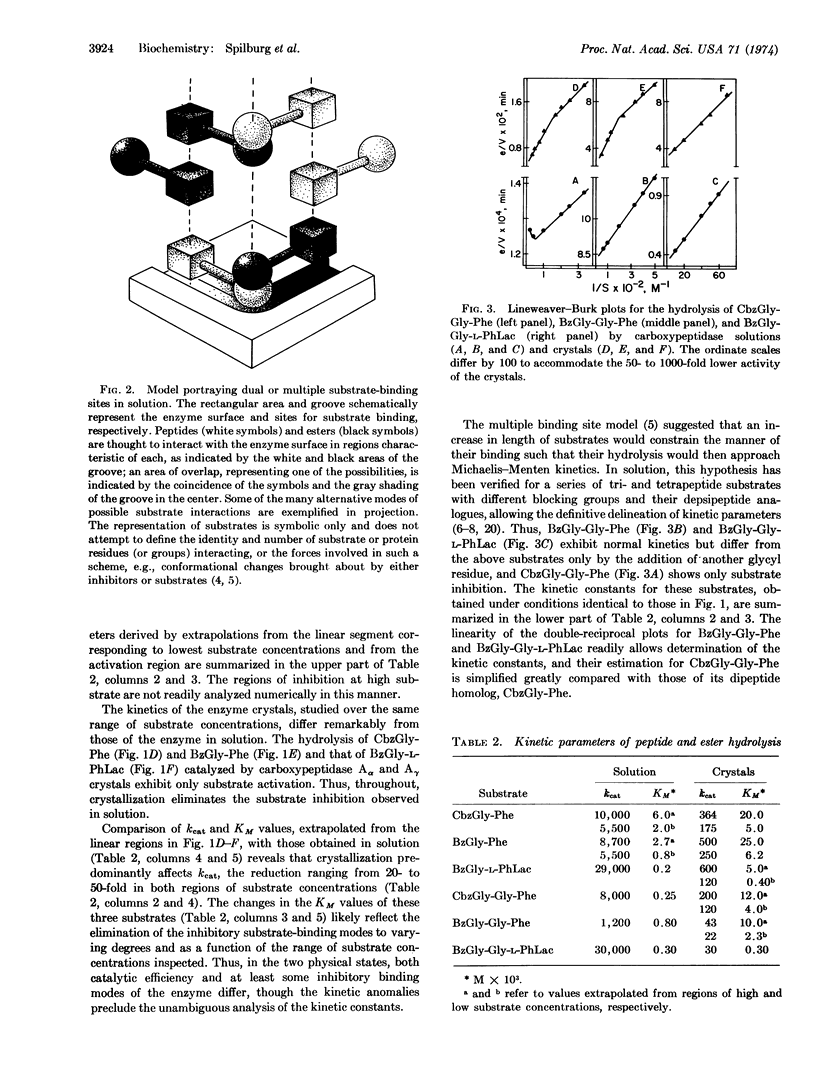
Spectrochemical probes have demonstrated that the conformations of carboxypeptidase A (EC 3.4.12.2) differ in solution and in the crystalline state. Detailed kinetic studies of carboxypeptidase Aα and Aγ crystals and solutions now show that the physical state of the enzyme is also a critical parameter that affects the function of the Aα and Aγ enzymes in the same manner. The kinetic profiles and the corresponding kinetic constants of substrate hydrolysis are, therefore, important functional indices of the known conformational differences of the enzyme in these two physical states. The complex kinetic behavior of this enzyme, however, precludes meaningful comparisons of activity measurements for crystals and solutions obtained at only one substrate concentration. Underlying differences in varying substrate-inhibiting or -activating binding modes can result in either high or low activity ratios, concealing the true, functional consequences of the change in physical state. Thus, for all substrates examined, crystallization of the enzyme markedly reduces catalytic efficiency, kcat, from 20- to 1000-fold. Equally as important, the substrate inhibition, apparent in solution for some di- and depsipeptides, is abolished with crystals, while for longer substrates the normal solution kinetics may acquire activation with the crystals. Hypothetical modes of substrate-enzyme interaction, generated by superimposing substrate models on the crystal structure of carboxypeptidase to simulate kinetics in solution, have failed to detect both of these changes, which affect inhibitory or activating binding modes. The only structure of carboxypeptidase yet published and that of its functionally inert complex with the pseudosubstrate, glycyl-L-tyrosine, derive from a unique form of carboxypeptidase Aα crystals. These crystals differ from all others with regard both to their spectral properties and activity toward carbobenzoxy-glycyl-L-phenylalanine, which is 30% of that in solution, though the significance of this value cannot be gauged without knowledge of the relevant kinetic constants. The rapidly accumulating evidence for functional and conformational differences between crystals and solutions and the recent stress on the nonproductive aspects of the carboxypeptidase Aα-glycyl-L-tyrosine complex, based on 30% site occupancy, suggest that the functional implications of its structural features require reevaluation.
Keywords: enzyme kinetics, conformation, crystals, solution
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auld D. S., Holmquist B. Carboxypeptidase A. Differences in the mechanisms of ester and peptide hydrolysis. Biochemistry. 1974 Oct 8;13(21):4355–4361. doi: 10.1021/bi00718a018. [DOI] [PubMed] [Google Scholar]
- Auld D. S., Vallee B. L. Kinetics of carboxypeptidase A, pH and Temperature dependence of tripeptide hydrolysis. Biochemistry. 1971 Jul 20;10(15):2892–2897. doi: 10.1021/bi00791a015. [DOI] [PubMed] [Google Scholar]
- Auld D. S., Vallee B. L. Kinetics of carboxypeptidase A. II. Inhibitors of the hydrolysis of oligopeptides. Biochemistry. 1970 Feb 3;9(3):602–609. doi: 10.1021/bi00805a022. [DOI] [PubMed] [Google Scholar]
- Auld D. S., Vallee B. L. Kinetics of carboxypeptidase A. The pH dependence of tripeptide hydrolysis catalyzed by zinc, cobalt, and manganese enzymes. Biochemistry. 1970 Oct 27;9(22):4352–4359. doi: 10.1021/bi00824a016. [DOI] [PubMed] [Google Scholar]
- Davies R. C., Auld D. S., Vallee B. L. The effect of modifiers on the hydrolysis of esters and peptides by carboxypeptidase A. Biochem Biophys Res Commun. 1968 May 23;31(4):628–633. doi: 10.1016/0006-291x(68)90525-1. [DOI] [PubMed] [Google Scholar]
- Davies R. C., Riordan J. F., Auld D. S., Vallee B. L. Kinetics of carboxypeptidase A. I. Hydrolysis of carbobenzoxyglycyl-l-phenylalanine, benzoylglycyl-l-phenylalanine, and hippuryl-dl-beta-phenyllactic acid by metal-substituted and acetylated carboxypeptidases. Biochemistry. 1968 Mar;7(3):1090–1099. doi: 10.1021/bi00843a029. [DOI] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Conformations of arsanilazotyrosine-248 carboxypeptidase A alpha, beta, gamma, comparison of crystals and solution. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2006–2010. doi: 10.1073/pnas.70.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Differences between the conformation of arsanilazotyrosine 248 of carboxypeptidase A in the crystalline state and in solution. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2532–2535. doi: 10.1073/pnas.68.10.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lipscomb W. N. Enzymatic activities of carobxypeptidase A's in solution and in crystals. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3797–3801. doi: 10.1073/pnas.70.12.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb W. N., Hartsuck J. A., Reeke G. N., Jr, Quiocho F. A., Bethge P. H., Ludwig M. L., Steitz T. A., Muirhead H., Coppola J. C. The structure of carboxypeptidase A. VII. The 2.0-angstrom resolution studies of the enzyme and of its complex with glycyltyrosine, and mechanistic deductions. Brookhaven Symp Biol. 1968 Jun;21(1):24–90. [PubMed] [Google Scholar]
- Lipscomb W. N., Reeke G. N., Jr, Hartsuck J. A., Quiocho F. A., Bethge P. H. The structure of carboxypeptidase A. 8. Atomic interpretation at 0.2 nm resolution, a new study of the complex of glycyl-L-tyrosine with CPA, and mechanistic deductions. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):177–214. doi: 10.1098/rstb.1970.0020. [DOI] [PubMed] [Google Scholar]
- QUIOCHO F. A., RICHARDS F. M. INTERMOLECULAR CROSS LINKING OF A PROTEIN IN THE CRYSTALLINE STATE: CARBOXYPEPTIDASE-A. Proc Natl Acad Sci U S A. 1964 Sep;52:833–839. doi: 10.1073/pnas.52.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiocho F. A., McMurray C. H., Lipscomb W. N. Similarities between the conformation of arsanilazotyrosine 248 of carboxypeptidase A in the crystalline state and in solution. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2850–2854. doi: 10.1073/pnas.69.10.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. F., Muszynska G. Differences between the conformations of nitrotyrosyl-248 carboxypeptidase A in the crystalline state and in solution. Biochem Biophys Res Commun. 1974 Mar 25;57(2):447–451. doi: 10.1016/0006-291x(74)90951-6. [DOI] [PubMed] [Google Scholar]
- Shaklai N., Zisapel N., Sokolovsky M. The role of a tyrosyl residue in the mechanism of action of carboxypeptidase B: luminescence studies. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2025–2028. doi: 10.1073/pnas.70.7.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F., Auld D. S., Latt S. A. Chemical approaches to the mode of action of carboxypeptidase A. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):215–230. doi: 10.1098/rstb.1970.0021. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F., Bethune J. L., Coombs T. L., Auld D. S., Sokolovsky M. A model for substrate binding and kinetics of carboxypeptidase A. Biochemistry. 1968 Oct;7(10):3547–3556. doi: 10.1021/bi00850a032. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F. Chemical approaches to the mode of action of carboxypeptidase A. Brookhaven Symp Biol. 1968 Jun;21(1):91–119. [PubMed] [Google Scholar]


