Abstract
The phospholipid cardiolipin (CL) plays a role in many cellular functions and signaling pathways both inside and outside of mitochondria. This review focuses on the role of CL in energy metabolism. Many reactions of electron transport and oxidative phosphorylation, the transport of metabolites required for these processes, and the stabilization of electron transport chain supercomplexes require CL. Recent studies indicate that CL is required for the synthesis of iron-sulfur (Fe-S) co-factors, which are essential for numerous metabolic pathways. Activation of carnitine shuttle enzymes that are required for fatty acid metabolism is CL dependent. The presence of substantial amounts of CL in the peroxisomal membrane suggests that CL may be required for peroxisomal functions. Understanding the role of CL in energy metabolism may identify physiological modifiers that exacerbate the loss of CL and underlie the variation in symptoms observed in Barth syndrome, a genetic disorder of CL metabolism.
1. Introduction
Cardiolipin (CL) (1,3-diphosphatidyl-sn-glycerol) is the signature phospholipid of the mitochondrial membrane. First isolated from beef heart (Pangborn, 1942), it is ubiquitous in eukaryotes and also present in prokaryotes. Studies in yeast utilizing well-characterized deletion mutants of CL synthesis (Fig. 1) indicate that CL regulates many cellular functions and signaling pathways, both inside and outside of the mitochondria. The ubiquitous association of CL with energy transducing membranes is consistent with the role of this lipid in bioenergetics (reviewed by Joshi et al., 2009). In fact, CL synthesis and mitochondrial bioenergetics are inter-dependent, as CL synthesis is both required for and stimulated by oxidative phosphorylation (Gohil et al., 2004). Within the mitochondria, the effects of CL deficiency extend beyond bioenergetics to decreased mitochondrial protein import and perturbation of mitochondrial fusion (Jiang et al., 2000; Gebert et al., 2009; Joshi et al., 2012). The deleterious effects of CL deficiency outside the mitochondria include perturbation of the PKC-Slt2 cell integrity and high osmolarity glycerol (HOG) signaling pathways and decreased vacuolar function (Zhong et al., 2005; Zhong et al., 2007; Chen et al., 2008b; Zhou et al., 2009). Perturbation of CL synthesis has long been associated with aging (Paradies et al., 2010), and loss of CL was found to decrease longevity in yeast cells (Zhou et al., 2009). The significance of CL in human health is apparent from clinical findings that perturbation of CL metabolism leads to the life-threatening disorder known as Barth syndrome (BTHS).
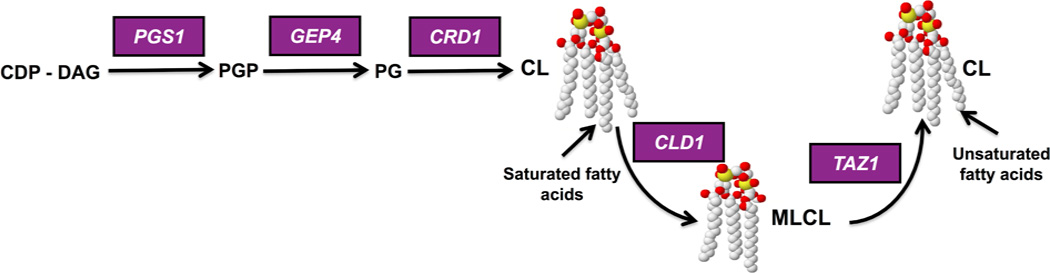
Figure 1. Synthesis and remodeling of cardiolipin (CL) in yeast.
CDP-DAG is converted to phosphatidylglycerolphosphate (PGP) by phosphatidylglycerolphosphate synthase (PGPS), encoded by PGS1 (Chang et al., 1998; Dzugasova et al., 1998). PGP phosphatase (Gep4) catalyzes the conversion of PGP to phosphatidylgylcerol (PG) (Osman et al., 2010). PG is converted to cardiolipin (CL) by CL synthase (Crd1) (Jiang et al., 1997; Chang et al., 1998; Tuller et al., 1998). CL is remodeled in a two-step process in which the CL specific deacylase encoded by CLD1 removes a fatty acyl group, forming monolysocardiolipin (MLCL) (Beranek et al., 2009), and tafazzin (Taz1) reacylates MLCL to form a generally more unsaturated CL (Xu et al., 2003). In mammalian cells, CL is deacylated by more than one enzyme (Kiebish et al., 2013). Tafazzin is the enzyme that is mutated in Barth syndrome.
In addition to the cellular functions listed above, recent studies indicate that CL is intricately involved in cellular metabolism (Fig. 2). These studies are the focus of the current review. CL interacts with components of the electron transport chain and is required for stabilization of electron transport chain supercomplexes and for optimal respiratory control (Bazan et al., 2013; Pfeiffer et al., 2003; Zhang et al., 2002; Zhang et al., 2005). Perturbation of iron-sulfur (Fe-S) biogenesis has been reported in CL deficient yeast cells, suggesting that iron homeostasis as well as enzymatic activities requiring Fe-S cofactors are dependent on CL biosynthesis (Patil et al., 2013). CL is also required for activities of carrier proteins that transport metabolites for energy metabolism (Kadenbach et al., 1982; Fiermonte et al., 1998; Sedlak et al., 1999; Lange et al., 2001; Hoffmann et al., 1994; Jiang et al., 2000; Bisaccia and Palmieri, 1984), as well as for enzymes in the carnitine shuttle (Pande et al., 1986; Noel and Pande, 1986). In addition, CL might also be required for cellular metabolism outside mitochondria. CL is present in the membrane of peroxisomes (Zinser et al., 1991) and may affect β-oxidation and other metabolic activities of this organelle. The role of CL in mitochondrial protein import is discussed as a potential mechanism underlying the metabolic defects associated with CL deficiency. We speculate that defects in these functions may be physiological modifiers that account for the wide disparity of clinical phenotypes observed in BTHS, and conclude with a discussion of important unanswered questions that are exciting directions for future research.
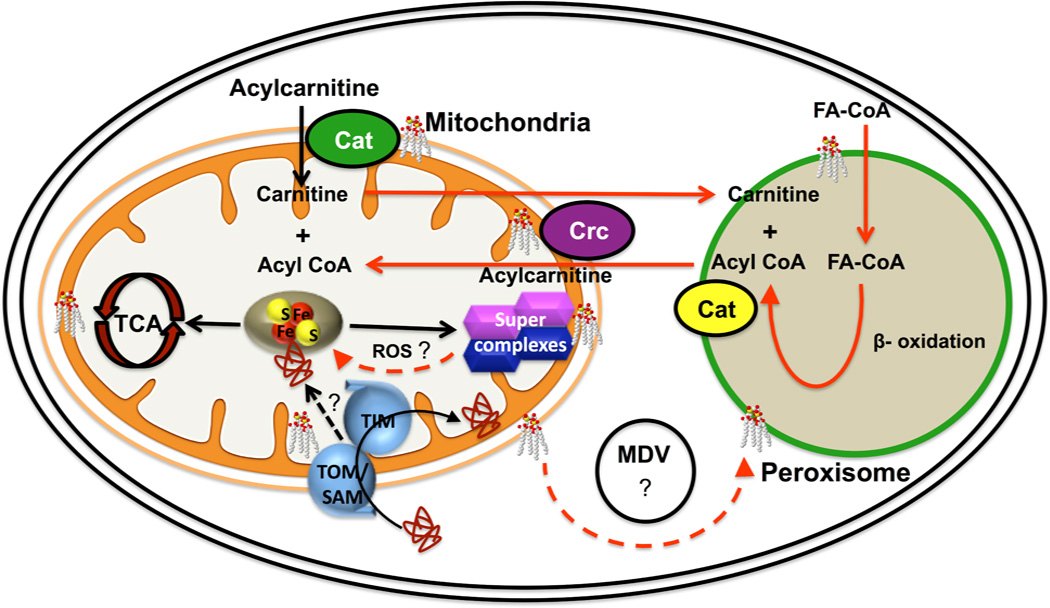
Figure 2. Functions of cardiolipin (CL) in metabolic pathways.
CL is most abundant in the inner membrane and is also present in the outer membrane of mitochondria. It is required for activities of transporters and electron transport chain enzymes and for stabilization of electron transport supercomplexes. Loss of CL leads to perturbation of Fe-S biogenesis, resulting in decreased activity of Fe-S enzymes in the TCA cycle, electron transport, and other pathways. The mechanism linking CL and Fe-S biogenesis is unknown. Because CL is required for the import of proteins through mitochondrial import complexes (TOM, SAM and TIM), it is possible that import of specific proteins required for Fe-S synthesis is defective in CL deficient cells. Alternatively, increased ROS generated by inefficient electron transport in CL deficient cells may damage Fe-S proteins. CL is also present in the membrane of the peroxisome, an organelle that carries out β-oxidation of fatty acids, ether lipid synthesis, and reactions of the glyoxylate cycle. The route whereby CL is transported from mitochondria to peroxisomes is unclear, but may involve mitochondria derived vesicles (MDVs). Acyl CoA produced by β-oxidation of long chain fatty acids in peroxisomes is transported to the mitochondria via the carnitine shuttle. The acyl CoA is transferred to carnitine in the peroxisome by carnitine acyltransferase (Cat). Acylcarnitine from the peroxisome crosses the mitochondrial membrane, facilitated by the carnitine/acylcarnitine translocase (Crc). CL is required for efficient activity of both mitochondrial carnitine enzymes in mammalian cells.
2. CL and mitochondrial bioenergetics
CL is enriched in the membranes of bacteria, mitochondria, and hydrogenosomes, which play a role in ATP synthesis through the generation of a transmembrane electrochemical gradient (Daum et al., 1985; Dowhan et al., 1997; de Andrade Rosa et al., 2006). The association of CL with energy transducing membranes is consistent with the crucial role of this lipid in cellular bioenergetics (reviewed by Schlame et al., 2000; Hoch, 1992). The physical interaction between CL and mitochondrial respiratory chain complexes and other components of membranes also helps in the formation of a lipid scaffold, which functions to stabilize, tether, and increase the enzyme activity of interacting proteins (Beyer et al., 1985; Beyer et al., 1996; Sedlak et al., 1999). In this light, it is not surprising that perturbation of CL synthesis affects the structure and function of mitochondrial respiratory chain complexes and transporters.
2.1 CL and respiration
Analyses of CL function in yeast have been facilitated by the availability of yeast mutants of each step in CL synthesis (Fig. 1). In particular, the CL synthase null mutant crd1Δ, which lacks CL (Jiang et al., 1997; Tuller et al., 1998; Chang et al., 1998), has been the focus of numerous studies. Although crd1Δ cells can grow in non-fermentable carbon sources, indicating that CL is not essential for respiration, the ADP/O and respiratory control ratios (RCR) of crd1Δ mitochondria are reduced in these conditions (Koshkin et al., 2002). CL is required for optimal RCR and ADP/O ratios and for maintenance of the mitochondrial membrane potential (Jiang et al., 2000; Claypool et al., 2008), especially during unfavorable conditions such as increased temperature and osmolarity (Koshkin et al., 2002; Koshkin et al., 2000). The role of CL in respiration has been recently reviewed (Joshi et al., 2009; Patil and Greenberg, 2013).
2.2 CL is required for stabilization of supercomplexes
The electron transport chain complexes are organized into supramolecular structures referred to as supercomplexes (Schagger and Pfeiffer, 2000). S. cerevisiae lacks complex I (NADH complex) but contains NADH dehydrogenase composed of a single subunit (Ndi1). Yeast supercomplexes are formed by the association of two units of complex III with units of complex IV. Supercomplexes in mammalian cells are formed by the association of complex I with two units of complex III and multiple units of complex IV (Schagger, 2002). The proposed role of supercomplexes is that of efficient substrate channeling between the individual complexes. The crd1Δ mutant exhibits destabilization of the supercomplexes (Pfeiffer et al., 2003; Zhang et al., 2002; Zhang et al., 2005). Bazan and co-workers reported the in vitro reconstitution of supercomplexes and showed that supercomplex III2IV2 reconstitution is dependent on CL (Bazan et al., 2013). The loss of CL decreases activity of ADP/ATP carrier protein activity and its association with the supercomplexes (Claypool et al., 2008; Jiang et al., 2000). Destabilization of supercomplexes was also reported in tafazzin-deficient human fibroblasts (McKenzie et al., 2006) and, more recently, in tafazzin-deficient induced pluripotent stem cells (Dudek et al., 2013).
2.3 Loss of CL leads to increased generation of reactive oxygen species (ROS)
Destabilization of supercomplexes is expected to result in increased electron leakage and ROS production (Maranzana et al., 2013). Not surprisingly, the absence of CL in yeast cells leads to increased protein carbonylation, a hallmark of increased ROS (Chen et al., 2008a). The primary sites of ROS generation are complexes I and III (Turrens et al., 1985; Barja, 1999; Kushnareva et al., 2002; Grivennikova and Vinogradov, 2006). The CL acyl chains, which are in close proximity to these ROS generating sites, are susceptible to peroxidation (Li et al., 2010; Li et al., 2012; Liu et al., 2012). The superoxides generated by respiratory complex III were shown to cause peroxidation of CL and to reduce the activity of cytochrome c oxidase (Paradies et al., 1998; Paradies et al., 2000; Paradies et al., 2001). The exogenous supplementation of CL, but not peroxidized CL or other phospholipids, rescued both reduced activity of cytochrome c oxidase and increased generation of ROS in reperfused heart (Paradies et al., 2001; Petrosillo et al., 2007). As CL is extensively remodeled by the transacylase tafazzin (Malhotra et al., 2009), we speculate that this may be a mechanism whereby damaged fatty acyl chains are replaced. Under oxidative stress conditions, acyl-CoA:lysocardiolipin acyltransferase-1 (ALCAT1) may also be involved in remodeling of CL. Increased expression of ALCAT1 leads to aberrant remodeling of CL with long polyunsaturated fatty acyl chains, which are sensitive to ROS (Watkins et al., 1998; Hong et al., 2002; Li et al., 2010). Increased expression of ALCAT1 is linked to diabetes and diet-induced obesity in humans and to hyperthyroid cardiomyopathy in mice (Cao et al., 2009; Li et al., 2010; Li et al., 2012; Liu et al., 2012). In summary, CL deficiency is linked to increased generation of ROS, which in turn damage CL by peroxidation of CL acyl chains.
3. Perturbation of iron homeostasis in CL deficient cells
A recent report indicates that CL is required for Fe-S biogenesis and iron homeostasis (Patil et al., 2013). Fe-S clusters are present in all kingdoms of life (Lill and Muhlenhoff, 2008; Johnson et al., 2005). They are cofactors for many biochemical reactions, including those required for electron transport and for the TCA cycle (Hausmann et al., 2008; Gerber et al., 2004; Li et al., 1999; Lill and Muhlenhoff. 2008). In eukaryotes, three Fe-S cluster biogenesis systems have been characterized, including the iron-sulfur cluster (ISC) import system in mitochondria (required for the generation of all cellular Fe-S proteins), the cytosolic Fe-S protein assembly (CIA) machinery, and the ISC export apparatus in mitochondria. The latter two processes are also involved in maturation of Fe-S proteins in the cytosol and nucleus (Balk et al., 2004; Ye et al., 2006; Kessler et al., 2005; Balk et al., 2005).
A role for CL in iron homeostasis was first suggested by a microarray analysis of genes exhibiting altered expression in the crd1Δ mutant (Patil et al., 2013). Most notably, the genes for iron uptake were greatly upregulated in crd1Δ cells, which exhibited increased mitochondrial iron as well as increased sensitivity to iron and to oxidative stress. Perturbation of iron homeostasis is a demonstrated outcome of defective Fe-S biogenesis (Rutherford et al., 2005; Hausmann et al., 2008). Consistent with an Fe-S defect, the crd1Δ mutant exhibits decreased activities of both mitochondrial and cytosolic enzymes that require Fe-S co-factors, including mitochondrial enzymes succinate dehydrogenase, aconitase, and ubiquinol cytochrome c oxidoreductase, and cytosolic enzymes sulfite reductase and isopropylmalate isomerase (Patil et al., 2013). The CL deficient mutant also exhibited synthetic interaction with the Fe-S scaffold mutant isu1.
The mechanism linking CL to Fe-S biogenesis is not currently understood. We speculate that defective import of proteins required for Fe-S biogenesis may underlie the defect, as several studies have shown that mitochondrial protein import as well as assembly of outer membrane complexes are decreased in yeast CL mutants and in lymphoblasts derived from BTHS patients (Jiang et al., 2000; Gebert et al., 2009). Additional evidence for the role of CL in mitochondrial protein import comes from functional studies of the translocator assembly and maintenance protein Tam41, which is required for the assembly and maintenance of the TIM mitochondrial import complex (Gallas et al., 2006; Tamura et al., 2006). Interestingly, the phenotypes of the tam41Δ mutant were found to be similar to those of the CL mutant crd1Δ. These include defective protein translocases and respiratory chain supercomplexes, decreased assembly of the ADP/ATP carrier (AAC), and decreased CL levels (Tamura et al., 2006; Gallas et al., 2006; Kutik et al., 2008). These findings suggested that protein import defects in the tam41Δ mutant were due to the loss of CL. Consistent with this possibility, Tamura and co-workers demonstrated that Tam41 is the mitochondrial CDP-DG synthase that catalyzes the synthesis of CDP-DG, which is required for CL synthesis (Tamura et al., 2013).
An alternative mechanism is that Fe-S proteins may be damaged by the increase in ROS in CL deficient cells, as the inactivation of Fe-S enzymes by superoxide has been demonstrated (Flint et al., 1993; Gardner, 1997). Increasing antioxidants by overexpression of the YAP1 gene did not rescue the iron sensitivity phenotypes of the CL mutant (Patil et al., 2013), a finding that might argue against Fe-S damage due to increased ROS. However, it is quite possible that free radicals that are not scavenged by Yap1-induced antioxidants may cause Fe-S damage in CL deficient cells.
In light of the role of Fe-S clusters in a wide variety of cellular functions (Rouault et al., 2012), perturbation of Fe-S biogenesis in CL deficient cells has far-reaching implications.
4. CL and mitochondrial transporters
Soluble molecules and substrates are transferred across organelle membranes via carrier proteins. Transporters that play major roles in energy transfer include the ADP/ATP translocase (AAC), phosphate carrier (PiC), pyruvate carrier, fatty acid transport protein (FATP), tricarboxylate transporter, and 2-oxoglutarate/malate carrier protein, among others (Nury et al., 2006; Klingenberg, 1990; Walker, 1992; Kuan et al., 1993; Nelson et al., 1993; Palmieri, 1994). The reader is referred to an excellent review of the role of CL in the stabilization of mitochondrial carrier proteins (Claypool, 2009). Membrane lipids play an important role in the assembly of carrier proteins (Hunte, 2005). Both the AAC and PiC, which are required for oxidative phosphorylation, interact with CL (Kadenbach et al., 1982; Fiermonte et al., 1998; Sedlak et al., 1999; Lange et al., 2001). Of the three isoforms of AAC that have been identified in yeast and humans, activity of the major isoform, AAC2, requires CL (Hoffmann et al., 1994). Furthermore, activity of AAC purified from the yeast crd1Δ mutant is decreased (Jiang et al., 2000). A recent report indicates that AAC also interacts with the TIM mitochondrial protein import complex, and this interaction is dependent on CL (Gebert et al., 2011).
In addition to oxidative phosphorylation, transporters play a role in two other sources of energy production, the oxidation of pyruvate and β-oxidation of fatty acids. Pyruvate is transported into the mitochondria of yeast, Drosophila, and humans by the pyruvate carrier proteins MPC1 & MPC2 (Bricker et al., 2012). The purification of pyruvate carrier proteins requires phospholipids, especially CL (Bisaccia and Palmieri, 1984). In the absence of CL, transport activity of the pyruvate carrier protein was not observed (Nalecz et al., 1986). Fatty acids are taken up by fatty acid transport proteins (FATP1 – FATP 6) in the plasma membrane (Van der Vusse et al., 2000; Gimeno, 2007; Jia et al., 2007). While a role for CL in FATP activation has not been reported,Mitchell et al. (2009) demonstrated that knockdown of FATPs in HEK 293 cells inhibits de novo CL synthesis, suggesting that fatty acid transport and CL synthesis may be interdependent. In summary, CL is required for the transport of metabolites utilized in the major cellular energy producing metabolic pathways.
5. Inter-relationship between CL and carnitine
Activated fatty acids (fatty acyl-CoAs) are metabolized to acetyl-CoA by the enzymes of β-oxidation. As membranes are impermeable to activated acyl-CoAs, their transport into mitochondria is facilitated by the quaternary ammonium compound L-carnitine (3- hydroxy-4-N-trimethylaminobutanoate) (Reuter and Evans, 2012). The transfer of acyl residues from CoA to carnitine is catalyzed by carnitine acyltransferase (van der Leij et al., 2000; Jogl and Tong, 2003; Franken et al., 2008). Carnitine/acylcarnitine translocase catalyzes the transport of acylcarnitine across the mitochondrial membrane (Murthy and Pande, 1984; Van Roermund et al., 1995). The carnitine shuttle is conserved throughout the eukaryotic kingdom (Bremer, 1983).
A role for CL in the carnitine shuttle is suggested by reports that CL is required for the activities of the carnitine shuttle enzymes. Carnitine acyltransferase (carnitine palmitoyltransferase) purified from rat liver cells was shown to contain CL (Fiol et al., 1984), and enzymatic activity of the enzyme was stimulated by CL (Pande et al., 1986). Carnitine/acylcarnitine translocase activity was also shown to require CL (Noel and Pande, 1986). Furthermore, the stimulating effect of carnitine on state 2 respiration in rat liver mitochondria was abolished by doxorubicin, which binds CL, and activity was restored by adding CL (Battelli et al., 1992).
In addition to its function in the transport of acyl groups, carnitine also plays a role in oxidative stress and aging. In S. cerevisiae, carnitine was shown to improve growth in the presence of oxidative stress (Franken et al., 2010). Several features of aging in rats are reversed by supplementation with carnitine, including both decreased CL and mitochondrial dysfunction. Decreased levels and pathological remodeling of CL with polyunsaturated fatty acids (arachidonic and docosahexaenoic acids) have been described in old rats (Lee et al., 2006; Sparagna et al., 2009; Maftah et al., 1994; Lewin et al., 1984; Paradies et al., 1993; Paradies et al., 1997; Lenaz et al., 1997; Paradies et al., 1990). Mitochondrial dysfunction in old animals is also associated with defects in ATP synthesis and oxidative phosphorylation (Maftah et al., 1994; Hoch, 1992; Hagen et al., 1998; Sen et al., 2006; Sen et al., 2007). Dietary supplementation of old rats with acylcarnitine significantly improved cellular respiration and mitochondrial membrane potential and, interestingly, also increased CL levels (Hagen et al., 1998).
These studies suggest an inter-relationship between CL and carnitine in regulating mitochondrial functions. CL is required for optimal activity of the carnitine shuttle enzymes, with implications for the generation of energy from β-oxidation and mitochondrial functions associated with aging. In addition, CL levels may be affected by carnitine supplementation.
6. CL, peroxisomes, and β-oxidation
The peroxisome is a unique, versatile, single membrane bound organelle found in all eukaryotes. The number, size, and function of peroxisomes vary among cell types and in response to physiological conditions (Van den Bosch et al., 1992; Veenhuis et al., 1988). Studies of peroxisomal biogenesis have been hampered by the fragility and low abundance of the organelle (Platta et al., 2007). Peroxisomal enzymes carry out β- oxidation of fatty acids, ether lipid synthesis, and reactions of the glyoxylate cycle (Van den Bosch et al., 1992). In eukaryotes, fatty acids are degraded by α-, β-, and ω- oxidation (Wanders et al., 2003). The major pathway, β-oxidation, is conserved from yeast to higher eukaryotes (Houten et al., 2010; Wanders et al., 2010), although localization of the pathways differs. In yeast, peroxisomes are the sole site of β- oxidation. In mammals, β-oxidation takes place in both mitochondria and peroxisomes (Barlett and Eaton, 2004; Van der Klei and Veenhuis, 1997). β-oxidation of fatty acids plays a key role in energy homeostasis in liver, heart, and skeletal muscle (Houten et al., 2010). The depletion of glucose during fasting is compensated by β-oxidation of fatty acids in many tissues (but not brain) to generate energy (Houten et al., 2010). Fatty acids are also converted to ketone bodies, an additional source of energy that can be utilized by all tissues, including the brain (Houten et al., 2010). The reader is referred to excellent recent reviews of β-oxidation in peroxisomes and mitochondria (Houten et al., 2010; Wanders et al., 2010).
Zinser et al. (1991) reported that the peroxisomal membrane of S. cerevisiae grown in rich media (yeast extract-peptone-dextrose) contains considerable levels of CL (7% of total phospholipid). This amount of CL is slightly more than half the level observed in mitochondria (13% of total phospholipid). The CL content of peroxisomal membranes of Pichia pastoris cells grown under conditions that induce peroxisomes (methanol or oleic acid as the sole source of carbon) was reported to be about a third of the level observed in mitochondria (Wriessneggar et al., 2007).
It is unclear how CL, which is synthesized in mitochondria, is transported to peroxisomes. Neuspiel et al. (2008) showed that, in mammalian cells, mitochondria derived vesicles (MDV) fuse to a fraction of pre-existing peroxisomes. The MDVs formed are of two types, single-membrane MDVs (mitochondrial outer membrane derived vesicles) and double-membrane MDVs (mitochondrial inner and outer membrane derived vesicles). Both types of MDVs fuse to form single membrane enclosed peroxisomes (Neuspiel et al., 2008). MDVs are thought to be involved in vesicular trafficking of membrane lipids (possibly including CL) and proteins from the intermembrane space of mitochondria to peroxisomes (Schumann et al., 2008).
In summary, while there is currently no direct evidence for a role of CL in peroxisomal metabolism, CL is clearly present at significant levels in the peroxisomal membrane. As discussed above, it is now well established that CL affects numerous mitochondrial functions by interacting with proteins and complexes in the mitochondrial membrane. We speculate that this structurally unique lipid also affects peroxisomal function as a consequence of interacting with peroxisomal membrane enzymes and transporters.
7. Implications for Barth syndrome
Barth syndrome (BTHS) is a severe X-linked disorder characterized by a decrease in total CL, an accumulation of MLCL, and the absence of the predominant unsaturated CL species due to the loss of the CL remodeling enzyme tafazzin (Schlame and Ren, 2006). The phenotypes observed in BTHS patients include dilated cardiomyopathy, neutropenia, skeletal myopathy, growth retardation, 3-methylglutaconic aciduria, defective oxidative phosphorylation, hypercholesterolemia, arrhythmia, and exercise intolerance (Clarke et al., 2013). The molecular basis underlying the pathology observed in BTHS patients is not understood. Interestingly, tafazzin mutations do not correlate with the extent of clinical abnormalities in BTHS, and substantial phenotypic variation occurs even for a single tafazzin mutation. The high degree of variation in the symptoms of BTHS patients with the same tafazzin mutation suggests that physiological factors influence the outcome of defective tafazzin (Johnston et al., 1997). We suggest that the functions of CL discussed above may be potential physiological modifiers of the BTHS phenotype.
7.1 Fatty acid metabolism, peroxisome biogenesis and the carnitine shuttle
In a landmark study, Spencer et al. (2011) observed that impaired oxygen utilization directly contributes to exercise intolerance in BTHS patients. It is well-established that mild or moderate exercise is driven by a significant increase in fat oxidation. This suggests the possibility that BTHS patients exhibit exercise intolerance due to the inability to adequately metabolize fat.
The clinical outcomes observed in many human disorders of β-oxidation are similar to those seen in BTHS. Perturbation of β-oxidation of fatty acids in mitochondria was shown to cause cardiomyopathy and arrhythmia (Bonnet et al., 1999; Saudubray et al., 1999). The role of CL in the carnitine shuttle suggests that the loss of CL may lead to defects in β-oxidation and or carnitine mediated transport of acyl-CoA. Consistent with this possibility, carnitine mutations lead to clinical outcomes similar to those seen in BTHS (Wanders et al., 2010). Exercise intolerance has been demonstrated in carnitine palmitoyltransferase deficiency (Ørngreen et al., 2003). Carnitine-acylcarnitine translocase deficiency results in cardiomyopathy with arrhythmia (Longo et al., 2006). Cardiomyopathy and skeletal myopathy are observed in deficiencies of the OCTN2- encoded carnitine transporter and very long chain acyl-CoA dehydrogenase (Rinaldo et al., 2002).
In summary, numerous disorders of fatty acid metabolism lead to clinical phenotypes similar to those found in BTHS, including cardiomyopathy, skeletal myopathy, arrhythmia, and exercise intolerance. Elucidating the role of CL in β-oxidation and carnitine-mediated transport of fatty acids may identify potential avenues for treatment of BTHS.
7.2 Bioenergetics and Fe-S biogenesis
Three human genetic disorders of Fe-S biogenesis have been described (Rouault and Tong, 2008). These include defects in the iron chaperone frataxin, the iron sulfur cluster assembly protein ISCU, and glutaredoxin. Mutations in frataxin in the disorder Friedreich ataxia lead to hypertrophic cardiomyopathy, heart failure, and deficiencies in enzymes requiring Fe-S cofactors, including aconitase and complex I-III enzymes. Interestingly, mitochondrial deficiencies similar to those seen in CL deficient cells can be rescued by overexpression of frataxin, including decreased mitochondrial membrane potential and ATP levels, sensitivity to oxidative stress, and reduced life span (Ristow et al., 2000; Runko et al., 2008). Cardiomyopathy and cardiac failure in mice were also shown to be relieved by overexpression of frataxin (Schulz et al., 2010). A mutation in the Fe-S scaffold protein ISCU has been described in an inherited skeletal muscle disorder characterized by muscle weakness, exercise-induced lactic acidosis, deficiencies in aconitase and succinate dehydrogenase, and iron overload (Mochel at al., 2008; Kollberg et al., 2009). This finding was consistent with earlier reports of aconitase and succinate dehydrogenase deficiencies in muscle disorders (Haller et al., 1991; Hall et al., 1993). In light of the role of CL in Fe-S biogenesis, we speculate that the clinical presentation in BTHS may be exacerbated by additional deficiencies in Fe-S metabolism.
7.3 Mitochondrial protein import
The clinical presentation of a disorder known as dilated cardiomyopathy with ataxia (DCMA) syndrome is very similar to BTHS despite normal CL metabolism in DCMA. Symptoms include cardiomyopathy, elevated 3-methylglutaconic acid, and neutropenia. DCMA syndrome is due to a mutation in a gene thought to function in protein import, TIM14 (which shares homology with yeast Tim14p) (Davey et al., 2006). The similarity in the clinical outcomes of DCMA and BTHS suggests that BTHS may be caused or exacerbated by defective mitochondrial import of specific proteins, the identities of which may shed light on the pathology of the disorders. We speculate that proteins required for Fe-S biogenesis may be likely candidates.
8. Future directions and unanswered questions
While it is clear that CL is required for numerous bioenergetic and metabolic pathways, many questions remain to be addressed to fully understand the role of CL in energy metabolism:
What are the implications of Fe-S perturbation in CL deficient cells? Fe-S clusters are essential for numerous metabolic pathways, including those of electron transport, the TCA cycle, heme synthesis, and amino acid synthesis, among others. To what extent are these pathways impaired during CL deficiency?
What is the mechanism underlying defective Fe-S biogenesis in CL deficient cells? The integrity and stability of the protein complexes in the mitochondrial membrane that drive protein import are altered in CL deficient cells, which could lead to defective import of proteins required for Fe-S biogenesis. The loss of CL also leads to increased ROS production (Chen et al., 2008a), which can result in damage to Fe-S proteins. The contributions of these (or other) potential mechanisms remain to be elucidated.
Significant levels of CL in the peroxisomal membrane suggest that this lipid plays a role in peroxisomal function. The many roles of CL in the mitochondrial membrane result from the direct interactions of CL with proteins, as well as from indirect effects on membrane function and membrane curvature (Acehan et al., 2011). Based on the physical properties of CL that underlie these interactions, we speculate that this lipid is also involved in peroxisomal activities, including import of peroxisomal proteins and β- oxidation of fatty acids, among other functions. It is also important to note that the mechanism underlying transport of CL from the mitochondria to the peroxisome is not known. Understanding the role of MDVs might address this question.
To what extent can other lipids compensate for CL deficiency? Studies in yeast indicate that cells that cannot synthesize CL are viable, although they exhibit numerous defects, especially when stressed. However, mutants blocked in the synthesis of the precursor lipid phosphatidylglycerol exhibit dramatically diminished growth and cannot respire (Chang et al., 1998). These findings suggest that phosphatidylglycerol can compensate for many functions of CL. At least some functions of CL may be compensated by phosphatidylethanolamine, which is also a non-bilayer forming lipid capable of forming hexagonal structures. This is supported by findings that the loss of both CL and mitochondrial phosphatidylethanolamine is lethal in yeast cells (Gohil et al., 2005) and that at least one common function of these lipids is in mitochondrial fusion (Joshi et al., 2012). The identification of compensating lipids may shed light on BTHS, as it is not clear if the pathology results from decreased CL, increased MLCL, and/or aberrant acylation of CL.
One of the enigmas of BTHS (and other monogenic disorders) is that the disorder is characterized by a wide disparity of symptoms ranging from asymptomatic to newborn death, even in the presence of identical tafazzin mutations. This indicates that physiological modifiers affect the clinical outcome in BTHS. We suggest that deficiencies in specific CL functions may act as physiological modifiers that exacerbate the loss of CL. In this light, the elucidation of mechanisms underlying CL functions may lead to new treatment options for BTHS, and for other disorders in which CL plays a role.
Highlights.
Barth syndrome, a disorder of cardiolipin (CL) remodeling caused by mutations in tafazzin, is characterized by a wide range of phenotypes.
Loss of CL-dependent functions may exacerbate phenotypes associated with tafazzin deficiency.
The requirement for CL in metabolism is reflected in its role in mitochondrial bioenergetics, transporter activity, and iron-sulfur biogenesis.
The presence of CL in peroxisomes suggests that it may also be required for peroxisomal function and the carnitine shuttle.
Perturbation of these metabolic functions may exacerbate the loss of tafazzin and other conditions of CL deficiency.
Acknowledgements
The Greenberg laboratory acknowledges support from the National Institutes of Health (HL 084218) and the Barth Syndrome Foundation, Barth Syndrome Foundation of Canada, and Association Barth France.
Abbreviations
- CL
cardiolipin
- Fe-S
iron-sulfur
- PKC-Slt2
Protein kinase C-Slt2 mitogen activated protein kinase
- HOG
high osmolarity glycerol
- BTHS
Barth syndrome
- RCR
respiratory control ratio
- ROS
reactive oxygen species
- ALCAT1
acyl-CoA:lysocardiolipin acyltransferase-1
- ISC
iron-sulfur cluster
- CIA
cytosolic Fe-S protein assembly
- TIM
translocase of the inner membrane
- CDP-DG
CDP-diacylglycerol
- AAC
ADP/ATP translocase
- PiC
phosphate carrier
- FATP
fatty acid transport protein
- MDV
mitochondrial derived vesicles
- DMCA
dilated cardiomyopathy with ataxia
- PGP
phosphatidylglycerolphosphate
- PGPS
phosphatidylglycerolphosphate synthase
- PG
phosphatidylglycerol
- MLCL
monolysocardiolipin
- TOM
translocase of the outer membrane
- SAM
sorting and assembly machinery
- CAT
carnitine acyltransferase
- CRC
carnitine/acylcarnitine translocase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acehan D, Malhotra A, Xu Y, Ren M, Stokes DL, Schlame M. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophysical journal. 2011;100:2184–2192. doi: 10.1016/j.bpj.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk J, Aguilar Netz DJ, Tepper K, Pierik AJ, Lill R. The essential WD40 protein Cia1 is involved in a late step of cytosolic and nuclear iron-sulfur protein assembly. Molecular and cellular biology. 2005;25:10833–10841. doi: 10.1128/MCB.25.24.10833-10841.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk J, Pierik AJ, Netz DJ, Muhlenhoff U, Lill R. The hydrogenase-like Nar1p is essential for maturation of cytosolic and nuclear iron-sulphur proteins. The EMBO journal. 2004;23:2105–2115. doi: 10.1038/sj.emboj.7600216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barja G. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. Journal of bioenergetics and biomembranes. 1999;31:347–366. doi: 10.1023/a:1005427919188. [DOI] [PubMed] [Google Scholar]
- Bartlett K, Eaton S. Mitochondrial beta-oxidation. European journal of biochemistry / FEBS. 2004;271:462–469. doi: 10.1046/j.1432-1033.2003.03947.x. [DOI] [PubMed] [Google Scholar]
- Battelli D, Bellei M, Arrigoni-Martelli E, Muscatello U, Bobyleva V. Interaction of carnitine with mitochondrial cardiolipin. Biochimica et biophysica acta. 1992;1117:33–36. doi: 10.1016/0304-4165(92)90158-q. [DOI] [PubMed] [Google Scholar]
- Bazan S, Mileykovskaya E, Mallampalli VK, Heacock P, Sparagna GC, DW Cardiolipindependent reconstitution of respiratory supercomplexes from purified Saccharomyces cerevisiae complexes III and IV. The Journal of biological chemistry. 2013;288:401–411. doi: 10.1074/jbc.M112.425876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beranek A, Rechberger G, Knauer H, Wolinski H, Kohlwein SD, Leber R. Identification of a cardiolipin-specific phospholipase encoded by the gene CLD1 (YGR110W) in yeast. The Journal of biological chemistry. 2009;284:11572–11578. doi: 10.1074/jbc.M805511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer K, Klingenberg M. ADP/ATP carrier protein from beef heart mitochondria has high amounts of tightly bound cardiolipin, as revealed by 31P nuclear magnetic resonance. Biochemistry. 1985;24:3821–3826. doi: 10.1021/bi00336a001. [DOI] [PubMed] [Google Scholar]
- Beyer K, Nuscher B. Specific cardiolipin binding interferes with labeling of sulfhydryl residues in the adenosine diphosphate/adenosine triphosphate carrier protein from beef heart mitochondria. Biochemistry. 1996;35:15784–15790. doi: 10.1021/bi9610055. [DOI] [PubMed] [Google Scholar]
- Bisaccia F, Palmieri F. Specific elution from hydroxylapatite of the mitochondrial phosphate carrier by cardiolipin. Biochimica et biophysica acta. 1984;766:386–394. doi: 10.1016/0005-2728(84)90254-8. [DOI] [PubMed] [Google Scholar]
- Bonnet D, Martin D, Pascale DL, Villain E, Jouvet P, Rabier D, Brivet M, Saudubray JM. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation. 1999;100:2248–2253. doi: 10.1161/01.cir.100.22.2248. [DOI] [PubMed] [Google Scholar]
- Bremer J. Carnitine--metabolism and functions. Physiological reviews. 1983;63:1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N, Redin C, Boudina S, Gygi SP, Brivet M, Thummel CS, Rutter J. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337:96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Shen W, Chang Z, Shi Y. ALCAT1 is a polyglycerophospholipid acyltransferase potently regulated by adenine nucleotide and thyroid status. American journal of physiology. Endocrinology and metabolism. 2009;296:E647–E653. doi: 10.1152/ajpendo.90761.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SC, Heacock PN, Clancey CJ, Dowhan W. The PEL1 gene (renamed PGS1) encodes the phosphatidylglycero-phosphate synthase of Saccharomyces cerevisiae. The Journal of biological chemistry. 1998;273:9829–9836. doi: 10.1074/jbc.273.16.9829. [DOI] [PubMed] [Google Scholar]
- Chen S, He Q, Greenberg ML. Loss of tafazzin in yeast leads to increased oxidative stress during respiratory growth. Molecular microbiology. 2008a;68:1061–1072. doi: 10.1111/j.1365-2958.2008.06216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Tarsio M, Kane PM, Greenberg ML. Cardiolipin mediates cross-talk between mitochondria and the vacuole. Molecular biology of the cell. 2008b;19:5047–5058. doi: 10.1091/mbc.E08-05-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SLN, Bowron A, Gonzalez IL, Groves SJ, Newbury-Ecob R, Clayton N, Martin RP, Tsa-Goodman B, Garratt V, Ashworth M, Bowen VM, McCurdy KR, Damin MK, Spencer CT, Toth MJ, Kelley RI, Steward CG. Barth syndrome. Orphanet journal of rare diseases. 2013;8:23. doi: 10.1186/1750-1172-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool SM. Cardiolipin, a critical determinant of mitochondrial carrier protein assembly and function. Biochimica et biophysica acta. 2009;1788:2059–2068. doi: 10.1016/j.bbamem.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool SM, Oktay Y, Boontheung P, Loo JA, Koehler CM. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. The Journal of cell biology. 2008;182:937–950. doi: 10.1083/jcb.200801152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G. Lipids of mitochondria. Biochimica et biophysica acta. 1985;822:1–42. doi: 10.1016/0304-4157(85)90002-4. [DOI] [PubMed] [Google Scholar]
- Davey KM, Parboosingh JS, McLeod DR, Chan A, Casey R, Ferreira P, Snyder FF, Bridge PJ, Bernier FP. Mutation of DNAJC19, a human homologue of yeast inner mitochondrial membrane co-chaperones, causes DCMA syndrome, a novel autosomal recessive Barth syndromelike condition. Journal of medical genetics. 2006;43:385–393. doi: 10.1136/jmg.2005.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrade Rosa I, Einicker-Lamas M, Roney Bernardo R, Previatto LM, Mohana-Borges R, Morgado-Diaz JA, Benchimol M. Cardiolipin in hydrogenosomes: evidence of symbiotic origin. Eukaryotic cell. 2006;5:784–787. doi: 10.1128/EC.5.4.784-787.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowhan W. Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annual review of biochemistry. 1997;66:199–232. doi: 10.1146/annurev.biochem.66.1.199. [DOI] [PubMed] [Google Scholar]
- Dudek J, Cheng IF, Balleininger M, Vaz FM, Streckfuss-Bomeke K, Hubscher D, Vukotic M, Wanders RJ, Rehling P, Guan K. Cardiolipin deficiency affects respiratory chain function and organization in an induced pluripotent stem cell model of Barth syndrome. Stem cell research. 2013;11:806–819. doi: 10.1016/j.scr.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Dzugasova V, Obernauerova M, Horvathova K, Vachova M, Zakova M, Subik J. Phosphatidylglycerolphosphate synthase encoded by the PEL1/PGS1 gene in Saccharomyces cerevisiae is localized in mitochondria and its expression is regulated by phospholipid precursors. Current genetics. 1998;34:297–302. doi: 10.1007/s002940050399. [DOI] [PubMed] [Google Scholar]
- Fiermonte G, Dolce V, Palmieri F. Expression in Escherichia coli, functional characterization, and tissue distribution of isoforms A and B of the phosphate carrier from bovine mitochondria. The Journal of biological chemistry. 1998;273:22782–22787. doi: 10.1074/jbc.273.35.22782. [DOI] [PubMed] [Google Scholar]
- Fiol CJ, Bieber LL. Sigmoid kinetics of purified beef heart mitochondrial carnitine palmitoyltransferase. Effect of pH and malonyl-CoA. The Journal of biological chemistry. 1984;259:13084–13088. [PubMed] [Google Scholar]
- Flint DH, Tuminello JF, Emptage MH. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. The Journal of biological chemistry. 1993;268:22369–22376. [PubMed] [Google Scholar]
- Franken J, Bauer FF. Carnitine supplementation has protective and detrimental effects in Saccharomyces cerevisiae that are genetically mediated. FEMS yeast research. 2010;10:270–281. doi: 10.1111/j.1567-1364.2010.00610.x. [DOI] [PubMed] [Google Scholar]
- Franken J, Kroppenstedt S, Swiegers JH, Bauer FF. Carnitine and carnitine acetyltransferases in the yeast Saccharomyces cerevisiae: a role for carnitine in stress protection. Current genetics. 2008;53:347–360. doi: 10.1007/s00294-008-0191-0. [DOI] [PubMed] [Google Scholar]
- Gallas MR, Dienhart MK, Stuart RA, Long RM. Characterization of Mmp37p, a Saccharomyces cerevisiae mitochondrial matrix protein with a role in mitochondrial protein import. Molecular biology of the cell. 2006;17:4051–4062. doi: 10.1091/mbc.E06-04-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner PR. Superoxide-driven aconitase FE-S center cycling. Bioscience reports. 1997;17:33–42. doi: 10.1023/a:1027383100936. [DOI] [PubMed] [Google Scholar]
- Gebert N, Gebert M, Oeljieklaus S, von der Malsburg K, Stroud DA, Kulawiak B, Wirth C, Zahedi RP, Dolezal P, Wiese S, Simon o, Schulze-Specking A, Truscott KN, Sickmann A, Rehling P, Guiard B, Hunte C, Warscheid B, van der Laan M, Pfanner N, Wiedemann N. Dual function of Sdh3 in the respiratory chain and TIM22 protein translocase of the mitochondrial inner membrane. Molecular cell. 2011;44:811–818. doi: 10.1016/j.molcel.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Gebert N, Joshi AS, Kutik S, Becker T, McKenzie M, Guan XL, Mooga VP, Stroud DA, Kulkarni G, Wenk MR, Rehling P, Meisinger C, Ryan MT, Wiedemann N, Greenberg ML, Pfanner N. Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: implications for Barth syndrome. Current biology : CB. 2009;19:2133–2139. doi: 10.1016/j.cub.2009.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber J, Neumann K, Prohl C, Muhlenhoff U, Lill R. The yeast scaffold proteins Isu1p and Isu2p are required inside mitochondria for maturation of cytosolic Fe/S proteins. Molecular and cellular biology. 2004;24:4848–4857. doi: 10.1128/MCB.24.11.4848-4857.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno RE. Fatty acid transport proteins. Current opinion in lipidology. 2007;18:271–276. doi: 10.1097/MOL.0b013e3281338558. [DOI] [PubMed] [Google Scholar]
- Gohil VM, Hayes P, Matsuyama S, Schagger H, Schlame M, Greenberg ML. Cardiolipin biosynthesis and mitochondrial respiratory chain function are interdependent. The Journal of biological chemistry. 2004;279:42612–42618. doi: 10.1074/jbc.M402545200. [DOI] [PubMed] [Google Scholar]
- Gohil VM, Thompson MN, Greenberg ML. Synthetic lethal interaction of the mitochondrial phosphatidylethanolamine and cardiolipin biosynthetic pathways in Saccharomyces cerevisiae. The Journal of biological chemistry. 2005;280:35410–35416. doi: 10.1074/jbc.M505478200. [DOI] [PubMed] [Google Scholar]
- Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochimica et biophysica acta. 2006;1757:553–561. doi: 10.1016/j.bbabio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Hagen TM, Wehr CM, Ames BN. Mitochondrial decay in aging. Reversal through supplementation of acetyl-L-carnitine and N-tert-butyl-alpha-phenyl-nitrone. Annals of the New York Academy of Sciences. 1998;854:214–223. doi: 10.1111/j.1749-6632.1998.tb09904.x. [DOI] [PubMed] [Google Scholar]
- Hall RE, Henriksson KG, Lewis SF, Haller RG, Kennaway NG. Mitochondrial myopathy with succinate dehydrogenase and aconitase deficiency. Abnormalities of several iron-sulfur proteins. The Journal of clinical investigation. 1993;92:2660–2666. doi: 10.1172/JCI116882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller RG, Henriksson KG, Jorfeldt L, Hultman E, Wiborn R, Sahlin K, Areskog NH, Gunder M, Ayyad K, Blomqvist CG. Deficiency of skeletal muscle succinate dehydrogenase and aconitase. Pathophysiology of exercise in a novel human muscle oxidative defect. The Journal of clinical investigation. 1991;88:1197–1206. doi: 10.1172/JCI115422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann A, Samans B, Lill R, Muhlenhoff U. Cellular and mitochondrial remodeling upon defects in iron-sulfur protein biogenesis. The Journal of biological chemistry. 2008;283:8318–8330. doi: 10.1074/jbc.M705570200. [DOI] [PubMed] [Google Scholar]
- Hoch FL. Cardiolipins and biomembrane function. Biochimica et biophysica acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Stockl A, Schlame M, Beyer K, Klingenberg M. The reconstituted ADP/ATP carrier activity has an absolute requirement for cardiolipin as shown in cysteine mutants. The Journal of biological chemistry. 1994;269:1940–1944. [PubMed] [Google Scholar]
- Hong MY, Chapkin RS, Barhoumi R, Burghardt RC, Turner ND, Henderson CE, Sanders LM, Fan YY, Davidson LA, Murphy ME, Spinka CM, Carroll RJ, Lupton JR. Fish oil increases mitochondrial phospholipid unsaturation, upregulating reactive oxygen species and apoptosis in rat colonocytes. Carcinogenesis. 2002;23:1919–1925. doi: 10.1093/carcin/23.11.1919. [DOI] [PubMed] [Google Scholar]
- Houten SM, Wanders RJ. A general introduction to the biochemistry of mitochondrial fatty acid betaoxidation. Journal of inherited metabolic disease. 2010;33:469–477. doi: 10.1007/s10545-010-9061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunte C. Specific protein-lipid interactions in membrane proteins. Biochemical Society transactions. 2005;33:938–942. doi: 10.1042/BST20050938. [DOI] [PubMed] [Google Scholar]
- Jia Z, Pei Z, Maiguel D, Toomer CJ, Watkins PA. The fatty acid transport protein (FATP) family: very long chain acyl-CoA synthetases or solute carriers? Journal of molecular neuroscience : MN. 2007;33:25–31. doi: 10.1007/s12031-007-0038-z. [DOI] [PubMed] [Google Scholar]
- Jiang F, Rizavi HS, Greenberg ML. Cardiolipin is not essential for the growth of Saccharomyces cerevisiae on fermentable or non-fermentable carbon sources. Molecular microbiology. 1997;26:481–491. doi: 10.1046/j.1365-2958.1997.5841950.x. [DOI] [PubMed] [Google Scholar]
- Jiang F, Ryan MT, Schlame M, Zhao M, Gu Z, Klingenberg M, Pfanner N, Greenberg ML. Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. The Journal of biological chemistry. 2000;275:22387–22394. doi: 10.1074/jbc.M909868199. [DOI] [PubMed] [Google Scholar]
- Jogl G, Tong L. Crystal structure of carnitine acetyltransferase and implications for the catalytic mechanism and fatty acid transport. Cell. 2003;112:113–122. doi: 10.1016/s0092-8674(02)01228-x. [DOI] [PubMed] [Google Scholar]
- Johnson DC, Dean DR, Smith AD, Johnson MK. Structure, function, and formation of biological iron-sulfur clusters. Annual review of biochemistry. 2005;74:247–281. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- Johnston J, Kelley RI, Feigenbaum A, Cox GF, Iyer GS, Funanage VL, Proujansky R. Mutation characterization and genotype-phenotype correlation in Barth syndrome. American journal of human genetics. 1997;61:1053–1058. doi: 10.1086/301604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AS, Thompson MN, Fei N, Huttemann M, Greenberg ML. Cardiolipin and mitochondrial phosphatidylethanolamine have overlapping functions in mitochondrial fusion in Saccharomyces cerevisiae. The Journal of biological chemistry. 2012;287:17589–17597. doi: 10.1074/jbc.M111.330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AS, Zhou J, Gohil VM, Chen S, Greenberg ML. Cellular functions of cardiolipin in yeast. Biochimica et biophysica acta. 2009;1793:212–218. doi: 10.1016/j.bbamcr.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadenbach B, Mende P, Kolbe HV, Stipani I, Palmieri F. The mitochondrial phosphate carrier has an essential requirement for cardiolipin. FEBS letters. 1982;139:109–112. doi: 10.1016/0014-5793(82)80498-5. [DOI] [PubMed] [Google Scholar]
- Kessler D, Papenbrock J. Iron-sulfur cluster biosynthesis in photosynthetic organisms. Photosynthesis research. 2005;86:391–407. doi: 10.1007/s11120-005-5913-2. [DOI] [PubMed] [Google Scholar]
- Kiebish MA, Yang K, Liu X, Mancuso DJ, Guan S, Zhao Z, Sims HF, Cerqua R, Cade WT, Han X, Gross RW. Dysfunctional cardiac mitochondrial bioenergetic, lipidomic, and signaling in a murine model of Barth syndrome. Journal of lipid research. 2013;54:1312–1325. doi: 10.1194/jlr.M034728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. Mechanism and evolution of the uncoupling protein of brown adipose tissue. Trends in biochemical sciences. 1990;15:108–112. doi: 10.1016/0968-0004(90)90194-g. [DOI] [PubMed] [Google Scholar]
- Kollberg G, Tulinius M, Melberg A, Darin N, Anderson O, Holmgren D, Oldfors A, Holme E. Clinical manifestation and a new ISCU mutation in iron-sulphur cluster deficiency myopathy. Brain : a journal of neurology. 2009;132:2170–2179. doi: 10.1093/brain/awp152. [DOI] [PubMed] [Google Scholar]
- Koshkin V, Greenberg ML. Cardiolipin prevents rate-dependent uncoupling and provides osmotic stability in yeast mitochondria. The Biochemical journal. 2002;364:317–322. doi: 10.1042/bj3640317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshkin V, Greenberg ML. Oxidative phosphorylation in cardiolipin-lacking yeast mitochondria. The Biochemical journal. 2000;347(Pt 3):687–691. [PMC free article] [PubMed] [Google Scholar]
- Kuan J, Saier MH., Jr Expansion of the mitochondrial carrier family. Research in microbiology. 1993;144:671–672. doi: 10.1016/0923-2508(93)90073-b. [DOI] [PubMed] [Google Scholar]
- Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. The Biochemical journal. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutik S, Rissler M, Guan XL, Guiard B, Shui G, Gebert N, Heacock PN, Rehling P, Dowhan W, Wenk MR, Pfanner N, Wiedemann N. The translocator maintenance protein Tam41 is required for mitochondrial cardiolipin biosynthesis. The Journal of cell biology. 2008;183:1213–1221. doi: 10.1083/jcb.200806048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Nett JH, Trumpower BL, Hunte C. Specific roles of protein-phospholipid interactions in the yeast cytochrome bc1 complex structure. The EMBO journal. 2001;20:6591–6600. doi: 10.1093/emboj/20.23.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Mayette J, Rapoport SI, Bazinet RP. Selective remodeling of cardiolipin fatty acids in the aged rat heart. Lipids in health and disease. 2006;5:2. doi: 10.1186/1476-511X-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaz G, Bovina C, Castelluccio C, Fato R, Formiggini G, Genova ML, Marchetti M, Pich MM, Pallotti F, Parenti Castelli G, Biagini G. Mitochondrial complex I defects in aging. Molecular and cellular biochemistry. 1997;174:329–333. [PubMed] [Google Scholar]
- Lewin MB, Timiras PS. Lipid changes with aging in cardiac mitochondrial membranes. Mechanisms of ageing and development. 1984;24:343–351. doi: 10.1016/0047-6374(84)90119-2. [DOI] [PubMed] [Google Scholar]
- Li J, Kogan M, Knight SA, Pain D, Dancis A. Yeast mitochondrial protein, Nfs1p, coordinately regulates iron-sulfur cluster proteins, cellular iron uptake, and iron distribution. The Journal of biological chemistry. 1999;274:33025–33034. doi: 10.1074/jbc.274.46.33025. [DOI] [PubMed] [Google Scholar]
- Li J, Liu X, Wang H, Zhang W, Chan DC, Shi Y. Lysocardiolipin acyltransferase 1 (ALCAT1) controls mitochondrial DNA fidelity and biogenesis through modulation of MFN2 expression. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6975–6980. doi: 10.1073/pnas.1120043109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Romestaing C, Han X, Li Y, Hao X, Wu Y, Sun C, Liu X, Jefferson LS, Xiong J, Lanoue KF, Chang Z, Lynch CJ, Wang H, Shi Y. Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell metabolism. 2010;12:154–165. doi: 10.1016/j.cmet.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R, Muhlenhoff U. Maturation of iron-sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annual review of biochemistry. 2008;77:669–700. doi: 10.1146/annurev.biochem.76.052705.162653. [DOI] [PubMed] [Google Scholar]
- Liu X, Ye B, Miller S, Yuan H, Zhang H, Tian L, Nie J, Imae R, Arai H, Li Y, Cheng Z, Shi Y. Ablation of ALCAT1 mitigates hypertrophic cardiomyopathy through effects on oxidative stress and mitophagy. Molecular and cellular biology. 2012;32:4493–4504. doi: 10.1128/MCB.01092-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo N, Amat di San Filippo C, Pasquali M. Disorders of carnitine transport and the carnitine cycle. American journal of medical genetics. Part C, Seminars in medical genetics. 2006;142C:77–85. doi: 10.1002/ajmg.c.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maftah A, Ratinaud MH, Dumas M, Bonte F, Meybeck A, Julien R. Human epidermal cells progressively lose their cardiolipins during ageing without change in mitochondrial transmembrane potential. Mechanisms of ageing and development. 1994;77:83–96. doi: 10.1016/0047-6374(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Xu Y, Ren M, Schlame M. Formation of molecular species of mitochondrial cardiolipin. 1. A novel transacylation mechanism to shuttle fatty acids between sn-1 and sn-2 positions of multiple phospholipid species. Biochimica et biophysica acta. 2009;1791:314–320. doi: 10.1016/j.bbalip.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranzana E, Barbero G, Falasca AI, Lenaz G, Genova ML. Mitochondrial Respiratory Supercomplex Association Limits Production of Reactive Oxygen Species from Complex I. Antioxidants & redox signaling. 2013 doi: 10.1089/ars.2012.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie M, Lazarou M, Thorburn DR, Ryan MT. Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. Journal of molecular biology. 2006;361:462–469. doi: 10.1016/j.jmb.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Mitchell RW, Hatch GM. Regulation of cardiolipin biosynthesis by fatty acid transport protein-1 IN HEK 293 cells. Biochimica et biophysica acta. 2009;1788:2015–2021. doi: 10.1016/j.bbamem.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Mochel F, Knight MA, Tong WH, Hernandez D, Ayyad K, Taivassalo T, Andersen PM, Singleton A, Rouault TA, Fischbeck KH, Haller RG. Splice mutation in the iron-sulfur cluster scaffold protein ISCU causes myopathy with exercise intolerance. American journal of human genetics. 2008;82:652–660. doi: 10.1016/j.ajhg.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy MS, Pande SV. Mechanism of carnitine acylcarnitine translocase-catalyzed import of acylcarnitines into mitochondria. The Journal of biological chemistry. 1984;259:9082–9089. [PubMed] [Google Scholar]
- Nalecz KA, Bolli R, Wojtczak L, Azzi A. The monocarboxylate carrier from bovine heart mitochondria: partial purification and its substrate-transporting properties in a reconstituted system. Biochimica et biophysica acta. 1986;851:29–37. doi: 10.1016/0005-2728(86)90245-8. [DOI] [PubMed] [Google Scholar]
- Nelson DR, Lawson JE, Klingenberg M, Douglas MG. Site-directed mutagenesis of the yeast mitochondrial ADP/ATP translocator. Six arginines and one lysine are essential. Journal of molecular biology. 1993;230:1159–1170. doi: 10.1006/jmbi.1993.1233. [DOI] [PubMed] [Google Scholar]
- Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, Andrade-Navarro MA, McBride HM. Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Current biology : CB. 2008;18:102–108. doi: 10.1016/j.cub.2007.12.038. [DOI] [PubMed] [Google Scholar]
- Noel H, Pande SV. An essential requirement of cardiolipin for mitochondrial carnitine acylcarnitine translocase activity. Lipid requirement of carnitine acylcarnitine translocase. European journal of biochemistry / FEBS. 1986;155:99–102. doi: 10.1111/j.1432-1033.1986.tb09463.x. [DOI] [PubMed] [Google Scholar]
- Nury H, Dahout-Gonzalez C, Trezeguet V, Lauquin GJ, Brandolin G, Pebay-Peyroula E. Relations between structure and function of the mitochondrial ADP/ATP carrier. Annual review of biochemistry. 2006;75:713–741. doi: 10.1146/annurev.biochem.75.103004.142747. [DOI] [PubMed] [Google Scholar]
- Orngreen MC, Ejstrup R, Vissing J. Effect of diet on exercise tolerance in carnitine palmitoyltransferase II deficiency. Neurology. 2003;61:559–5561. doi: 10.1212/01.wnl.0000078195.05396.20. [DOI] [PubMed] [Google Scholar]
- Osman C, Haag M, Wieland FT, Brugger B, Langer T. A mitochondrial phosphatase required for cardiolipin biosynthesis: the PGP phosphatase Gep4. The EMBO journal. 2010;29:1976–1987. doi: 10.1038/emboj.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri F. Mitochondrial carrier proteins. FEBS letters. 1994;346:48–54. doi: 10.1016/0014-5793(94)00329-7. [DOI] [PubMed] [Google Scholar]
- Pande SV, Murthy MS, Noel H. Differential effects of phosphatidylcholine and cardiolipin on carnitine palmitoyltransferase activity. Biochimica et biophysica acta. 1986;877:223–230. doi: 10.1016/0005-2760(86)90298-5. [DOI] [PubMed] [Google Scholar]
- Pangborn M. Isolation and purification of a serologically active phospholipid from beef heart. Journal of biological chemistry. 1942;143:247–256. [Google Scholar]
- Paradies G, Ruggiero FM. Age-related changes in the activity of the pyruvate carrier and in the lipid composition in rat-heart mitochondria. Biochimica et biophysica acta. 1990;1016:207–212. doi: 10.1016/0005-2728(90)90060-h. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Oxidative stress, mitochondrial bioenergetics, and cardiolipin in aging. Free radical biology & medicine. 2010;48:1286–1295. doi: 10.1016/j.freeradbiomed.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. Reactive oxygen species generated by the mitochondrial respiratory chain affect the complex III activity via cardiolipin peroxidation in beef-heart submitochondrial particles. Mitochondrion. 2001;1:151–159. doi: 10.1016/s1567-7249(01)00011-3. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. The effect of reactive oxygen species generated from the mitochondrial electron transport chain on the cytochrome c oxidase activity and on the cardiolipin content in bovine heart submitochondrial particles. FEBS letters. 2000;466:323–326. doi: 10.1016/s0014-5793(00)01082-6. [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Age-dependent decrease in the cytochrome c oxidase activity and changes in phospholipids in rat-heart mitochondria. Archives of gerontology and geriatrics. 1993;16:263–272. doi: 10.1016/0167-4943(93)90037-i. [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Age-dependent decline in the cytochrome c oxidase activity in rat heart mitochondria: role of cardiolipin. FEBS letters. 1997;406:136–138. doi: 10.1016/s0014-5793(97)00264-0. [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Peroxidative damage to cardiac mitochondria: cytochrome oxidase and cardiolipin alterations. FEBS letters. 1998;424:155–158. doi: 10.1016/s0014-5793(98)00161-6. [DOI] [PubMed] [Google Scholar]
- Patil VA, Greenberg ML. Cardiolipin-mediated cellular signaling. Advances in experimental medicine and biology. 2013;991:195–213. doi: 10.1007/978-94-007-6331-9_11. [DOI] [PubMed] [Google Scholar]
- Patil VA, Fox JL, Gohil VM, Winge DR, Greenberg ML. Loss of cardiolipin leads to perturbation of mitochondrial and cellular iron homeostasis. The Journal of biological chemistry. 2013;288:1696–1705. doi: 10.1074/jbc.M112.428938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosillo G, Portincasa P, Grattagliano I, Casanova G, Matera M, Ruggiero FM, Ferri D, Paradies G. Mitochondrial dysfunction in rat with nonalcoholic fatty liver Involvement of complex I, reactive oxygen species and cardiolipin. Biochimica et biophysica acta. 2007;1767:1260–1267. doi: 10.1016/j.bbabio.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Pfeiffer K, Gohil V, Stuart RA, Hunte C, Brandt U, Greenberg ML, Schagger H. Cardiolipin stabilizes respiratory chain supercomplexes. The Journal of biological chemistry. 2003;278:52873–52880. doi: 10.1074/jbc.M308366200. [DOI] [PubMed] [Google Scholar]
- Platta HW, Magraoui FE, Schlee D, Grunau S, Girzalsky W, Erdmann R. Ubiquitination of the peroxisomal import receptor Pex5p is required for its recycling. The Journal of cell biology. 2007;177:197–204. doi: 10.1083/jcb.200611012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter SE, Evans AM. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clinical pharmacokinetics. 2012;51:553–572. doi: 10.1007/BF03261931. [DOI] [PubMed] [Google Scholar]
- Rinaldo P, Matern D, Bennett MJ. Fatty acid oxidation disorders. Annual review of physiology. 2002;64:477–502. doi: 10.1146/annurev.physiol.64.082201.154705. [DOI] [PubMed] [Google Scholar]
- Ristow M, Pfister MF, Yee AJ, Schubert M, Michael L, Zhang CY, Ueki K, Michael MD, Lowell BB, Kahn CR. Frataxin activates mitochondrial energy conversion and oxidative phosphorylation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12239–12243. doi: 10.1073/pnas.220403797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault TA, Tong WH. Iron-sulfur cluster biogenesis and human disease. Trends in genetics : TIG. 2008;24:398–407. doi: 10.1016/j.tig.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault TA. Biogenesis of iron-sulfur clusters in mammalian cells: new insights and relevance to human disease. Disease models & mechanisms. 2012;5:155–164. doi: 10.1242/dmm.009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runko AP, Griswold AJ, Min KT. Overexpression of frataxin in the mitochondria increases resistance to oxidative stress and extends lifespan in Drosophila. FEBS letters. 2008;582:715–719. doi: 10.1016/j.febslet.2008.01.046. [DOI] [PubMed] [Google Scholar]
- Rutherford JC, Ojeda L, Balk J, Muhlenhoff U, Lill R, Winge DR. Activation of the iron regulon by the yeast Aft1/Aft2 transcription factors depends on mitochondrial but not cytosolic iron-sulfur protein biogenesis. The Journal of biological chemistry. 2005;280:10135–10140. doi: 10.1074/jbc.M413731200. [DOI] [PubMed] [Google Scholar]
- Saudubray JM, Martin D, de Lonlay P, Touati G, Poggi-Travert F, Bonnet D, Jouvet P, Boutron M, Slama A, Vianey-Saban C, Bonnefont JP, Rabier D, Kamoun P, Brivet M. Recognition and management of fatty acid oxidation defects: a series of 107 patients. Journal of inherited metabolic disease. 1999;22:488–502. doi: 10.1023/a:1005556207210. [DOI] [PubMed] [Google Scholar]
- Schagger H, Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. The EMBO journal. 2000;19:1777–1783. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagger H. Respiratory chain supercomplexes of mitochondria and bacteria. Biochimica et biophysica acta. 2002;1555:154–159. doi: 10.1016/s0005-2728(02)00271-2. [DOI] [PubMed] [Google Scholar]
- Schlame M, Ren M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS letters. 2006;580:5450–5455. doi: 10.1016/j.febslet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Progress in lipid research. 2000;39:257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Westermann D, Isken F, Voigt A, Laube B, Thierbach R, Kuhlow D, Zarse K, Schomburg L, Pfeiffer AF, Tschope C, Ristow M. Activation of mitochondrial energy metabolism protects against cardiac failure. Aging. 2010;2:843–853. doi: 10.18632/aging.100234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann U, Subramani S. Special delivery from mitochondria to peroxisomes. Trends in cell biology. 2008;18:253–256. doi: 10.1016/j.tcb.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak E, Robinson NC. Phospholipase A(2) digestion of cardiolipin bound to bovine cytochrome c oxidase alters both activity and quaternary structure. Biochemistry. 1999;38:14966–14972. doi: 10.1021/bi9914053. [DOI] [PubMed] [Google Scholar]
- Sen T, Sen N, Jana S, Khan FH, Chatterjee U, Chakrabarti S. Depolarization and cardiolipin depletion in aged rat brain mitochondria: relationship with oxidative stress and electron transport chain activity. Neurochemistry international. 2007;50:719–725. doi: 10.1016/j.neuint.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Sen T, Sen N, Tripathi G, Chatterjee U, Chakrabarti S. Lipid peroxidation associated cardiolipin loss and membrane depolarization in rat brain mitochondria. Neurochemistry international. 2006;49:20–27. doi: 10.1016/j.neuint.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Sparagna GC, Lesnefsky EJ. Cardiolipin remodeling in the heart. Journal of cardiovascular pharmacology. 2009;53:290–301. doi: 10.1097/FJC.0b013e31819b5461. [DOI] [PubMed] [Google Scholar]
- Spencer CT, Byrne BJ, Bryant RM, Margossian R, Maisenbacher M, Breitenger P, Benni PB, Redfearn S, Marcus E, Cade WT. Impaired cardiac reserve and severely diminished skeletal muscle O(2) utilization mediate exercise intolerance in Barth syndrome. American journal of physiology. Heart and circulatory physiology. 2011;301:H2122–H2129. doi: 10.1152/ajpheart.00479.2010. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Harada Y, Nishikawa S, Yamano K, Kamiya M, Shiota T, Kuroda T, Kuge O, Sesaki H, Imai K, Tomil K, Endo T. Tam41 is a CDP-diacylglycerol synthase required for cardiolipin biosynthesis in mitochondria. Cell metabolism. 2013;17:709–718. doi: 10.1016/j.cmet.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Harada Y, Yamano K, Watanabe K, Ishikawa D, Ohshima C, Nishikawa S, Yamamoto H, Endo T. Identification of Tam41 maintaining integrity of the TIM23 protein translocator complex in mitochondria. The Journal of cell biology. 2006;174:631–637. doi: 10.1083/jcb.200603087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuller G, Hrastnik C, Achleitner G, Schiefthaler U, Klein F, Duam G. YDL142c encodes cardiolipin synthase (Cls1p) and is non-essential for aerobic growth of Saccharomyces cerevisiae. FEBS letters. 1998;421:15–18. doi: 10.1016/s0014-5793(97)01525-1. [DOI] [PubMed] [Google Scholar]
- Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Archives of biochemistry and biophysics. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- van den Bosch H, Schutgens RB, Wanders RJ, Tager JM. Biochemistry of peroxisomes. Annual review of biochemistry. 1992;61:157–197. doi: 10.1146/annurev.bi.61.070192.001105. [DOI] [PubMed] [Google Scholar]
- van der Klei IJ, Veenhuis M. Yeast peroxisomes: function and biogenesis of a versatile cell organelle. Trends in microbiology. 1997;5:502–509. doi: 10.1016/S0966-842X(97)01156-6. [DOI] [PubMed] [Google Scholar]
- van der Leij FR, Huijkman NC, Boomsma C, Kuipers JR, Bartelds B. Genomics of the human carnitine acyltransferase genes. Molecular genetics and metabolism. 2000;71:139–153. doi: 10.1006/mgme.2000.3055. [DOI] [PubMed] [Google Scholar]
- van der Vusse GJ, van Bilsen M, Glatz JF. Cardiac fatty acid uptake and transport in health and disease. Cardiovascular research. 2000;45:279–293. doi: 10.1016/s0008-6363(99)00263-1. [DOI] [PubMed] [Google Scholar]
- van Roermund CW, Elgersma Y, Singh N, Wanders RJ, Tabak HF. The membrane of peroxisomes in Saccharomyces cerevisiae is impermeable to NAD(H) and acetyl-CoA under in vivo conditions. The EMBO journal. 1995;14:3480–3486. doi: 10.1002/j.1460-2075.1995.tb07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenhuis M, Harder W. Microbodies in yeasts: structure, function and biogenesis. Microbiological sciences. 1988;5:347–351. [PubMed] [Google Scholar]
- Walker JE. The mitochondrial transporter family. Current opinion in structural Biology. 1992;2(4):519–526. [Google Scholar]
- Wanders RJ, Jansen GA, Lloyd MD. Phytanic acid alpha-oxidation, new insights into an old problem: a review. Biochimica et biophysica acta. 2003;1631:119–135. doi: 10.1016/s1388-1981(03)00003-9. [DOI] [PubMed] [Google Scholar]
- Wanders RJ, Ruiter JP, L IJ, Waterham HR, Houten SM. The enzymology of mitochondrial fatty acid beta-oxidation and its application to follow-up analysis of positive neonatal screening results. Journal of inherited metabolic disease. 2010;33:479–494. doi: 10.1007/s10545-010-9104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SM, Carter LC, German JB. Docosahexaenoic acid accumulates in cardiolipin and enhances HT-29 cell oxidant production. Journal of lipid research. 1998;39:1583–1588. [PubMed] [Google Scholar]
- Wriessnegger T, Gubitz G, Leitner E, Ingolic E, Cregg J, de la Cruz BJ, Daum G. Lipid composition of peroxisomes from the yeast Pichia pastoris grown on different carbon sources. Biochimica et biophysica acta. 2007;1771:455–461. doi: 10.1016/j.bbalip.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Xu Y, Kelley RI, Blanck TJ, Schlame M. Remodeling of cardiolipin by phospholipid transacylation. The Journal of biological chemistry. 2003;278:51380–51385. doi: 10.1074/jbc.M307382200. [DOI] [PubMed] [Google Scholar]
- Ye H, Pilon M, Pilon-Smits EA. CpNifS-dependent iron-sulfur cluster biogenesis in chloroplasts. The New phytologist. 2006;171:285–292. doi: 10.1111/j.1469-8137.2006.01751.x. [DOI] [PubMed] [Google Scholar]
- Zhang M, Mileykovskaya E, Dowhan W. Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. The Journal of biological chemistry. 2005;280:29403–29408. doi: 10.1074/jbc.M504955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Mileykovskaya E, Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. The Journal of biological chemistry. 2002;277:43553–43556. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Gvozdenovic-Jeremic J, Webster P, Zhou J, Greenberg ML. Loss of function of KRE5 suppresses temperature sensitivity of mutants lacking mitochondrial anionic lipids. Molecular biology of the cell. 2005;16:665–675. doi: 10.1091/mbc.E04-09-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Li G, Gvozdenovic-Jeremic J, Greenberg ML. Up-regulation of the cell integrity pathway in saccharomyces cerevisiae suppresses temperature sensitivity of the pgs1Delta mutant. The Journal of biological chemistry. 2007;282:15946–15953. doi: 10.1074/jbc.M701055200. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhong Q, Li G, Greenberg ML. Loss of cardiolipin leads to longevity defects that are alleviated by alterations in stress response signaling. The Journal of biological chemistry. 2009;284:18106–18114. doi: 10.1074/jbc.M109.003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinser E, Sperka-Gottlieb CD, Fasch EV, Kohlwein SD, Paltauf F, Daum G. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. Journal of bacteriology. 1991;173:2026–2034. doi: 10.1128/jb.173.6.2026-2034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]