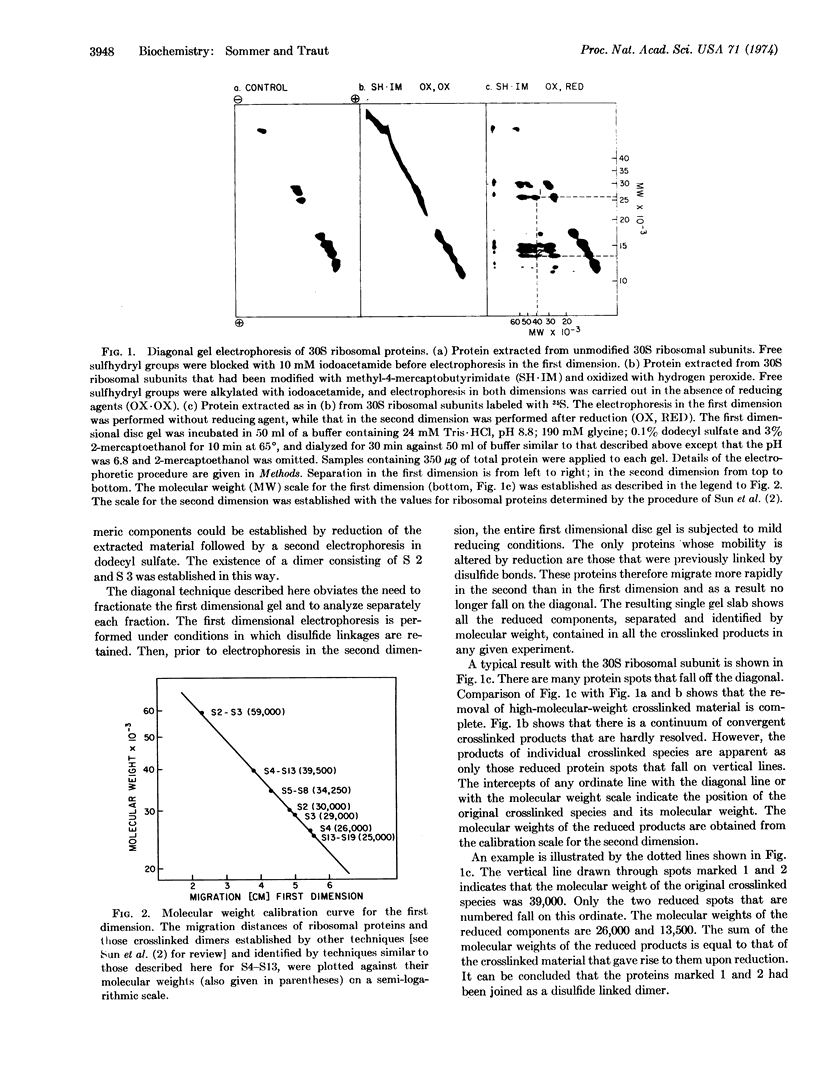
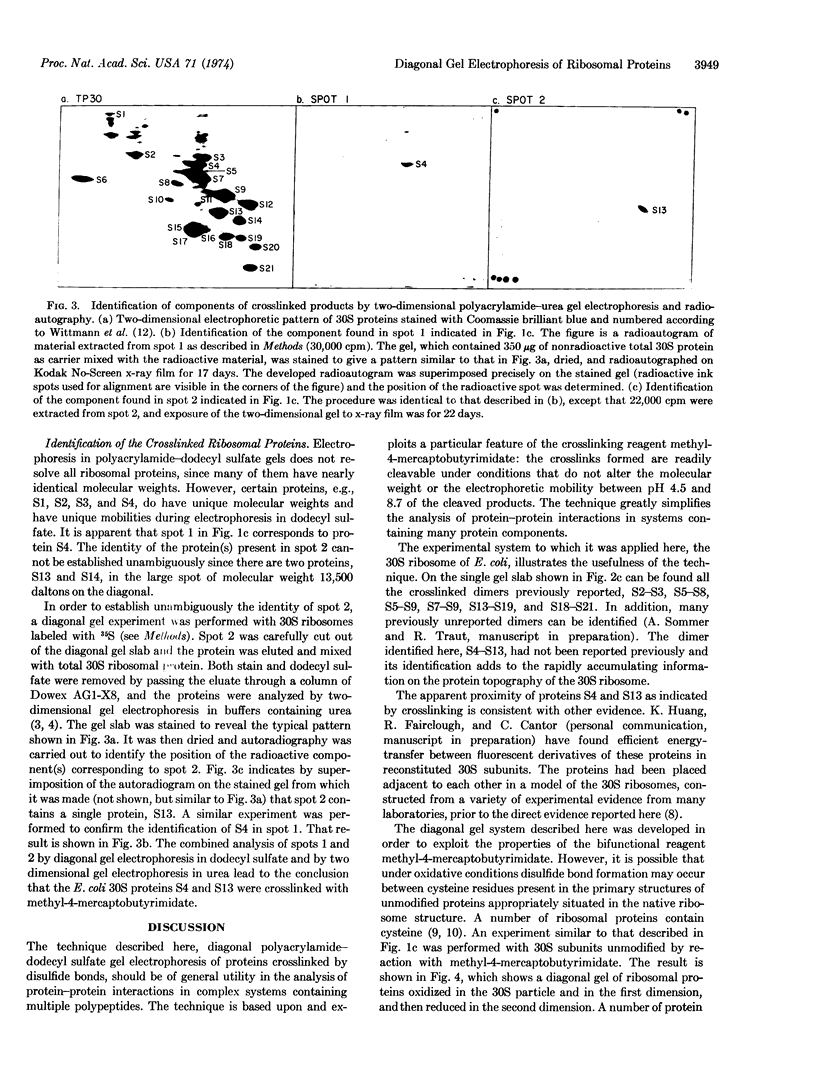
Abstract
A diagonal polyacrylamide-dodecyl sulfate gel electrophoresis procedure is described. Its utility is related to the use of the reagent methyl-4-mercaptobutyrimidate as a protein-protein crosslinking reagent. Crosslinking with this reagent occurs through the formation of intermolecular disulfide bonds. Oxidized proteins are separated in one dimension by electrophoresis under non-reducing conditions and in the second dimension under reducing conditions. All proteins except those derived from crosslinked species fall on a diagonal. Methods are described for the identification of the separated monomeric components of crosslinked species. The technique has been applied to the 30S ribosomal subunit of Escherichia coli, and the new crosslinked dimer, S4-S13, has been identified.
Keywords: protein-protein interaction, ribosome topography
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya A. S., Moore P. B. Reaction of ribosomal sulfhydryl groups with 5,5'-dithiobis(2-nitrobenzoic acid). J Mol Biol. 1973 May 15;76(2):207–221. doi: 10.1016/0022-2836(73)90385-9. [DOI] [PubMed] [Google Scholar]
- Ginzburg I., Miskin R., Zamir A. N-ethyl maleimide as a probe for the study of functional sites and conformations of 30 S ribosomal subunits. J Mol Biol. 1973 Sep 25;79(3):481–494. doi: 10.1016/0022-2836(73)90400-2. [DOI] [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Traut R. R. Separation and radioautography of microgram quantities of ribosomal proteins by two-dimensional polyacrylamide gel electrophoresis. FEBS Lett. 1973 Jan 15;29(2):177–180. doi: 10.1016/0014-5793(73)80555-1. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. VII. Two-dimensional polyacrylamide gel electrophoresis for fingerprinting of ribosomal proteins. Anal Biochem. 1970 Aug;36(2):401–412. doi: 10.1016/0003-2697(70)90376-3. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Bollen A., Kahan L., Traut R. R. Topography of ribosomal proteins of the Escherichia coli 30S subunit as studied with the reversible cross-linking reagent methyl 4-mercaptobutyrimidate. Biochemistry. 1974 May 21;13(11):2334–2340. doi: 10.1021/bi00708a015. [DOI] [PubMed] [Google Scholar]
- Traut R. R., Bollen A., Sun T. T., Hershey J. W., Sundberg J., Pierce L. R. Methyl 4-mercaptobutyrimidate as a cleavable cross-linking reagent and its application to the Escherichia coli 30S ribosome. Biochemistry. 1973 Aug 14;12(17):3266–3273. doi: 10.1021/bi00741a019. [DOI] [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]
- Wittmann H. G., Stöfflet G., Hindennach I., Kurland C. G., Birge E. A., Randall-Hazelbauer L., Nomura M., Kaltschmidt E., Mizushima S., Traut R. R. Correlation of 30S ribosomal proteins of Escherichia coli isolated in different laboratories. Mol Gen Genet. 1971;111(4):327–333. doi: 10.1007/BF00569784. [DOI] [PubMed] [Google Scholar]
- Zamir A., Miskin R., Elson D. Inactivation and reactivation of ribosomal subunits: amino acyl-transfer RNA binding activity of the 30 s subunit of Escherichia coli. J Mol Biol. 1971 Sep 14;60(2):347–364. doi: 10.1016/0022-2836(71)90299-3. [DOI] [PubMed] [Google Scholar]