Abstract
Cognitive deficits occur in over half of multiple sclerosis patients, with hippocampal-dependent learning and memory commonly impaired. Data from in vivo MRI and post-mortem studies in MS indicate that the hippocampus is targeted. However the relationship between structural pathology and dysfunction of the hippocampus in MS remains unclear. Hippocampal neuropathology also occurs in experimental autoimmune encephalomyelitis (EAE), the most commonly used animal model of MS. While estrogen treatment of EAE has been shown to be anti-inflammatory and neuroprotective in the spinal cord, it is unknown if estrogen treatment may prevent hippocampal pathology and dysfunction. In the current study we examined excitatory synaptic transmission during EAE and focused on pathological changes in synaptic protein complexes known to orchestrate functional synaptic transmission in the hippocampus. We then determined if estriol, a candidate hormone treatment, was capable of preventing functional changes in synaptic transmission and corresponding hippocampal synaptic pathology. Electrophysiological studies revealed altered excitatory synaptic transmission and paired-pulse facilitation during EAE. Neuropathological experiments demonstrated that there were decreased levels of pre-and postsynaptic proteins in the hippocampus, diffuse loss of myelin staining and atrophy of the pyramidal layers of hippocampal cornu ammonis 1 (CA1). Estriol treatment prevented decreases in excitatory synaptic transmission and lessened the effect of EAE on paired-pulse facilitation. In addition, estriol treatment prevented several neuropathological alterations that occurred in the hippocampus during EAE. Cross-modality correlations revealed that deficits in excitatory synaptic transmission were significantly correlated with reductions in trans-synaptic protein binding partners known to modulate excitatory synaptic transmission. To our knowledge, this is the first report describing a functional correlate to hippocampal neuropathology in any MS model. Furthermore, a treatment was identified which prevented both deficits in synaptic function and hippocampal neuropathology.
Keywords: MS, EAE, hippocampus, synaptic transmission, estriol
More than 50% of MS patients experience cognitive deficits (1–2), and hippocampal-dependent learning and memory is often impaired (3–4). Hippocampal atrophy has been shown in MS patients using in vivo MRI (5–6), and this atrophy was correlated with impaired performance on visuospatial memory testing, a hippocampal-dependent function (7). Data from post-mortem studies in MS have demonstrated that hippocampal demyelination and neuropathology occur (8–9). This demyelination has been associated with changes in molecules modulating synaptic integrity, axonal transport and glutamate homeostasis (10). While evidence from both in vivo MRI and post-mortem pathological studies indicates that hippocampal pathology occurs during MS, the relationship between such pathology and dysfunction remains unclear. Moreover, there are currently no treatments available to prevent hippocampal dysfunction during MS.
Demyelination, axonal loss and inflammation have been demonstrated within the central nervous system of mice with experimental autoimmune encephalomyelitis, (EAE). While most EAE studies have focused on spinal cord, only recently have studies investigated neurodegeneration within the brain (11–14). The functional correlates of these described pathologies in brain have not been elucidated. Specifically, little is known about the pathology that occurs in the hippocampus during EAE and an understanding of how it may lead to dysfunction is lacking.
In MS, estriol, an estrogen elevated during pregnancy, is a therapeutic candidate because it has widespread effects on the immune system and the central nervous system (15). Studies have shown that MS patients have significantly decreased relapse rates during the third trimester of pregnancy, when estriol levels are most elevated, and that relapse rates rebound during the postpartum period coinciding with an abrupt decline in serum estriol levels (16). In non-pregnant MS patients, estriol treatment significantly reduced gadolinium-enhancing lesion number and volumes measured by MRI (17). The study further showed that estriol treatment significantly improved cognitive function as measured by the paced auditory serial addition task (PASAT) in relapsing-remitting MS patients, however this could have been due, at least in part, to a practice effect.
Estrogen treatment can have both anti-inflammatory (18–19) and neuroprotective effects during EAE (20–21). Estrogen treatment is clinically beneficial in several neurodegenerative disease models including those for Alzheimer’s and Parkinson’s diseases, (22), and in other conditions such as traumatic brain injury and stroke (23–24). In vitro and in vivo studies have used estrogen to protect neurons against degeneration due to excitotoxicity, oxidative stress, and apoptosis (25–27). Finally, estrogen treatment may affect hippocampal learning and memory, as previous studies have shown that estrogen treatment in rodents led to up regulated synaptic protein synthesis (28), increased activation of neuronal signaling cascades (29), and increased dendritic arborizations (30). Evidence demonstrates that estrogen treatment enhanced long-term potentiation, altered neuronal excitability and increased synaptic transmission in the hippocampus (31–33).
In this study, we determine whether hippocampal synaptic protein complexes known to orchestrate functional synaptic transmission and the excitatory synaptic transmission itself, are affected during EAE. Furthermore, we determine if estriol, a candidate hormone treatment, is capable of preventing hippocampal synaptic pathology and corresponding functional changes in synaptic transmission.
MATERIALS AND METHODS
Animals & Treatment
Gonadally intact female C57Bl/6 mice, age 12-16 weeks, bred in-house from animals purchased from Jackson Laboratories. All animals were maintained in accordance with guidelines of UCLA's Chancellor’s Animal Research Committee (ARC), and the PHS Policy on Humane Care and Use of Laboratory Animals. One week prior to disease induction, 90-day release pellets of estriol (ESTRIOL) at 5 mg dose, as well as placebo (PLAC) pellets that contain carrier binder alone (Innovative Research of America, Sarasota, FL) were implanted into the scapular region subcutaneously.
EAE Induction and clinical disease scoring
One week after pellet implantation, active EAE was induced by immunizing with 200ug of Myelin Oligodendrocyte Glycoprotein (MOG) peptide, amino acids 35-55 (Chiron Mimotopes, San Diego, CA), and 300ug of Mycobacterium tuberculosis in complete Freund’s Adjuvant, over two sites: the right draining inguinal and axillary lymph nodes. Seven days later, a booster immunization was delivered subcutaneously, over the contralateral draining lymph nodes, as described (34). For MOG immunizations mice were temporarily anesthetized with isofluorane. In addition, EAE mice received intraperitoneal injections of Bordatella pertussis toxin (500ng/mouse; Ptx) on days 0 and 2. Mice were monitored daily, and clinical disease severity was measured using the standard EAE grading scale (35). Clinical scores were averaged across all animals per day, yielding a mean clinical disease index per group.
Slice electrophysiology
Mice were randomly selected for electrophysiological recording, daily, corresponding to EAE day 21 through 45. Hippocampi were obtained from mice that were deeply anesthetized with halothane and then killed by cervical dislocation. The brain was removed and placed in cold (4 °C), oxygenated (95% O2/5% CO2) artificial CSF (ACSF) containing: 124mM NaCl, 25mM Na2HCO3, 4.4mM KCl, 1mM NaH2PO4, 1.2mM MgSO4, 2mM CaCl2, and 10mM glucose. The left hemisphere was collected for subsequent immunohistochemical staining (see below) and the hippocampus from the right hemisphere of each animal was then dissected free from the rest of the brain. The right hippocampus was then cut into 400 µm thick slices perpendicular to its long axis and 3 – 4 slices from the extreme dorsal and ventral ends were discarded. The remaining slices were maintained in interface-type recording chambers perfused at a constant rate (2–3 ml/min) with a warmed (30°C), oxygenated aCSF and allowed to recover for at least 2 hours prior to each experiment. Low resistance (5–10 MΩ) glass microelectrodes filled with aCSF were placed into stratum radiatum of the hippocampal CA1 region to record field excitatory postsynaptic potentials (fEPSPs). Presynaptic stimulation pulses were delivered once every 50 sec to the Schaffer collateral/commissural fibers via a bipolar nichrome wire stimulating electrode. Basal synaptic transmission was examined by generating input/output curves where fiber volley amplitude (input) and fEPSPs slopes (output) were compared across a range of presynaptic fiber stimulation intensities (36–38). To assess short-term plasticity, hippocampal slices were stimulated to trigger half-maximal (50%) fEPSPs. Paired-pulse facilitation (PPF) was measured by delivering pairs of presynaptic fiber stimulation pulses with varying inter-pulse intervals (25ms, 50ms, 100ms, 200ms and 275ms) and measured as the ratio of the slope of the fEPSP evoked by the second stimulation pulse relative to that produced by the first stimulation pulse. After a 20-minute baseline recording, long-term potentiation (LTP) was elicited with high-frequency stimulation, where two trains of 100 Hz, each with duration of 1 second, were delivered with an inter-train interval of 10 seconds. LTP was monitored and recorded for 60 minutes post HFS. Data acquisition and analysis were performed using the Experimenter's Workbench software package from DataWave Technologies (Loveland, CO).
Immunohistochemistry
Left hemispheres were collected and fixed overnight in 4% paraformaldehyde, for each animal, then transferred to a 30% sucrose/saline solution. Once tissue from all animals in experiment had been collected all samples were gelatin-embedded, frozen, and sliced thin using a cryostat at -20°C, as previously described (34). To determine hippocampal CA1 volume, tissue sections were uniform randomly collected and nissl stained using standard protocols. Specifically, every fifth sagittal 20um-thick tissue section was collected, to sample approximately 640 microns of the left hemisphere, (from Bregma lateral coordinates 0.12mm to 1.08mm, from Paxinos & Franklin Mouse Brain Atlas, [39]). For all other immunostaining conditions, three sagittal sections were used from each mouse in each condition. Tissue was first permeabilized in either 2% normal goat or normal rabbit serum (NGS or NRS) in 0.3% Triton X-100 in phosphate buffer solution, for 30 min at room temperature. Sections were then blocked in 10-15% NGS/NRS for 2 hours at room temperature. To detect microglia, myelin basic protein, and synaptic proteins, the following primary antibodies were used: polyclonal anti-Iba1 1:1000 (Wako Chemicals, Richmond, VA); monoclonal myelin basic protein (MBP; 1:500; polyclonal anti-Synapsin-1 (Syn-1; 1:500); monoclonal postsynaptic density protein 95 (PSD-95; 1:500; Chemicon, Temecula, CA); polyclonal neurexin IIβ (NRXIIβ; 1:500); and polyclonal neuroligin 1 (NLG1; 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA). Fluorescent-conjugated secondary antibodies (goat anti-mouse Cy5 [1:750; Chemicon]; goat anti-rabbit Cy3 IgG [1:1000; Vector Laboratories, Burlingame, CA] and rabbit anti-goat Cy5 [1:750; Vector Labs] in 2% NGS/NRS in TBS solution) were used to visualize staining. Positive and negative controls were performed for each staining, to ensure antibody specificity. 4’, 6-Diamidine-2’-pheynylindole dihydrochloride, (DAPI; 1:1000; Invitrogen, Eugene, OR) staining was used in all fluorescence staining conditions to identify nuclear DNA in all cell types.
Microscopy and Stereological analysis
CA1 volume was estimated using the rigorous Cavalieri method (40–41). Iba1+ cells morphologically representative of reactive microglia in CA1 were counted using unbiased stereology methods and presented as number of cells by area (42). By carefully delineating CA1 regions by anatomical landmarks, the area of myelin and PSD-95 staining within the CA1 were measured as percent area of immunoreactivity, using ImageJ (NIH http://rsb.info/nih/gov/ij). To quantify Synapsin-1, neurexin IIβ and neuroligin-1 immunoreactive puncta in CA1, puncta size threshold range was determined by measuring puncta size range in several tissues from each staining condition in many animals, then averaging both upper and lower threshold limits. For Syn-1 this yielded a puncta size range from 0.816um2 to 3.264um2. NRXIIβ puncta size ranged from 0.1015um2 to 0.5075um2, and NLG1 puncta size ranged from 0.0508um2 to 0.5075um2. These ranges were then used to filter for puncta particle size through out the experiment for all three of these synaptic stains. The number of puncta in each optical image was averaged across z-stack, brain section, and animal, to attain a composite average of synaptic puncta within the sampled hippocampal area in each condition.
Statistical analysis
All quantitative measures are presented as mean ± S.E.M. and analyzed by Student’s t-test or one-way ANOVA. Post-hoc analyses were conducted only if ANOVA yielded p < 0.05, with Bonferroni analysis. Pearson’s linear regression analysis was used to determine correlations between electrophysiology and pathology using GraphPad Software Version 4.0, (San Diego CA). Cross-modality correlations have been made in other previously published work (43–45).
RESULTS
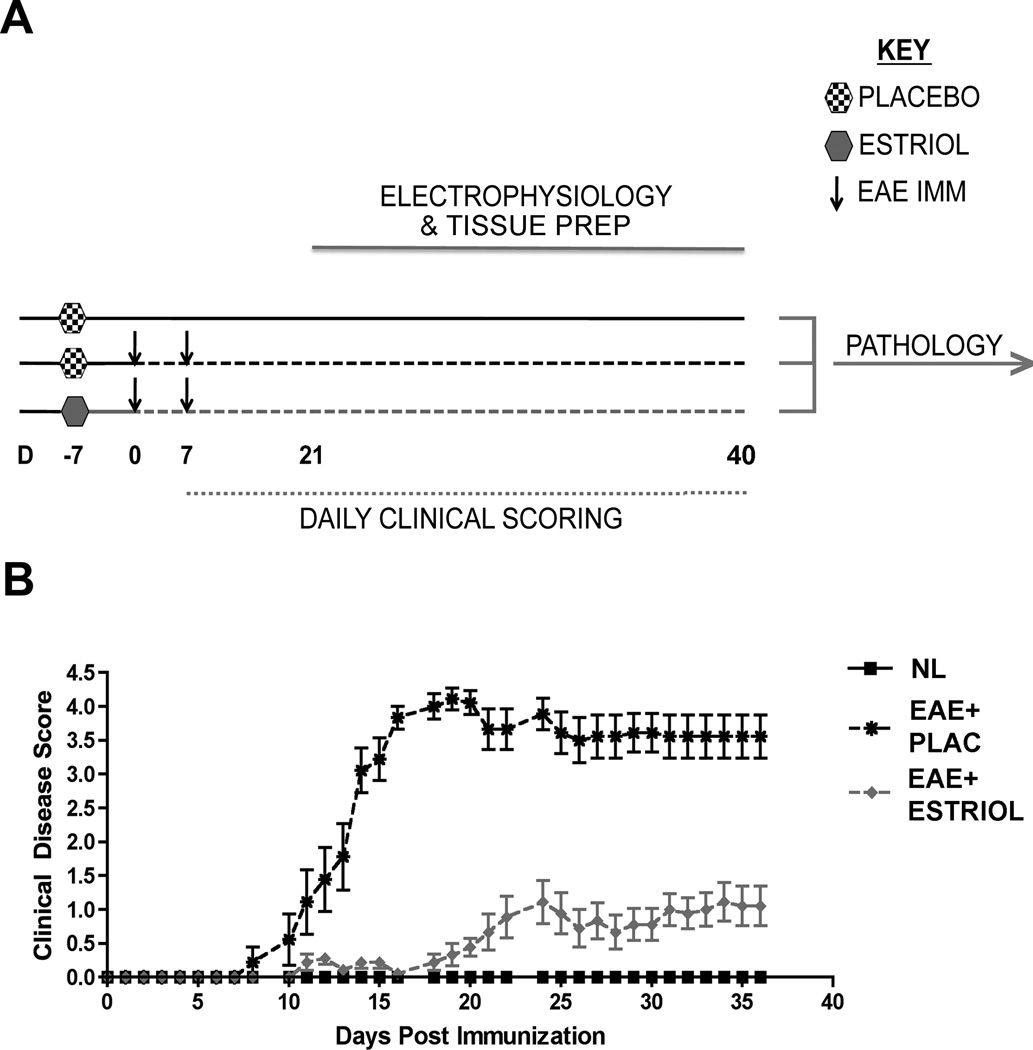
Active EAE was induced using MOG 35-55 peptide and standard EAE clinical scores were attained, primarily reflecting the level of motor disability. There were three groups: placebo-treated healthy control females, placebo-treated females with EAE, and estriol-treated females with EAE (Fig 1A). Estriol-treated females with EAE had significantly decreased clinical disability scores compared to placebo-treated females with EAE, while healthy control females did not display clinical disease (Fig 1B). These findings were consistent with previous work from our lab and others (18–19, 46), demonstrating that estriol treatment ameliorates motor disability in EAE.
Figure 1. Experimental design and standard clinical scores in EAE.
(A) Experimental design depicting the timing of placebo or estriol pellet implantation (D -7), EAE induction (D 0 & D 7), daily clinical scoring (D 7-40) and electrophysiology (D 21-40). Pertussis injections (intraperitoneal, i.p.) were given to both EAE groups on D0 and D2, as part of standard EAE induction, not shown. All mice were age-matched C57Bl/6 adult females, and were uniform randomly selected (one per day) for electrophysiological recording. On each day of electrophysiology, the right hemisphere was prepared for electrophysiology, while the left hemisphere of each mouse was prepared for pathology studies and kept until all tissue from all conditions had been collected. (B) EAE clinical scores were recorded in placebo-treated (EAE + PLAC, black star, dashed black line) or estriol-treated (EAE + ESTRIOL, gray triangle, dashed gray line) mice as well as in non-EAE induced healthy controls (NL, black square, solid black line). Placebo-treated mice with EAE exhibited a severe clinical disease course, while estriol-treated mice exhibited significantly reduced clinical severity. Data are representative of two separate experiments, and experiment has been repeated more four times. Repeated measures ANOVA with post-hoc pair-wise comparisons, revealed that placebo-treated EAE mice were significantly different from other two groups beginning at D12 of disease, * p < 0.05, n = 5 per group per experiment.
Synaptic transmission and paired-pulse facilitation are altered in the hippocampus during EAE
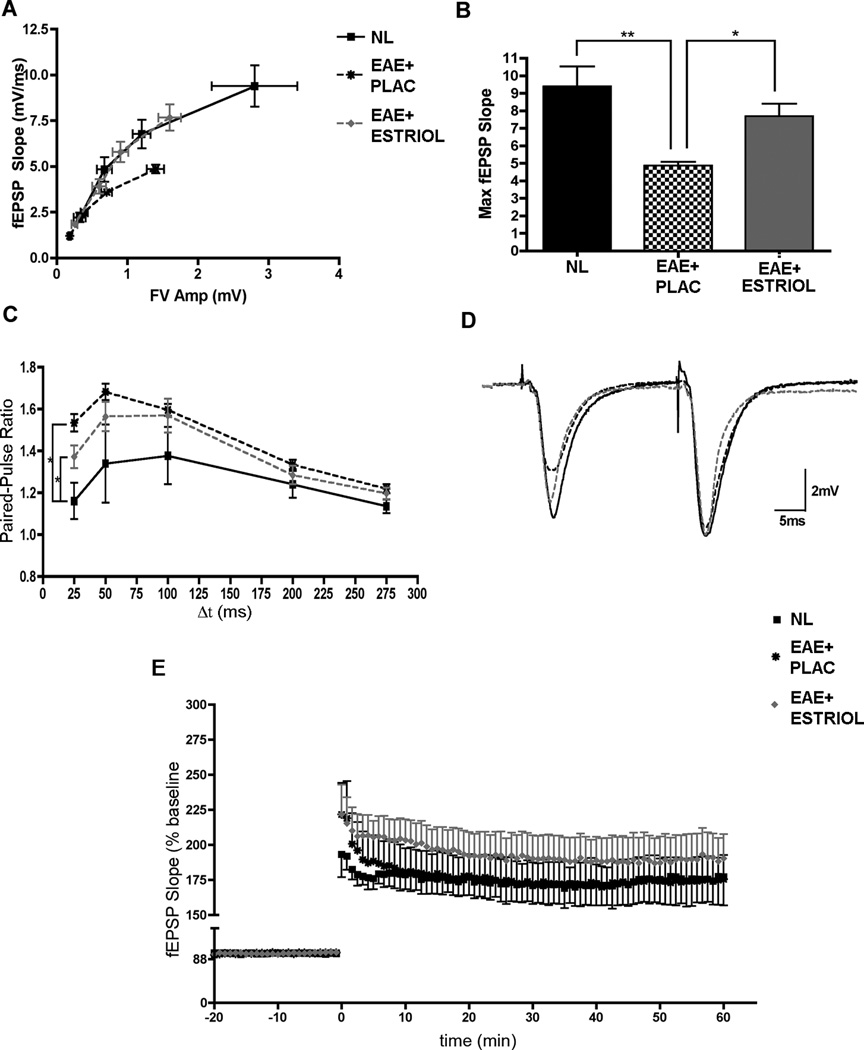
Excitatory synaptic transmission in the hippocampus during EAE has not been previously investigated. Synaptic transmission is dependent on functional and appropriately located synaptic protein families. Thus, basal synaptic transmission and paired-pulse facilitation were assessed using in vitro hippocampal slices. To determine if EAE caused an effect in excitatory synaptic transmission, input/output (IO) relationships were compared in hippocampal slices from the right hemispheres of mice in each experimental group. The amplitude of presynaptic fiber volleys and the slope of the EPSPs evoked by different intensities of Schaffer Collateral fiber stimulation were analyzed. Here, hippocampal slices from placebo-treated EAE mice had a significantly altered IO curve compared to those from healthy controls (Fig 2A). Hippocampal slices from EAE mice had FV Amps and fEPSP slopes that were both decreased by nearly 50%, suggesting that basal synaptic transmission was significantly impaired during EAE. When treated with estriol, however, hippocampal slices from mice with EAE displayed input/output relationships more similar to healthy controls, (Fig 2A-B). When maximal fEPSP slopes were compared across the three groups, there was a significant deficit in postsynaptic responses in slices from EAE mice compared to healthy controls, and this decrease in the postsynaptic response was prevented with estriol treatment (Fig 2B). Next, to examine short-term plasticity we measured paired-pulse facilitation (PPF) at Schaffer collateral fiber inputs onto CA1 pyramidal cells. Paired-pulse facilitation is inversely correlated with the probability of neurotransmitter release from presynaptic terminals, such that an increase in PPF is indicative of a decrease in transmitter release probability, whereas a decrease in PPF is indicative of an increase in transmitter release probability. Compared to healthy controls, placebo-treated EAE mice displayed significantly increased PPF at the shortest paired-pulse interval tested (Fig 2C-D). Interestingly, although PPF in slices from estriol-treated EAE mice was also significantly elevated compared to healthy controls, the increase tended to be lower than placebo-treated EAE mice at the same time interval (Fig 2C-D). Since hippocampal slices from placebo-treated EAE mice had significantly increased paired-pulse ratios, our results suggest that EAE caused a decrease in the probability of presynaptic neurotransmitter release. With estriol treatment during EAE there was a trend of partially restored release probability. To examine if EAE impaired synaptic plasticity, long-term potentiation (LTP) was induced with high frequency stimulation (HFS, 2x100Hz) in hippocampal slices from mice in each condition. Two separate components of LTP were analyzed: post-tetanic potentiation (PTP; the immediate potentiated response of each slice after HFS stimulation), and early phase LTP (eLTP; the average of the last five minutes of LTP in each slice). When compared across groups, PTP was not significantly affected by disease, or by estriol treatment during disease. eLTP was also unaffected by EAE or estriol treatment during EAE (Fig 2E).
Figure 2. Excitatory synaptic transmission and paired-pulse facilitation are altered during EAE.
(A) Input/output (IO) curves were created by comparing fiber volley amplitude (FV Amp; mV) to field excitatory postsynaptic potential (fEPSP; mV/ms) during four different stimulation intensities of presynaptic fiber stimulation that caused fEPSP responses of 25, 50, 75 and 100% maximal fEPSP amplitude. One-way ANOVA analyses demonstrated that placebo-treated EAE (EAE+PLAC; black star, dashed black line) fEPSP responses were significantly reduced at each point along the IO curve (percentage of maximal fEPSP slope: 25, 50 75 and 100%) in comparison to healthy controls (NL; black squares, solid black line), p < 0.05. With estriol treatment during EAE, (EAE+ESTRIOL; gray triangle, dashed gray line), the IO curve followed a similar trajectory to healthy controls. (B) A histogram demonstrating decreased maximal fEPSP responses in placebo-treated EAE compared to healthy controls, and maximal fEPSPs in estriol-treated EAE mice that were not significantly different from controls. One-way ANOVA revealed a significant effect of disease on maximal fEPSP slope (p = 0.0029) and follow up Bonferroni tests indicated that placebo-treated EAE mice (EAE+PLAC; n = 15 slices from 5 mice) were significantly different from healthy controls (NL, n = 7 slices from 3 mice; ** p < 0.01) and also significantly different from estriol-treated EAE (EAE+ESTRIOL, n = 16 slices from 5 mice; * p < 0.05). (C) Paired-pulse facilitation was increased in the hippocampal CA1 region of placebo-treated EAE mice. Paired presynaptic fiber stimulation pulses were delivered with varying inter-pulse intervals (25, 50, 100, 200 and 275 ms) to elicit postsynaptic responses with amplitudes that were then compared. At an inter-pulse interval of 25ms, one-way ANOVA demonstrated that condition had a significant effect on paired-pulse facilitation, (p = 0.0178). Bonferroni post-hoc analysis showed that placebo-treated mice (EAE+PLAC; black star, dashed black line, n = 12 slices from 5 mice) had significantly greater paired-pulse facilitation (* p < 0.05) compared to healthy controls (NL; black square, solid black line, n = 6 slices from 3 mice). Estriol-treated mice (EAE+ESTRIOL; gray triangle, dashed gray line, n = 11 slices from 5 mice) also had significantly increased PPF compared to healthy controls. (D) Sample waveforms depict representative paired-pulse responses recorded from animals in each experimental condition at a 25ms inter-pulse interval (NL: black solid line; EAE+PLAC: dashed black line; and EAE+ESTRIOL: gray dashed line). (E) Neither the post-tetanic potentiation (PTP; the first response after high-frequency stimulation), nor the early phase of LTP were significantly affected by EAE, as analyzed by one-way ANOVAs. Furthermore, one-way ANOVA’s revealed that estriol treatment during EAE did not have an effect on PTP or eLTP.
Pre- and postsynaptic proteins are differentially affected in the hippocampus during EAE and estriol treatment of EAE
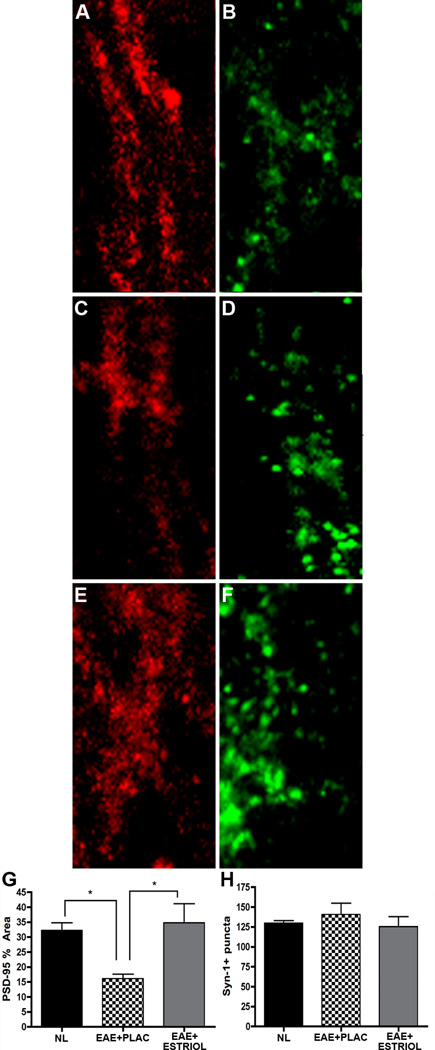
Synaptic health and transmission are critical to hippocampal functions including learning and memory. Because excitatory synaptic transmission and paired-pulse facilitation were both affected during EAE, we next assessed whether synaptic proteins were also affected in the hippocampus during EAE. Presynaptic (synapsin-1, Syn-1; and neurexin IIβ, NRXIIβ) and postsynaptic (postsynaptic density 95, PSD-95; and neuroligin 1, NLG1) protein immunoreactivity was measured in the hippocampus of normal, placebo-treated EAE and estriol-treated EAE mice. The percent area of PSD-95 staining was significantly decreased in EAE mice compared to healthy controls (Fig 3A & C vs. D & F, graph J). Estriol treatment during EAE reversed this effect on PSD-95 (Fig 3G, I & J). In contrast, the number of Syn-1 puncta was not significantly reduced during EAE (Fig 3B, E, H & K).
Figure 3. Hippocampal PSD-95 levels are decreased in EAE and preserved with estriol treatment.
(A-F) Fluorescent images depict the stratum radiatum with the CA1 region of representative hippocampal slices from healthy (panel A-B), placebo-treated EAE (panel C-D) and estriol-treated EAE (panel E-F) mice, where PSD-95 (A, C & E; Cy5-red), and Synapsin-1 (B, D & F; Syn-1; Cy3-green) staining are shown at 60x magnification. During EAE, PSD-95 % area was significantly decreased in the CA1 region of the hippocampus, (C), compared to healthy controls (A). This decrease was prevented with estriol treatment during EAE, (E), quantified in graph G. One-way ANOVA and Bonferroni post-hoc analysis revealed statistical significance in PSD-95 % area where, * indicates p = 0.0324, n = 5 per group. Average number of presynaptic Syn-1+ puncta within the CA1 was not significantly different across conditions. These experiments were repeated twice with similar results. Scale bar 10um.
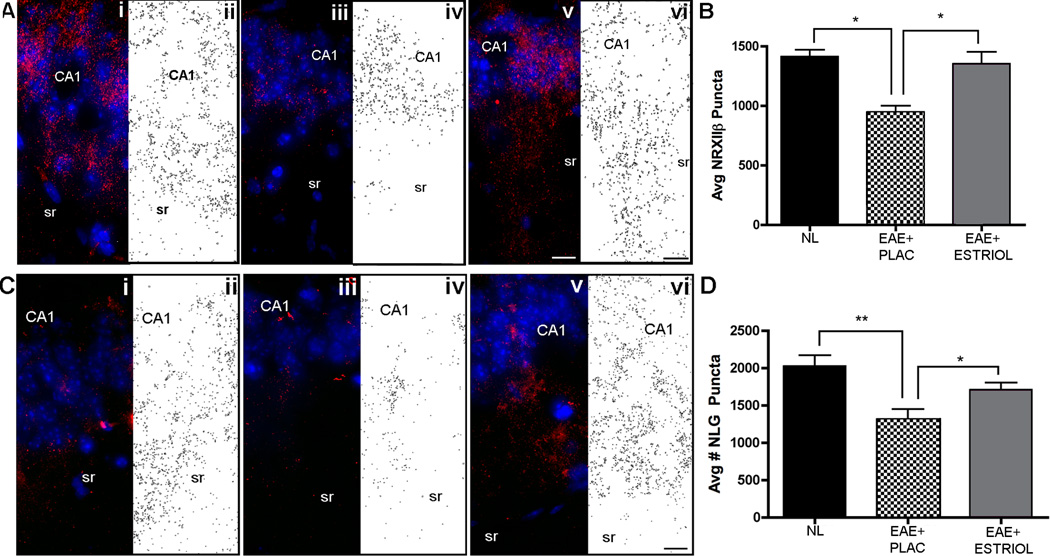
Recent evidence has demonstrated that PSD-95 can bind to neuroligin 1 to modulate presynaptic release probability in a retrograde trans-synaptic fashion by binding to β-neurexin II (47). Since we found that PSD-95 levels were significantly decreased in the hippocampus during EAE, we next investigated whether EAE affected levels of presynaptic β-neurexin II (NRXII) and postsynaptic neuroligin 1 (NLG1). We found that immunoreactive NRXIIβ puncta were significantly decreased in hippocampi from placebo-treated EAE compared to healthy controls (Fig 4A-B). Estriol treatment of EAE mice prevented this decrease in NRXIIβ and resulted in a similar amount of immunoreactive puncta within hippocampus as that found in healthy control mice. Immunoreactive NLG1 puncta were also significantly decreased in placebo-treated EAE compared to healthy controls, and estriol treatment of EAE also prevented this decrease in NLG 1 (Fig 4C-D).
Figure 4. Presynaptic β-neurexin II and postsynaptic neuroligin-1 are decreased during EAE, but preserved with estriol treatment.
Synaptic puncta were measured in the hippocampal CA1 region by counting immunoreactive NRXIIβ puncta (A-B) and NLG1 puncta (C-D) in healthy control, (panels i-ii), placebo-treated EAE, (panels iii-iv), and estriol-treated EAE mice (panels v-vi), respectively. Pseudo-colored confocal images depict synaptic staining (either NRXIIβ or NLG1, Cy3-red) in hippocampus at 60x magnification, while black and white images show outlined puncta from respective confocal images. One-way ANOVA revealed that groups differed significantly in quantity of NRXIIβ puncta, (p = 0.005) and Bonferroni post-hoc test indicated that placebo-treated EAE mice (EAE+PLAC, n = 5 mice) had significantly decreased (* p < 0.01) numbers of NRXIIβ puncta compared to healthy control (NL, n = 5 mice) and estriol-treated EAE mice (EAE+ESTRIOL, n = 4 mice), graph B. Simultaneously, NLG1 puncta quantities were also significantly different across conditions (one-way ANOVA, p = 0.0056) with follow up tests indicating significant difference between healthy controls (NL, n = 5 mice) and placebo-treated EAE mice (EAE+PLAC, ** p < 0.01, n = 5 mice), as well as significant difference between placebo-treated EAE and estriol-treated EAE mice (EAE+ESTRIOL, * p < 0.05, n = 5 mice), graph D. These experiments were repeated twice and similar results were found. Scale bars 10um.
Demyelination and microglial activation occur in the hippocampus during EAE
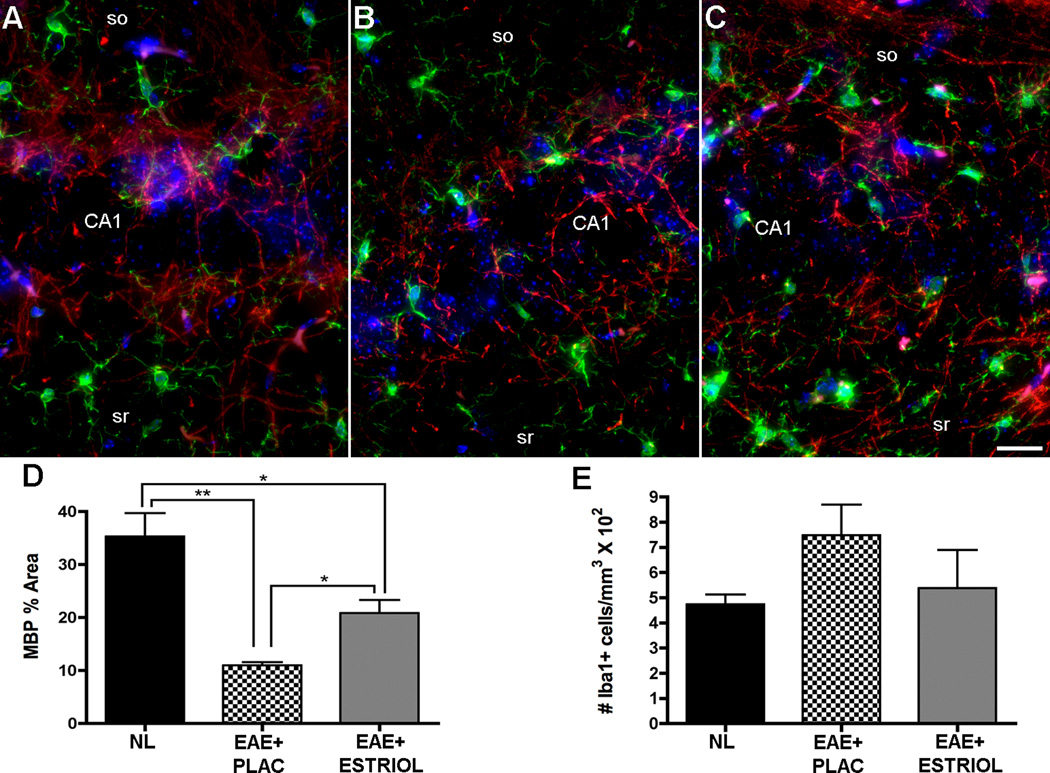
Demyelination is a classic neuropathological finding in spinal cords of mice with EAE and often leads to deficits in sensory and motor function. Demyelination has been observed in the hippocampus of MS patients (10) generally as focal demyelinating lesions. We have demonstrated in a previous report that there is a diffuse reduction in myelin staining in the hippocampus during EAE (34). Thus, myelin staining was assessed in this study. Myelin basic protein (MBP) fluorescence intensity measurements demonstrated a significant reduction in myelin immunoreactivity within the delineated CA1 region of placebo-treated EAE mice, compared to healthy controls (Fig 5A-B & D). Estriol treatment during EAE significantly preserved myelin staining in the CA1 region (Fig 5C & D) compared to placebo-treated EAE mice, but myelin staining levels were not similar to normal controls.
Figure 5. Decreased myelin staining and increased microglial activation occur within the hippocampus during EAE, and estriol treatment preserves myelin staining.
(A-C) Pseudo-colored confocal fluorescent images depict myelin basic protein immunoreactivity (MBP, Cy5-red) and cells of microglial/macrophage lineage stained with Iba1 (Cy3-green) and Dapi (blue) in the CA1 region within healthy (A), placebo-treated EAE (B) and estriol-treated EAE (C) mice at 40x magnification. (D) One-way ANOVA revealed significant differences in myelin staining across conditions, p = 0.003, n = 5 mice per group, and Bonferroni post-hoc tests indicated that EAE caused a significant reduction in myelin staining within the CA1 region of the hippocampus in placebo-treated mice (EAE+PLAC, ** p < 0.01, n = 5 mice) compared to myelin staining in hippocampus of healthy control mice (NL, n = 5 mice). Estriol-treated EAE mice (EAE+ESTRIOL, n = 5 mice) had myelin levels significantly increased compared to placebo-treated EAE mice (* p < 0.05), but were still significantly different from myelin staining levels in healthy controls. Analysis was conducted on 10x magnification images where MBP % area represents total MBP immunoreactivity as a percent of total delineated CA1 area imaged. Iba1+ cells with morphology characteristic of reactive microglia were relatively low in healthy controls (A), and increased in placebo-treated EAE mice (B). The number of Iba1+ cells was not significantly different in estriol-treated EAE mice (C), however. The number of Iba1+ cells per mm3 were counted and presented as whole numbers (graph E). Data are representative of two experiments. Scale bar 10um.
Reactive microglia with infiltration of T lymphocytes and macrophages characterize the inflammation that occurs in spinal cords of EAE mice. Within the hippocampus, however, we have previously demonstrated that immune cell infiltrates are minimal, with inflammation limited to resident reactive microglia (34). Reactive microglia and the inflammatory molecules they produce have also been shown to detrimentally affect synaptic transmission (14). Iba1 is a protein highly expressed by resident microglia in the hippocampus (48). Iba1 is also expressed by macrophages, and the two cell types may be distinguished by morphology, as we have previously described, (49). Thus, Iba1 staining was used to label, count and morphologically characterize cells of microglial/macrophage lineage. Hippocampal tissue sections from placebo-treated EAE mice demonstrated a trend for elevated numbers of highly branched, ramified microglia compared to healthy controls, which had small, finely branched quiescent microglia (Fig 5A-B & F). Estriol treatment in EAE mice tended to prevent this increase in ramified microglia (Fig 5C & D). Within the hippocampus, we did not find large, globoid Iba1 positive cells, suggesting that inflammation in the hippocampus consists primarily of reactive microglia and not immune cell infiltrates.
CA1 Pyramidal layer volume is decreased during disease and preserved with estriol treatment
The hippocampal CA1 region undergoes atrophy in humans with MS (7). As such, we next determined if atrophy was occurring in the hippocampal CA1 during, where altered synaptic transmission had also been found. CA1 volume was rigorously assessed using the Cavalieri method of volume estimation for histopathological data. EAE caused a significant decrease in CA1 pyramidal layer (CA1 pyr) volume compared to healthy controls (Fig 6A-B & D). Importantly, estriol treatment during EAE prevented CA1 pyramidal layer volume loss and preserved CA1 layer volume to levels similar to healthy controls (Fig 6C-D).
Figure 6. Hippocampal CA1 atrophy occurs during EAE and estriol treatment prevents this.
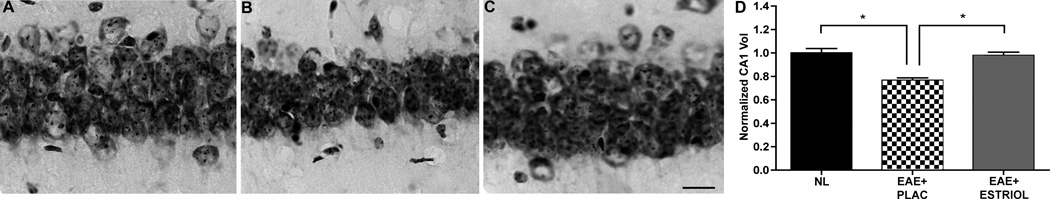
(A-C) Nissl-stained hippocampal sections representative of normal (NL; A), placebo-treated EAE (EAE+PLAC; B) and estriol-treated EAE (EAE+ESTRIOL; C) mice depict the CA1 pyramidal layer at 40x magnification. Compared to healthy controls, placebo-treated EAE mice had significantly reduced CA1 pyramidal layer volume, quantified in D. With estriol treatment during EAE, the CA1 pyramidal volume was preserved to levels similar to those in controls. One-way ANOVA and Bonferroni post-hoc analysis revealed significant difference between three groups, (*p = 0.05, n = 5 mice per group), scale bar 10um. This finding has been confirmed by repeated experiments.
Neuropathological outcome measures are significantly correlated with changes in electrophysiological outcome measures
Because we found that excitatory synaptic transmission was altered in hippocampal slices from placebo-treated EAE mice and also found neuropathology in tissue from those same animals, we next used cross-modality correlations to determine if functional and pathological outcomes were related during disease state. Specifically, we investigated if changes in PSD-95 and NRXII staining levels correlated with changes in excitatory synaptic transmission. Interestingly, PSD-95 % area in disease states was significantly correlated to fEPSP responses during disease states (Fig 7A). Further, the number of immunoreactive NRXII puncta was also significantly correlated to fEPSP (Fig 7B) during disease states. Thus, these cross-modality correlations together demonstrate that excitatory synaptic transmission during EAE coincides with levels of PSD-95 and NRXIIβ in the hippocampus during EAE.
Figure 7. Decreased pre- and postsynaptic protein levels are significantly correlated with electrophysiological changes during EAE.
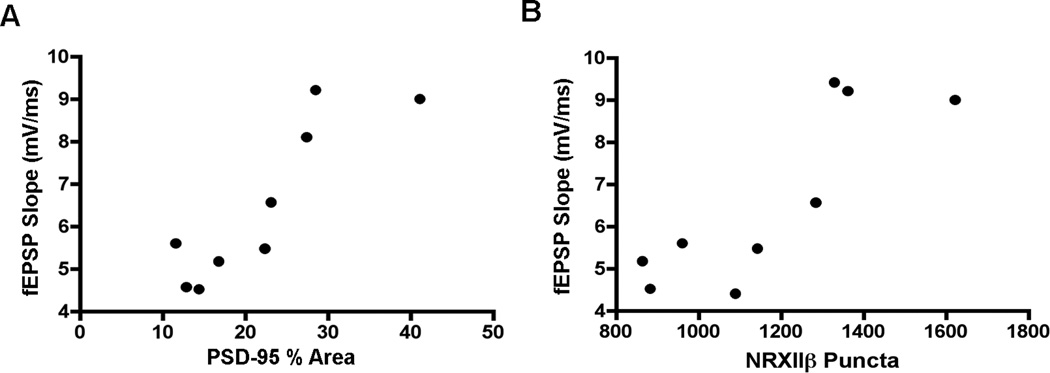
Cross-modality correlations were conducted to determine potential neuropathological substrates underlying changes in excitatory synaptic transmission and paired-pulse facilitation during EAE. (A) PSD-95% area was significantly correlated to maximal fEPSP slope throughout experiment, where the correlation coefficient rho = 0.8767, p = 0.019, n = 9 mice total. (B) The average number of presynaptic NRXIIβ puncta was also significantly correlated to postsynaptic responses (fEPSP) where rho = 0.8396, p = 0.0046, n = 9 mice total.
DISCUSSION
The results of the present study show, for the first time, that excitatory synaptic transmission and paired pulse facilitation are significantly impaired in the hippocampus during EAE and that estriol treatment is capable of preventing this. Furthermore, we demonstrate that these functional deficits occur within tissue that has neuropathological changes in expression of synaptic proteins known to play an important role in excitatory transmission in the hippocampus (PSD-95, neurexin IIβ and neuroligin I). Interestingly, estriol treatment prevented these changes in synaptic protein expression.
Our data show a reduction of both pre- and postsynaptic electrophysiological responses in hippocampal slices of mice with EAE. Presynaptic facilitation is inversely related to synaptic vesicle release probability at presynaptic terminals (50–51). Since we found elevated paired-pulse facilitation during disease, this suggested that EAE decreased the probability of presynaptic vesicle release. Presynaptic terminal structure and protein composition can directly mediate changes in the probability of synaptic vesicle release (52–53). Thus, it is plausible that EAE leads to detrimental changes in presynaptic terminal structure and protein composition. Postsynaptic excitatory responses were also reduced in this study, thus EAE may cause simultaneous alterations to pre- and postsynaptic terminals that lead to impaired synaptic transmission.
EAE induced a reduction in protein levels of trans-synaptic binding partners. PSD-95, NRXIIβ and NLGI are distinct, interacting scaffolding proteins known to have important roles in excitatory synaptic transmission (54). PSD-95 levels were significantly decreased in the hippocampus of mice with EAE. PSD-95 is abundantly expressed in healthy excitatory synapses and has been shown to recruit diverse proteins to sites of synaptic adhesion (55), and promote trans-synaptic signaling (56). PSD-95 also has extensive interactions with neuroligin 1 (NLG1; a postsynaptic cell adhesion protein) at excitatory synapses (57) and with beta-neurexin II (NRXIIβ; a presynaptic cell adhesion protein) to mediate presynaptic terminal assembly (58). Indeed, neurexin-neuroligin trans-synaptic binding is necessary for proper synapse formation, stability, maturation and function (59). Both NRXIIβ and NLG1 protein levels were reduced in the hippocampus during EAE, and moreover, cross-modality correlations demonstrated that the reductions in these protein levels were correlated with reductions in basal synaptic transmission and paired-pulse facilitation. PSD-95 and neuroligin can directly modulate the release probability of neurotransmitter vesicles in a retrograde fashion via direct binding with neurexin (47). Thus, the changes in these synaptic protein levels during EAE may affect probability of vesicle release, and result in impairment of hippocampal function.
Estrogens are known to have extensive effects on the hippocampus in healthy and disease states, making them likely candidates for neuroprotective treatments. Estrogens promote synaptogenesis (60) and can enhance synaptic transmission in healthy states (61) by facilitating presynaptic and postsynaptic plasticity (62–63). In addition, estrogens can significantly increase expression of synaptic proteins required for functional synaptic transmission, including PSD-95 (64), and neuroligin (65). Besides providing beneficial support to synaptic integrity and function in healthy conditions, estrogens have also been shown to induce other neuroprotective mechanisms in other diseases (66). In animal models of Alzheimer’s disease (AD), estrogen treatment reduced beta-amyloid protein accumulation (67), promoted neuron viability (68) and enhanced NMDA-mediated synaptic transmission and long-term plasticity (63). In EAE, estrogen treatment has been shown to reduce clinical disease severity (18–19) through both neuroprotective and anti-inflammatory actions (20–21, 42). Since EAE principally affects ambulation, the majority of these studies have been limited to spinal cord pathology. One recent publication has examined estrogen-mediated neuroprotection in the corpus callosum (12) and found that estrogen treatment prevented callosal axon loss and preserved axonal conductance across the corpus callosum. Our study is the first to report that estriol, a pregnancy estrogen, is capable of preventing EAE-mediated impairment hippocampal function and neuropathology. Important evidence from human clinical trials in MS suggest that estriol treatment may be beneficial in clinical application (15, 17, 69) and further investigation in clinical trials is underway (http://clinicaltrials.gov/ct2/show/NCT00451204). This report suggests that estriol treatment should be further investigated for its potential role in preventing hippocampal dysfunction and neuropathology in MS.
It remains to be determined where and how estriol might act to prevent the pathological changes that occur in the hippocampus during EAE. To understand the cellular and molecular mechanism of estrogen-mediated protection in the hippocampus during EAE, treatment with estrogen-receptor-specific ligands must be investigated. Estrogen-mediated protection, like other estrogenic actions, is mediated primarily through estrogen receptors, ERα and ERβ, although other non-genomic mechanisms have been identified. ERα and ERβ have distinct tissue distributions, (70), which may cause some tissue selectivity using selective estrogen receptor modifiers (SERMs). The two receptors act synergistically in some tissues, while they act antagonistically in others. These tissue-specific differences are thought to be due to tissue-specific differences in transcription factor activation upon ER-ligand binding. In a previous publication, our lab has shown that ERα ligand (PPT) treatment ameliorated clinical disease score through out disease, while treatment with an ERβ ligand (DPN) had no effect in early EAE, but promoted recovery during the late phase of disease, (21). In addition, EAE mice treated with ERα ligand had significantly reduced CNS inflammation, while EAE mice treated with ERβ ligand did not. Interestingly, treatment with either the ERα or the ERβ ligand was neuroprotective, as evidenced by axonal sparing within the white matter of the spinal cord. By using ER-specific ligands, the anti-inflammatory effects were dissociated from the neuroprotective effects of estrogen treatment during EAE, since only ERα ligand treatment significantly reduced inflammation in the spinal cord. These data suggested that the neuroprotective effects of estrogen treatment are not necessarily dependent on anti-inflammatory properties, but rather may be mediated by direct effects of estrogen binding to estrogen receptors on cells within the central nervous system. Estrogen receptors have been detected on several CNS cells, including neurons (71–72), astrocytes (73), oligodendrocytes and microglia (74). Recently, it was shown that when ERα was selectively removed from astrocytes, but not neurons, the protection from spinal cord pathology in ERα ligand treated EAE mice was lost. Both ERα and ERβ are expressed in the hippocampus (75). Future studies using CNS cell specific ER knockouts are needed to determine whether the astrocyte or other CNS cell is the target of estriol-mediated protection in the hippocampus during EAE.
In conclusion, this is the first report demonstrating that EAE leads to impaired synaptic transmission and corresponding changes in synaptic protein levels within the hippocampus. Moreover, our data argue strongly that estriol treatment is neuroprotective in the hippocampus by preventing both dysfunction and neuropathology. Although there are always limitations to the conclusions one can draw from animal models of disease, this report provides a foundation for further studies that focus on hippocampal synaptic proteins as they relate to hippocampal-dependent cognitive impairment in MS.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health [grant K24NS052117] and from the National Multiple Sclerosis Society [grants RG4033 and RG4364, to RRV], as well as funding from the Skirball Foundation, the Conrad Hilton Foundation and the Sherak Family Foundation. MZ was supported in part by the UCLA Laboratory of Neuroendocrinology (LNE), funded by the National Institutes of Health [grant T32 HD07228-26], and in part by the National Science Foundation GK-12 UCLA SEE-LA program, [grant DGE-074241]. This work was also supported by the National Institutes of Mental Health [grant MH609197, to TJO).
References
- 1.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 2.Benedict RHB, Cookfair D, Gavett R, et al. Validity of the minimal assessment of cognitive function in multiple sclerosis (MCFIMS) J Int Neuropsychol Soc. 2006;12(4):549–558. doi: 10.1017/s1355617706060723. [DOI] [PubMed] [Google Scholar]
- 3.Thornton AE, Raz N. Memory impairment in multiple sclerosis. Neuropsychology. 1997;11:357–366. doi: 10.1037//0894-4105.11.3.357. [DOI] [PubMed] [Google Scholar]
- 4.Benedict RHB, Ramasamy D, Munschauer F, et al. Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J Neurol Neurosurg Psychiatry. 2009;80:201–206. doi: 10.1136/jnnp.2008.148403. [DOI] [PubMed] [Google Scholar]
- 5.Roosendaal SD, Moraal B, Vrenken H, et al. In vivo imaging of hippocampal lesions in multiple sclerosis. J Magn Reson Imaging. 2008;27:726–731. doi: 10.1002/jmri.21294. [DOI] [PubMed] [Google Scholar]
- 6.Anderson VM, Fisniku LK, Khaleeli A, et al. Hippocampal atrophy in relapsing-remitting and primary progressive MS: a comparative study. Mult Scler. 2010;16:1083–1090. doi: 10.1177/1352458510374893. [DOI] [PubMed] [Google Scholar]
- 7.Sicotte NL, Kern KC, Giesser BS, et al. Regional hippocampal atrophy in multiple sclerosis. Brain. 2009;132:3072–3086. doi: 10.1093/brain/awn030. [DOI] [PubMed] [Google Scholar]
- 8.Geurts JJ, Bo L, Roosendaal SD, et al. Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol. 2007;66:819–827. doi: 10.1097/nen.0b013e3181461f54. [DOI] [PubMed] [Google Scholar]
- 9.Papadopoulos D, Dukes S, Patel R, et al. Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathol. 2009;19:238–253. doi: 10.1111/j.1750-3639.2008.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutta R, Chang A, Doud MK, et al. Demyelination causes synaptic alterations in hippocampi from Multiple Sclerosis patients. Ann Neurol. 2011;69:445–454. doi: 10.1002/ana.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen S, Wang Y, Kivisakk P, et al. Persistent activation of microglia is associated with neuronal dysfunction of callosal projecting pathways and multiple sclerosis-like lesions in relapsing remitting experimental autoimmune encephalomyelitis. Brain. 2007;130:2816–2829. doi: 10.1093/brain/awm219. [DOI] [PubMed] [Google Scholar]
- 12.Crawford DK, Mangiardi M, Song B, et al. Oestrogen receptor beta ligand: a novel treatment to enhance endogenous functional remyelination. Brain. 2010;133:2999–3016. doi: 10.1093/brain/awq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacKenzie-Graham A, Tiwari-Woodruff SK, Sharma G, et al. Purkinje cell loss in experimental autoimmune encephalomyelitis. Neuroimage. 2009;48:637–651. doi: 10.1016/j.neuroimage.2009.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centonze D, Muzio L, Rossi S, et al. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci. 2009;29:3442–3452. doi: 10.1523/JNEUROSCI.5804-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold SM, Voskuhl RR. Estrogen and testosterone therapies in multiple sclerosis. Prog Brain Res. 2009;175:239–251. doi: 10.1016/S0079-6123(09)17516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Confavreux C, Hutchinson M, Hours MM, et al. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. New Eng J of Med. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 17.Sicotte NL, Liva SM, Kluth R, et al. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann Neurol. 2002;52:421–428. doi: 10.1002/ana.10301. [DOI] [PubMed] [Google Scholar]
- 18.Kim S, Liva SM, Dalal MA, et al. Estriol ameliorates autoimmune demyelinating disease: implications for multiple sclerosis. Neurology. 1999;52(6):1230–1238. doi: 10.1212/wnl.52.6.1230. [DOI] [PubMed] [Google Scholar]
- 19.Bebo BF, Jr, Fyfe-Johnson A, Adlard K, et al. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two difference inbred mouse strains. J Immunol. 2001;166(3):2080–2089. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- 20.Morales LBJ, Loo KK, Liu HB, et al. Treatment with an estrogen receptor {alpha} ligand Is neuroprotective in experimental autoimmune encephalomyelitis. J Neurosci. 2006;26:6823–6823. doi: 10.1523/JNEUROSCI.0453-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiwari-Woodruff S, Morales LBJ, Lee R, et al. Differential neuroprotective and anti-inflammatory effects of estrogen receptor (ER)α and ERβ ligand treatment. PNAS. 2007;104(37):14813–14818. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vetego E, Benedusi V, Maggi A. Estrogen anti-inflammatory activity in the brain: A therapeutic opportunity for menopause and neurodegenerative diseases. Front Neuroendocrinol. 2008;29(4):507–519. doi: 10.1016/j.yfrne.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein DG, Hoffman SW. Estrogen and progesterone as neuroprotective agents in the treatment of acute brain injuries. Pediatr Rehabil. 2003;6(1):13–22. doi: 10.1080/1363849031000095279. [DOI] [PubMed] [Google Scholar]
- 24.Wise PM, Dubal DB, Rau SW, et al. Are estrogens protective or risk factors in brain injury and neurodegeneration? Reevaluation after the Women’s Health Initiative. Endocr Rev. 2005;26(3):308–312. doi: 10.1210/er.2004-0014. [DOI] [PubMed] [Google Scholar]
- 25.Behl C, Skutella T, Lezoualch F, et al. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol Pharmacol. 1997;51(4):535–541. [PubMed] [Google Scholar]
- 26.Murphy DD, Cole NB, Greenberger V, et al. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jover T, Tanaka H, Calderone A, et al. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J Neurosci. 2002;22:2115–2124. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kretz O, Fester L, Wehrenberg U, et al. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24(26):5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toran-Allerand CD. Novel sites and mechanism of oestrogen action in the brain. Novartis Found Symp. 2000;230:56–73. doi: 10.1002/0470870818.ch6. [DOI] [PubMed] [Google Scholar]
- 30.Kramar EA, Chen LY, Brandon NJ, et al. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29(41):12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yun SH, Park KA, Kwon S, et al. Estradiol enhances long-term potentiation in hippocampal slices from aged apoE4-TR mice. Hippocampus. 2007;17(12):1153–1157. doi: 10.1002/hipo.20357. [DOI] [PubMed] [Google Scholar]
- 32.Woolley CS. Effects of estrogen in the CNS. Curr Opin Neurobiol. 1999;9(3):349–354. doi: 10.1016/s0959-4388(99)80051-8. [DOI] [PubMed] [Google Scholar]
- 33.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 34.Ziehn MO, Avedisian AA, Tiwari-Woodruff S, et al. Hippocampal CA1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE. Lab Invest. 2010;90:774–786. doi: 10.1038/labinvest.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pettinelli C, McFarlin DE. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2- T lymphocytes. J Immunol. 1981;127:1420–1423. [PubMed] [Google Scholar]
- 36.Cuthbert PC, Stanford LA, Coba MP, et al. Synapse-associated protein 102/dlgh3 couples the NMDA receptor to specific plasticity pathways and learning strategies. J Neurosci. 2007;27(10):2673–2682. doi: 10.1523/JNEUROSCI.4457-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komiyama NH, Watabe AM, Carlisle HJ, et al. SynGAP regulates ERK/MAPK signaling, synaptic plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor. J Neurosci. 2002;22(22):9721–9732. doi: 10.1523/JNEUROSCI.22-22-09721.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiltgen BJ, Royle GA, Gray EE, et al. A role for calcium-permeable AMPA receptors in synaptic plasticity and learning. PLoS One. 2010;5(9):e12818. doi: 10.1371/journal.pone.0012818. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paxinos G, Franklin KBJ. The Mouse Brain in Stereological Coordinates. 2nd ed. San Diego, CA: Academic Press; 2001. [Google Scholar]
- 40.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 41.Cruz-Orive LM. Precision of Cavalieri sections and slices with local errors. J Microsc. 1999;193:182–198. doi: 10.1046/j.1365-2818.1999.00460.x. [DOI] [PubMed] [Google Scholar]
- 42.Spence RD, Hamby ME, Umeda E, et al. Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc Natl Acad Sci USA. 2011;108(21):8867–8872. doi: 10.1073/pnas.1103833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arezzo JC, Litwak MS, Zotova EG. Correlation and dissociation of electrophysiology and histopathology in the assessment of toxic neuropathy. Toxicol Pathol. 2011;39:46–51. doi: 10.1177/0192623310390231. [DOI] [PubMed] [Google Scholar]
- 44.Aubert S, Wendling F, Regis J, McGonigal A, Figarella-Branger D, Peragut JC, Girard N, Chauvel P, Bartolomei F. Local and remote epileptogenicity in focal cortical dysplasias and neurodevelopmental tumours. Brain. 2009;132:3072–3086. doi: 10.1093/brain/awp242. [DOI] [PubMed] [Google Scholar]
- 45.Lehmann HC, Zhang J, Mori S, et al. Diffusion tensor imaging to assess axonal regeneration in peripheral nerves. Exp Neurol. 2010;223(1):238–244. doi: 10.1016/j.expneurol.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palaszynski KM, Liu H, Loo KK, et al. Estriol treatment ameliorates disease in males with experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J Neuroimmunol. 2004;149(1-2):84–89. doi: 10.1016/j.jneuroim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 47.Futai K, Kim MJ, Hashikawa T, et al. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci. 2007;10(2):186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuzumaki N, Ikegami D, Imai S, et al. Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse. 2010;64:721–728. doi: 10.1002/syn.20800. [DOI] [PubMed] [Google Scholar]
- 49.Voskuhl RR, Peterson RS, Song B, et al. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci. 2009;29:11511–11522. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Debanne D, Guerineau NC, Gahwiler BH, et al. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol. 1996;15(Pt 1):163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 52.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 53.Conti R, Lisman J. The high variance of AMPA receptor- and NMDA receptor-mediated responses at single hippocampal synapses: evidence for multi-quantal release. Proc Natl Acad Sci. 2003;100:4885–4890. doi: 10.1073/pnas.0630290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dean C, Dresbach T. Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends in Neurosci. 2006;29(1):21–29. doi: 10.1016/j.tins.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5(10):771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 56.Han K, Kim E. Synaptic adhesion molecules and PSD-95. Prog Neurobiol. 2008;84(3):263–283. doi: 10.1016/j.pneurobio.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen T, Sudhof TC. Binding properties of neuroligin 1 and neurexin 1beta reveal function as heterophilic cell adhesion molecules. J Biol Chem. 1997;272:26032–26039. doi: 10.1074/jbc.272.41.26032. [DOI] [PubMed] [Google Scholar]
- 58.Dean C, Scholl FG, Choih J, et al. Neurexin mediates the assembly of presynaptic terminals. Nat Neusci. 2003;6(7):708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levinson JN, Chery N, Huang K, et al. Neuroligins mediate excitatory and inhibitory synapse formation: involvement of PSD-95 and neurexin-1beta in neuroligin-induced synaptic specificity. J Biol Chem. 2005;280(17):17312–17319. doi: 10.1074/jbc.M413812200. [DOI] [PubMed] [Google Scholar]
- 60.Jelks KB, Wylie R, Floyd CL, et al. Estradiol targets synaptic proteins to induce glutamatergic synapse formation in cultured hippocampal neurons: critical role of estrogen receptor-alpha. J Neurosci. 2007;27:6903–6913. doi: 10.1523/JNEUROSCI.0909-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teyler TJ, Vardaris RM, Lewis D, et al. Gonadal steroids: effects on excitability of hippocampal pyramidal cells. Science. 1980;209(4460):1017–1018. doi: 10.1126/science.7190730. [DOI] [PubMed] [Google Scholar]
- 62.Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30(48):16137–16148. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Foy MR, Xu J, Xie X, et al. 17β-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 64.Li C, Brake WG, Romeo RD, et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci. 2004;101(7):2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang HS, Lee CK, Kim JR, et al. Gene expression analysis of the pro-oestrous-stage rat uterus reveals neuroligin 2 as a novel steroid-regulated gene. Reprod Fertil Dev. 2004;16(8):763–772. doi: 10.1071/rd04040. [DOI] [PubMed] [Google Scholar]
- 66.Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63(1):29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 67.Carroll JC, Rosario ER, Chang L, et al. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J. Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pike CJ, Carroll JC, Rosario ER, et al. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol. 2009;30(2):239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Voskuhl RR. “Estrogens in the Treatment of MS” Multiple Sclerosis Therapeutics. 3rd Edition. United Kingdom: Informa Healthcare; 2007. pp. 645–657. [Google Scholar]
- 70.Enmark E, Gustafsson JA. Oestrogen receptors – an overview. J Intern Med. 1999;246(2):133–138. doi: 10.1046/j.1365-2796.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- 71.Mehra RD, Sharma K, Nyakas C, et al. Estrogen receptor alpha and beta immunoreactive neurons in normal adult and aged female rat hippocampus: a qualitative and quantitative study. Brain Res. 2005;1056(1):22–35. doi: 10.1016/j.brainres.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 72.Pfaff DW, Ribiero AC. Theoretical consequences of fluctuating versus constant liganding of oestrogen receptor-alpha in neurons. J Neuroendocrinol. 2010;22(6):486–491. doi: 10.1111/j.1365-2826.2009.01931.x. [DOI] [PubMed] [Google Scholar]
- 73.Azcoitia I, Santos-Galindo M, Arevalo MA, et al. Role of astroglia in the neuroplastic and neuroprotective actions of estradiol. Eur J Neurosci. 2010;32(12):1995–2002. doi: 10.1111/j.1460-9568.2010.07516.x. [DOI] [PubMed] [Google Scholar]
- 74.Arevalo MA, Santos-Galindo M, Bellini MJ, et al. Actions of estrogens on glial cells: implications for neuroprotection. Biochim Biophys Acta. 2010;1800(10):1106–1112. doi: 10.1016/j.bbagen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Mitterling KL, Spencer JL, Dziedzic N, et al. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol. 2010;518(14):2729–2743. doi: 10.1002/cne.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.