Abstract
AMP-activated protein kinase, AMPK, is a conserved serine/threonine kinase with a critical function in the regulation of metabolic pathways in eukaryotic cells. Recently, AMPK has been shown to play an additional role as a regulator of inflammatory activity in leukocytes. Treatment of macrophages with chemical AMPK activators, or forced expression of a constitutively active form of AMPK, results in polarization to an antiinflammatory phenotype. Additionally, we reported previously that stimulation of macrophages with antiinflammatory cytokines such as IL-10, IL-4 and TGF-β results in rapid activation of AMPK, suggesting that AMPK contributes to the suppressive function of these cytokines. In the current study we investigated the role of AMPK in IL-10-induced gene expression and antiinflammatory function. IL-10-stimulated wild-type macrophages displayed rapid activation of PI3K and its downstream targets Akt and mTORC1, an effect that was not seen in macrophages generated from AMPKα1-deficient mice. AMPK activation was not impacted by treatment with either the PI3K inhibitor LY294002 or the JAK inhibitor CP-690550, suggesting that IL-10-mediated activation of AMPK is independent of PI3K and JAK activity. IL-10 induced phosphorylation of both Tyr705 and Ser727 residues of STAT3 in an AMPKα1-dependent manner, and these phosphorylation events were blocked by inhibition of CaMKKβ, an upstream activator of AMPK, and by the mTORC1 inhibitor rapamycin, respectively. The impaired STAT3 phosphorylation in response to IL-10 observed in AMPKα1-deficient macrophages was accompanied by reduced SOCS3 expression and an inadequacy of IL-10 to suppress LPS-induced proinflammatory cytokine production. Overall, our data demonstrate that AMPKα1 is required for IL-10 activation of the PI3K/Akt/mTORC1 and STAT3-mediated antiinflammatory pathways regulating macrophage functional polarization.
Keywords: AMPKα, IL-10, PI3K, Akt, mTORC1, STAT3, SOCS3, TNFα, macrophages
Introduction
AMP-activated protein kinase (AMPK) is an evolutionarily conserved serine/ threonine kinase composed of 3 subunits with different isoforms in mammalian cells: the regulatory β (β1, β2), γ (γ1, γ2, γ3) subunits, and catalytic α (α1, α2) subunit. Phosphorylation of the 172-threonine residue within the catalytic subunit of AMPK is crucial for its activity (1, 2). AMPK is well established as a cellular energy sensor that regulates metabolic pathways and maintains energy homeostasis (3). When cellular AMP and ADP levels rise, AMPK is activated, promoting ATP-producing catabolic pathways while switching off ATP-consuming biosynthetic pathways. Recent work by us and others has also established an association of AMPK activity with inflammatory potential in leukocytes (4–7). For example, we reported that silencing of AMPKα1 in macrophages or forced expression of a dominant negative form of AMPKα1 results in amplification of inflammatory activity, whereas treatment with agents that activate AMPK is suppressive (4). Stimulation of macrophages with proinflammatory agents such as LPS reduces AMPK phosphorylation, whereas stimulation with antiinflammatory agents (e.g., IL-10, IL-4 and TGFβ) results in elevated levels of phospho-AMPK (4). Likewise, TLR activation by LPS in dendritic cells (DC) decreases AMPK activation and this effect is accompanied by increased cellular glucose consumption (5). We demonstrated that AMPKα1 deficiency in DC results in a heightened inflammatory response to CD40 stimulation, leading to an increased capacity to induce Th1 and Th17 differentiation during antigen presentation (6). AMPKα1 activity was also found to be responsible for T cell immunoglobulin-and mucin domain-containing molecule-4 (TIM-4)-mediated autophagic degradation of apoptotic tumor cells in macrophages and the subsequent immunosuppressive phenotype macrophages acquire during this process (7). Identified mechanisms of AMPK-induced suppression of inflammatory signaling in macrophages include activation of inhibitory PI3K-mediated pathways (4) and enhancement of SIRT1 activity and expression leading to NF-κB deacetylation (8).
Studies focusing on AMPK regulation of metabolic pathways in leukocytes have contributed to the emergent paradigm that increased glycolysis is associated with inflammatory activity and proliferation, whereas reduced glycolysis and enhanced oxidative metabolism is associated with suppressed inflammation and quiescence (9). However, activators of AMPK not only suppress inflammatory responses but re-polarize leukocytes by actively inducing programs of antiinflammatory gene expression. The observation that antiinflammatory cytokines, including IL-10, induce rapid activation of AMPK in macrophages (4) suggests that AMPK serves as an immediate upstream signaling molecule in the initiation of antiinflammatory signaling pathways.
IL-10 is a potent immunosuppressive cytokine that limits excessive inflammation and controls inflammatory disease progression (10). The antiinflammatory function of IL-10 has been well studied in murine models of inflammatory disease including inflammatory bowel disease (IBD), experimental allergic encephalomyelitis (EAE), and atherosclerosis (11–13). However, the signaling pathways activated through IL-10 receptor ligation have received less attention. Two important signaling cascades have been reported to mediate IL-10 suppressive functions in macrophages, the PI3K/Akt/GSK3β and JAK/ STAT3 pathways (14, 15). Pharmaceutical inhibition of PI3K in macrophages impairs IL-10-induced gene expression as well as IL-10-mediated suppression of LPS-induced proinflammatory gene expression. This effect is reversed by the expression of a constitutively active Akt (14). Although PI3K and Akt activity can be induced by IL-10 stimulation in pro-myeloid cells (16), the direct activation of PI3K by IL-10 in macrophages has not yet been reported. Class IA PI3Ks are composed of a catalytic subunit (p110α, p110β, or p110δ) and a tightly associated regulatory subunit (p85α, p85β, p55γ, p55α, or p50α). The regulatory p55 subunit results from the alternate transcription of the Pik3r1 gene (17). p55 associates with the catalytic subunit p110 and coordinates unique PI3K kinase functions such as the induction of cell cycle arrest (18) and prevention of xenograft tumor growth (19). Phosphorylation of the regulatory subunit of PI3K results in PI3K activation which promotes colocalization of phosphoinositide-dependent protein kinase 1 (PDK1) and Akt to the plasma membrane where PDK1 phosphorylates Akt directly on Thr308 (20). Phosphorylation of both Thr308 in the activation loop and Ser473 in the kinase tail enhances the activation of Akt (21). Activation of Akt mediates many downstream biological events including the direct activation of mammalian target of rapamycin complex 1 (mTORC1) (22). Relevant to the work presented herein, it has been reported that IL-10 stimulation of primary monocytes results in elevated mTORC1 activity and this effect is abrogated by PI3K inhibition (23).
Although the precise mechanism of activation of PI3K/Akt signaling via IL-10 in macrophages remains unclear, the direct association of IL-10R with JAK family tyrosine kinases and the subsequent phosphorylation/activation of STAT3 and STAT3-directed expression of SOCS3 is well-established (16, 24, 25). IL-10 induces the tyrosine phosphorylation of JAK1 that leads to STAT3 tyrosine phosphorylation and activation (26). The serine residue of STAT3 can be phosphorylated independent of JAK activity and STAT3 tyrosine phosphorylation (27), and the phosphorylation on serine residues within the IL-10 receptor intracellular domain and within STAT3 homodimer complexes are also important for IL-10/STAT3 suppressive function (28–30). Interestingly, mTORC1 was reported to mediate STAT3 serine phosphorylation in various cell types and data suggest that this serine phosphorylation is required for optimal activation of STAT3 (31–33). STAT3 activation induces the expression of several genes including Socs3, Bcl3, and Etv3, that encode proteins capable of suppressing TLR-mediated inflammatory function in a variety of cell types including macrophages (34, 35). The mTORC1 protein complex negatively regulates innate inflammatory responses in myeloid immune cells (36) and, in contrast to non-immune cells in which AMPK typically inhibits mTORC1 (37), evidence suggests that AMPK activity positively regulates mTORC1 activity in macrophages (38). Given the rapid and robust activation of AMPK in response to IL-10 stimulation, herein we addressed the hypothesis that AMPK orchestrates PI3K/Akt/mTORC1 and STAT3 signaling pathways to contribute to IL-10-mediated polarization of macrophages to an antiinflammatory phenotype.
Materials and Methods
Reagents
Recombinant mouse IL-10 was purchased from R&D Systems. LY294002 and rapamycin were purchased from Calbiochem (EMD Millipore). CP-690550 and STO-609 were purchased from Tocris Biosciences. Western blot detection of specific proteins used the following primary Abs: anti-phospho-AMPKα (Thr172), anti-AMPKα, anti-phospho-PI3 kinase p85 (Tyr458)/p55 (Tyr199), anti-PI3 kinase p55, anti-PI3 kinase p85, anti-phospho-Akt (Ser473), anti-phospho-Akt (Thr308), anti-Akt, anti-phospho-mTOR (Ser2448), anti-mTOR, anti-phospho-p70 S6K (Ser371), anti-p70 S6K, anti-phospho-TSC2 (Ser939), anti-phospho-TSC2 (Ser1387), anti-TSC2, anti-phospho-Stat3 (Tyr705), anti-phospho-Stat3 (Ser727), anti-Stat3, anti-phospho-JAK1 (Tyr1022/1023), anti-JAK1, anti-SOCS3, anti-phospho-NF-κB (Ser536), anti-NF-κB, anti-IκB (Cell Signaling Technology), anti-β-actin (Sigma-Aldrich), and HRP-conjugated secondary Ab (Jackson ImmunoResearch Laboratories).
Mice and Cell culture
AMPKα1 deficient (AMPKα1−/−) mice were generated as previously described and obtained with permission of Dr. Benoit Viollet (39). C57BL/6J mice were purchased from the Jackson Laboratory. C57BL/6J mice, AMPKα1−/− mice and their littermate controls, AMPKα1+/+ mice, were bred and maintained in the Research Resources Facility, University of Louisville. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee, University of Louisville. Bone marrow-derived macrophages (BMDM) were generated from C57BL/6J mice, AMPKα1+/+ and AMPKα1−/− mice following the protocol previously described (40). Bone marrow was collected from the femurs and tibias from 8- to 10-wk-old mice, washed in DPBS (Mediatech) supplemented with 2% FBS (Atlanta Biologicals), and plated overnight in RPMI 1640 (HyClone) supplemented with 5% FBS, 0.01M HEPES (Sigma), and 0.01mg/ml gentamycin (Atlanta Biologicals) (referred as R5 medium) with 10 ng/ml M-CSF (R&D Systems) in 100-mm tissue culture dishes (BD Biosciences). After overnight incubation, nonadherent cells were plated in R5 medium containing 25% filtered supernatant of L929 fibroblasts (American Type Culture Collection) with 10 ng/ml M-CSF in 6-well Ultra Low Cluster (low attach) plates (Corning). Cells were maintained in incubators set to 37°C and 5% CO2. BMDMs were harvested on day 7. For stimuli/reagents used in individual experiments, BMDM were harvested into a 24-well cell culture plate and rested overnight in R5 medium prior to stimulus. CSF-1 cultured BMDM were selected for this study as the most widely used reproducible model of resting macrophages (41).
Western blot analysis
Cell lysates were generated by lysis with a buffer containing 125 mM Tris (pH 6.8), 2% SDS, 20% glycerol, 100 µM PMSF, protease inhibitor mixture (Promega), and HALT™ phosphatase inhibitor cocktail (Thermo Scientific). Total protein content of the samples was assessed by BCA protein assay (Pierce). Equal amounts of protein were separated on 10% or 4%~20% Criterion gels (Bio-Rad) by SDS-PAGE. Medium and high molecular weight proteins (55-289KDa) were transferred to nitrocellulose membranes using a Trans-Blot® Turbo™ Nitrocellulose Transfer Pack and Trans-Blot®Turbo™ transfer system (Bio-Rad). Low molecular weight proteins (< 55kDa) were transferred to nitrocellulose membranes (Hybond; Amersham Biosciences) using a Trans-Blot SD semidry electrophoretic transfer cell (Bio-Rad). Ab-bound proteins were detected using an ECL Western blotting analysis system (Amersham Corp.), and the membranes were exposed to SRX-101A film (Konica Minolta). Densitometry analysis was performed using the UN-SCAN-IT gel (version 6.1) software. Scans of the films representing the Western blots of p-JAK1 levels, p-PI3K p55 levels, total TSC2 level, SOCS3 level and p-STAT3-Ser727 levels required use of the auto-equalize feature of CorelDraw software to optimize visualization of the bands in printed versions of the image.
Real-time RT-PCR analysis
mMACs™ One-step cDNA Kit (Miltenyi Biotech) was used for RNA isolation and cDNA synthesis. cDNAs were amplified in a 20 µl reaction volume containing SYBR Green (New England Biolabs) and analyzed using a DNA Opticon 2 Monitor (MJ Research). The expression levels of mRNA (apoe, socs3, socs1, abca1, nr1h3, arg1, il-10, tgfb, tnfa, il-6, and il-12b) were analyzed by Quantitect Primer Assays (Qiagen). The cDNA concentrations in each sample were normalized using transcripts for β-actin. The relative expression software tool (REST©) was used to quantify mRNA expression (42).
ELISA
Following stimulation in 24-well plates, supernatants were collected into 96-well plates and assayed by ELISA using OptEIA™ sets (BD Biosciences Pharmingen) according to the manufacturer’s instructions. Analysis was performed using an E-max precision micro plate reader (Molecular Devices).
Statistical Analysis
Statistical significance between groups was calculated with an unpaired Student’s t test, with a p value < 0.05 considered statistically significant.
Results
Influence of AMPK on IL-10-induced gene expression in macrophages
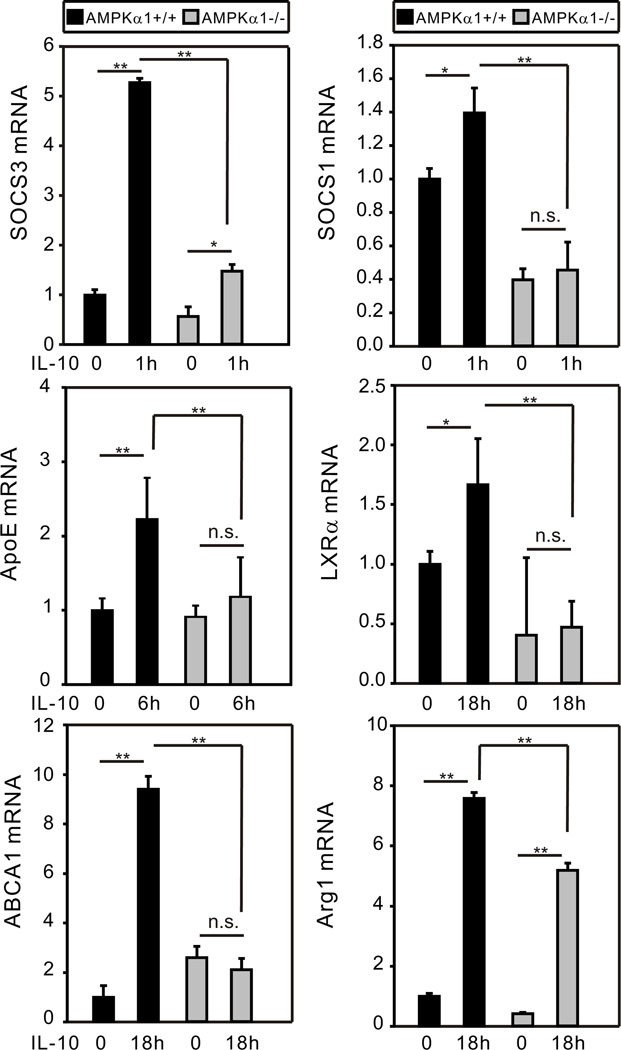
IL-10 can induce expression of antiinflammatory proteins such as arginase 1, TGFβ, IL-10 itself, and SOCS family members which serve as effective counter-regulators to inflammatory TLR and cytokine stimuli (35, 43). An atheroprotective role of IL-10 has been established (13) and atheroprotective proteins such as ATP-binding cassette transporter A1 (ABCA1) and liver X receptor α (LXRα) have been shown to be induced by IL-10 in macrophages (44). In line with our finding that IL-10 induces AMPK, AMPK activators such as metformin display protective effects in cardiovascular diseases (45). We examined expression of a panel of genes induced by IL-10 in BMDM generated from AMPKα1+/+ and AMPKα1−/− mice. Levels of mRNA were assessed at time points post stimulation ranging from 30 min to 18 h. The peak levels of mRNA detected post-stimulation are shown in Fig. 1. Robust expression of apolipoprotein E (ApoE), an important atheroprotective protein (46), was observed in AMPKα1+/+ BMDM stimulated with IL-10. Likewise, IL-10 stimulation increased mRNA expression of genes encoding SOCS1, SOCS3, LXRα, ABCA1, and Arg 1 in AMPKα1+/+ BMDM to varying degrees (1.4 to ~ 10 -fold). However, in AMPKα1−/− BMDM, IL-10 induction of expression of these genes was either abrogated (e.g., ApoE, SOCS3, SOCS1, LXRα, ABCA1) or reduced (Arg1). IL-10 induction of SOCS1 and LXRα mRNA expression in AMPKα1+/+ BMDM was quite modest. However, interestingly, AMPK-deficiency reduced the expression levels of these genes to below the baseline levels of AMPKα+/+ BMDM, suggesting a role of AMPK in maintenance of basal expression of SOCS1 and LXRα proteins. Similar results were observed for IL-10 and TGFβ (not shown).
Figure 1.
AMPK mediates IL-10-induced gene expression in macrophages. BMDM generated from AMPKα1+/+ and AMPKα1−/− mice were treated with recombinant mouse (rm)-IL-10 (20 ng/ml) for the duration of 30 min, 1 h, 3 h, 6 h, and 18 h. Total cellular lysates were collected for real-time PCR analysis. The time point representing the peak level of expression for each gene is shown (representative result of two or more independent experiments). The mRNA expression of each gene was normalized to β-actin and compared to the AMPKα1+/+ untreated group. Data shown are the mean ± SD of triplicate determinations. Statistical significance between groups was calculated with an unpaired Student’s t test, with a value of p < 0.050 considered statistically significant. (**, p < 0.001. *, p < 0.050. n.s., p > 0.050).
IL-10 activation of the PI3K/Akt pathway requires AMPK
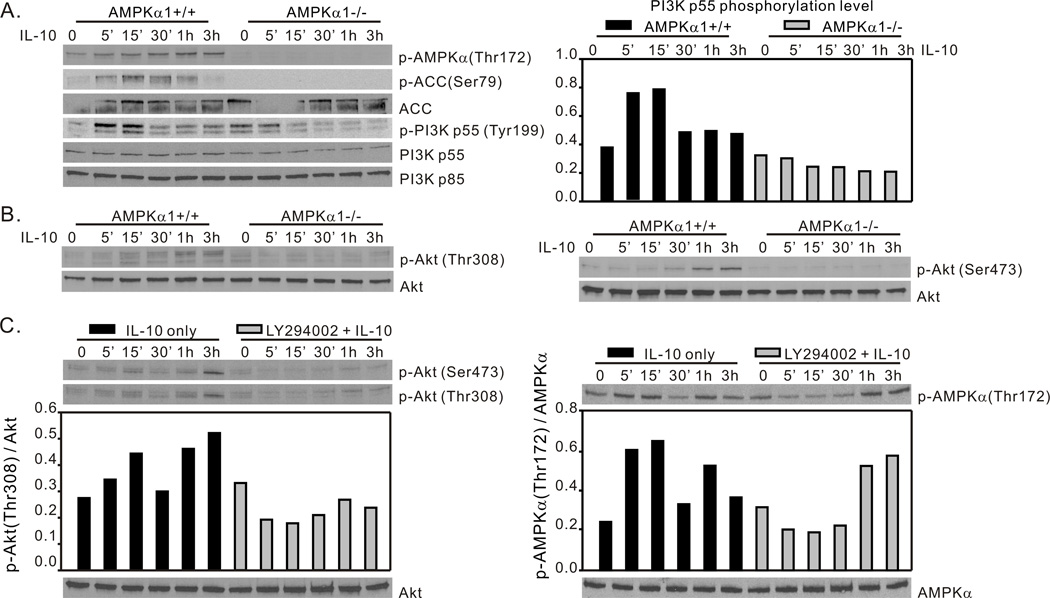
The data depicted in Fig. 1 demonstrate a role of AMPK in IL-10-mediated gene expression, including expression of SOCS3 which has been shown to be largely responsible for the ability of IL-10 to suppress TLR-mediated inflammatory responses (47). We investigated the means by which AMPK signaling contributes to this aspect of IL-10 function. IL-10 is reported to activate the PI3K/Akt pathway in macrophages (14, 16), however direct phosphorylation of PI3K in macrophages through IL-10 stimulation has not been shown. With the use of BMDM generated from AMPKα1+/+ mice, we demonstrated that IL-10 stimulation induced rapid AMPK activation indicated by elevated phosphorylation of AMPKα-Thr172 residue and phosphorylation of ACC-Ser79 residue within 5 minutes, and this effect was accompanied by more than 2 fold increase of the phosphorylation level of the PI3K p55 regulatory subunit within 15 minutes (Fig. 2A, left panel). Compared to the transient elevation of PI3K p55 phosphorylation, phosphorylation of the AMPKα-Thr172 residue persisted and increased during IL-10 stimulation in AMPKα1+/+ BMDM. IL-10 induced PI3K activation was not apparent in AMPKα1−/− BMDM (Fig. 2A). Interestingly, we noted that the overall expression level of the PI3K p55 subunit was ~1.5 fold lower in AMPKα1−/− BMDM compared to AMPKα1+/+ BMDM, while the level of p85 subunit expression was similar in both groups. We failed to detect phosphorylated PI3K p85 in both AMPKα1+/+ and AMPKα1−/− BMDM in repeated experiments, indicating a novel role of IL-10-induced AMPK in the activation of the PI3K p55 regulatory subunit, specifically.
Figure 2.
IL-10 activation of the PI3K/Akt pathway requires AMPK. (A and B), IL-10 activates PI3K and Akt in AMPKα1+/+ BMDM. BMDM generated from AMPKα1+/+ and AMPKα1−/− mice were treated with rm-IL-10 (20 ng/ml) for the time points indicated. Total cellular lysates were collected for Western blot assessment of (A) AMPK-Thr172, ACC-Ser79, PI3K-Tyr199, and (B) Akt-Thr308 and Akt-Ser473 phosphorylation. (C), PI3K inhibition does not block IL-10 activation of AMPK. BMDM generated from C57BL/6 mice were incubated with either media alone, or with LY294002 (20 µM) for 1 h, then exposed to rm-IL-10 (20 ng/ml) for the time points indicated. Total cellular lysates were collected for Western blot assessment of Akt-Thr308 and Akt-Ser473 and AMPK-Thr172 phosphorylation. Protein phosphorylation levels were analyzed by densitometry and are displayed as a bar histogram. Results shown are representative of three independent experiments.
The level of Akt phosphorylation in AMPKα1−/− BMDM was greatly reduced as compared with levels present in AMPKα1+/+ BMDM. Phosphorylation of Thr308 was reduced ~3.5 fold (Fig. 2B, left panel) and phosphorylation of Ser473 was reduced ~8.5 fold (Fig. 2B, right panel) indicating a significant impairment of IL-10’s ability to activate Akt in the absence of AMPKα1 expression. These data suggested that AMPK acts upstream of PI3K in IL-10-initiated signaling. To test this possibility, we pre-treated BMDM generated from C57BL/6J mice with the PI3K inhibitor LY294002, then stimulated the cells with IL-10. Cell lysates were collected at indicated time points for Western blot analysis of both p-Akt and p-AMPK levels. As showed in Fig. 2C, left panel, pre-treatment with LY294002 completely blocked IL-10-induced Akt phosphorylation of both serine and threonine residues. LY294002 pre-treatment resulted in a slight impairment of early phosphorylation of AMPK induced by IL-10 stimulation but phosphorylation reached control levels at 30 min (Fig. 2C, right panel). LY294002 is capable of suppressing calcium entry into the cells independent of its inhibition on PI3K activity (48) thus the early inhibition of AMPK by LY294002 may due to LY294002 inhibition of Ca2+ influx leading to the inhibition of the AMPK upstream regulator CaMKKβ (49). The demonstration that IL-10-induced PI3K and Akt activation require AMPKα1, and that AMPKα1 can be activated despite PI3K inhibition, suggests that AMPKα1 acts upstream of IL-10-induced PI3K/Akt signaling in macrophages.
IL-10-induced AMPK activity promotes mTORC1 activation
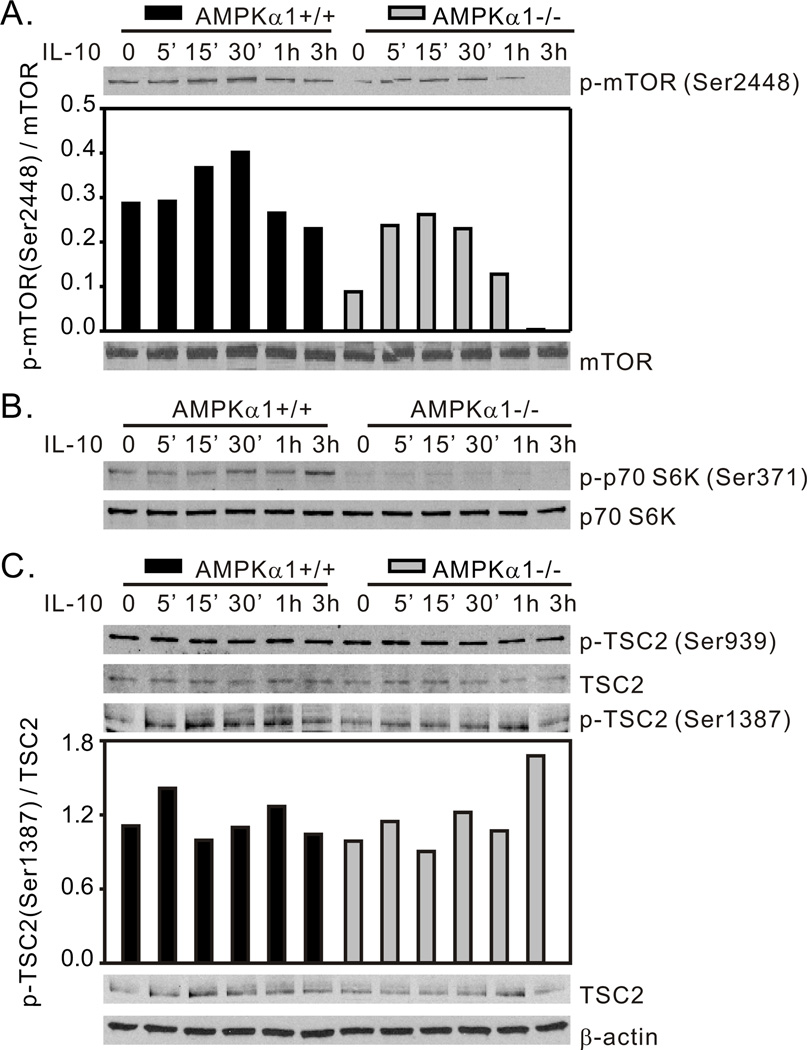
Activated Akt regulates numerous cellular functions including cytokine production, apoptosis, and proliferation (50). Activity of the kinase complex mTORC1 is promoted by Akt phosphorylation of mTOR on Ser2448 (22) and by Akt phosphorylation/inhibition of the mTORC1 inhibitory protein tuberous sclerosis complex protein 2 (TSC2) (residue Thr1462 and Ser939) (51, 52). In non-immune cells AMPK restricts mTORC1 activity by phosphorylation/activation of TSC2 (residue Ser1387 and Thr1227) under conditions of energy stress cells to limit energy consuming metabolic pathways (37). In contrast, in macrophages, transfection with a constitutively active form of CaMKIα, an AMPK upstream kinase, resulted in dramatically elevated AMPK activation accompanied by enhanced mTORC1-Ser2448 phosphorylation. Treatment with AMPK inhibitor Compound C abolished this effect, indicating positive regulation of mTORC1 by AMPK (38). Emerging evidence supports a role of mTORC1 in the negative regulation of inflammatory responses in myeloid immune cells (36). Given the AMPK-dependent Akt activation in response to IL-10 stimulation (Fig. 2), the role of mTORC1 in this signaling pathway was evaluated. We found that IL-10 stimulus induced phosphorylation of mTOR-Ser2448 in AMPKα1+/+ BMDM with a peak of ~1.5 fold increase at 30 min post-stimulus. However, in comparison with AMPKα1+/+ BMDM, both baseline and IL-10-induced levels of phospho-mTOR-Ser2448 were reduced in AMPKα1−/− cells (Fig. 3A). This positive influence of AMPK on mTOR activity was further demonstrated by the phosphorylation of p70 S6 Kinase (p70 S6K), a key downstream substrate of mTORC1, which, likewise, was efficiently activated by IL-10 stimulus in AMPKα1+/+, but not AMPKα1−/− BMDM (Fig. 3B). Meanwhile, there was no influence of IL-10 of the phosphorylation levels of either TSC2-Ser939 (Akt substrate) or Ser1387 (AMPK substrate) in either AMPKα1+/+ or AMPKα1−/− BMDM (Fig. 3C). These data suggest a positive role of AMPK in IL-10-induced mTORC1 activation via enhancement of PI3K/Akt activity, and this regulatory effect is TSC2-independent in macrophages.
Figure 3.
IL-10-induced AMPK activity promotes mTORC1 activation. BMDM generated from AMPKα1+/+ and AMPKα1−/− mice were treated with rm-IL-10 (20 ng/ml) for the time points indicated. Total cellular lysates were collected for Western blot assessment of (A) mTOR-Ser2448, (B) p70 S6K-Ser371, and (C) TSC2-Ser939 and TSC2-Ser1387 phosphorylation. Protein phosphorylation levels were analyzed by densitometry and are displayed as a bar histogram. Results shown are representative of two to four independent experiments.
AMPK is required for IL-10-induced JAK/STAT3 signaling
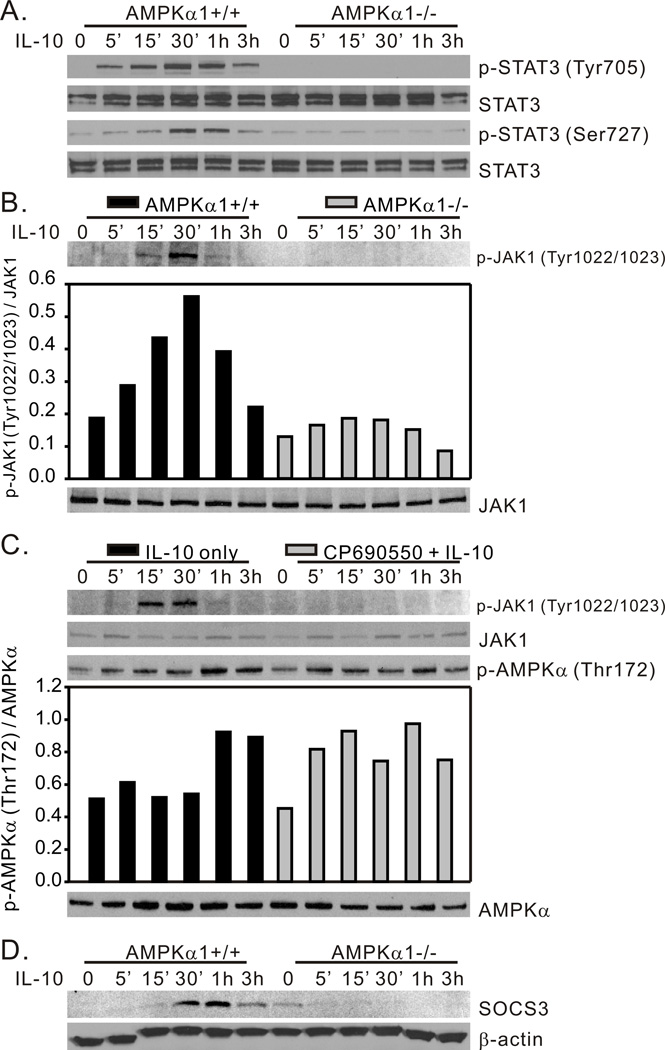
The JAK/STAT3 signaling cascade is an important contributor to the antiinflammatory activity of IL-10 (35, 47). JAK kinase activity is responsible for IL-10 induced STAT3 tyrosine phosphorylation. However, phosphorylation at both tyrosine and serine residues is necessary for STAT3 dimerization, translocation to the nucleus, and binding to the promoter regions of the socs3 gene to initiate SOCS3 protein expression. SOCS3 is a rapidly produced and quickly degraded protein demonstrated to contribute to IL-10-mediated suppression of TLR-induced inflammatory signaling (53, 54). In our evaluation of the role of AMPK in IL-10-induced STAT3 phosphorylation we found that IL-10 induced phosphorylation of both STAT3 Tyr705 (Fig. 4A, top panel) and Ser727 (Fig. 4A, bottom panel) in AMPKα1+/+ BMDM, whereas AMPKα1-deficiency completely abrogated this influence of IL-10. The phosphorylation of Tyr705 occurred within 5 min whereas the phosphorylation of Ser727 occurred with a slightly delayed onset, but both phosphorylation events peaked at 30 min – 1h (Fig. 4A). This IL-10-induced STAT3 activation is accompanied with ~3 fold increase of JAK1 phosphorylation in AMPKα1+/+ BMDM, an effect that was greatly reduced in AMPKα1−/− BMDM (Fig. 4B). To further address the relationship between IL-10 induced AMPK and JAK kinase activity, we pre-treated BMDM generated from wild-type C57BL/6J mice with the JAK kinase inhibitor CP-690550, then stimulated the cells with IL-10. Cell lysates were collected at the time points shown in Fig. 4C for Western blot analysis of phospho-AMPK levels. The efficacy of the inhibitor was demonstrated by its ability to abrogate JAK1 phosphorylation in response to IL-10 stimulation (Fig. 4C top panel). As shown in Fig. 4C, although the kinetics of IL-10-induced phosphorylation of AMPK in response to IL-10 are slightly altered by preincubation with CP-690550, the overall levels of phosphorylation achieved in the presence or absence of the inhibitor are identical. In our analysis of SOCS3 mRNA in response to IL-10, an example of which is shown in Fig. 1, we observed SOCS3 mRNA accumulation in less than 30 min in AMPKα1+/+ cells (data not shown). Consistent with this result, IL-10 treatment induced the rapid appearance of SOCS3 protein in AMPKα1+/+ cells, which peaked at 30 min – 1h, yet we were unable to detect SOCS3 protein in IL-10-stimulated AMPKα1−/− BMDM (Fig. 4D).
Figure 4.
AMPK is required for IL-10-induced activation of the JAK/STAT3/SOCS3 pathway. BMDM generated from AMPKα1+/+ and AMPKα1−/− mice were treated with rm-IL-10 (20 ng/ml) for the time points indicated. Total cellular lysates were collected for Western blot assessment of (A) STAT3-Tyr705 and STAT3-Ser727 phosphorylation, and (B) JAK1-Tyr1022/1023 phosphorylation. (C), BMDM generated from C57BL/6 mice were incubated with either media alone, or with JAK inhibitor CP-690550 (10 µM) for 1 h, then exposed to rm-IL-10 (20 ng/ml) for the time points indicated. Cell lysates were analyzed by Western blot for JAK1-Tyr1022/1023 and AMPK-Thr172 phosphorylation. (D), Cells were generated and treated as in (A). SOCS3 and β-actin expression were assessed by Western blot. Protein phosphorylation and expression levels were analyzed by densitometry and are displayed as a bar histogram. The results shown are representative of two to four independent experiments.
IL-10 induced STAT3 activation is dependent on AMPK, JAK1 and mTORC1
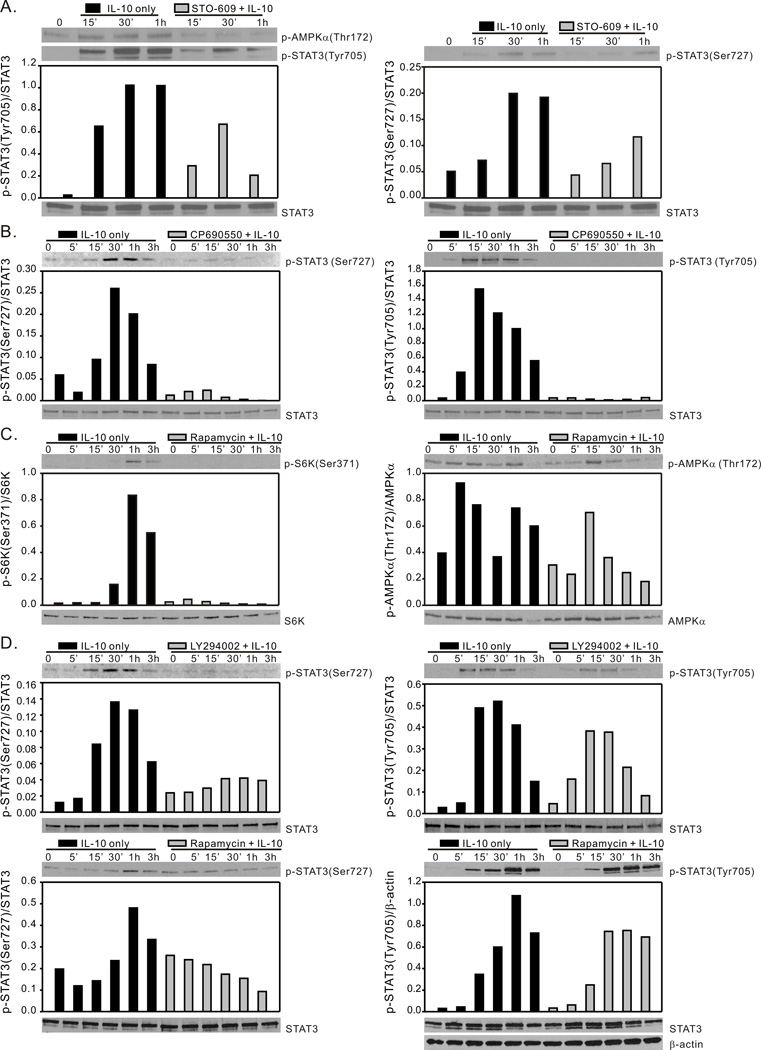
The AMPK-dependency of both tyrosine and serine phosphorylation of STAT3 suggested the possible convergence of AMPK-directed tyrosine kinase (JAK1) activities and serine kinase (mTORC1) activities in IL-10-directed STAT3 activation. To investigate this possibility we utilized the pharmacological inhibitors STO-609, CP-690550, LY294002, and rapamycin to block AMPK activity, JAK1 activity, PI3K activity and mTORC1 activity, respectively. STO-609 at a concentration between 1–10 µg/ml (2.6 – 26 µM) effectively blocks AMPK signaling cascades via inhibition of its upstream regulatory kinase CaMKKβ (55). BMDM derived from C57BL/6J mice were pre-incubated with STO-609 (5 µM) for 1h prior to stimulation with IL-10. Cell lysates were harvested at the indicated time points and were analyzed by Western blot for AMPKα1and STAT3 phosphorylation. Pre-incubation with STO-609 blocked IL-10 induced AMPKα-Thr172 phosphorylation (Fig. 5A, left panel) and inhibited IL-10-induced STAT3 phosphorylation of both Tyr705 (Fig. 5A, left panel) and Ser727 (Fig. 5A, right panel). The JAK1 inhibitor CP-690550 inhibited IL-10-induced phosphorylation on the tyrosine residue, as expected, but it also reduced phosphorylation of the serine residue (Fig. 5B), which may be due an ability of STAT tyrosine phosphorylation to positively influence phosphorylation on serine, as has been suggested in studies of STAT1 phosphorylation (27).
Figure 5.
IL-10 activation of STAT3 requires AMPK, JAK and mTORC1 activity. (A), BMDM generated from C57BL/6 mice were incubated with either media alone, or with the CaMKKβ inhibitor STO-609 (5 µM) for 1 h and then exposed to rm-IL-10 (20 ng/ml) for the time points indicated. Total cellular lysates were collected for Western blot assessment of AMPK-Thr172, STAT3-Tyr705 and STAT3-Ser727 phosphorylation. (B), BMDM generated from C57BL/6 mice were incubated with either media alone, or with JAK inhibitor CP-690550 (10 µM) for 1 h, then exposed to rm-IL-10 (20 ng/ml) for the time points indicated. Cell lysates were analyzed by Western blot for STAT3-Ser727 and STAT3-Tyr705 phosphorylation. (C), BMDM generated from C57BL/6 mice were incubated with either media alone, or with the mTORC1 inhibitor rapamycin (100 ng/ml) for 1 h and then exposed to rm-IL-10 (20 ng/ml) for the time points indicated. Total cellular lysates were collected for Western blot assessment of p70 S6K-Ser371 and AMPK-Thr172 phosphorylation. (D), BMDM generated from C57BL/6 mice were incubated with either media alone, with LY294002 (20 µM) for 1 h (top panels), or with the mTORC1 inhibitor rapamycin (100 ng/ml) for 1 h (bottom panels), then exposed to rm-IL-10 (20 ng/ml) for the time points indicated. Total cellular lysates were collected for Western blot assessment of STAT3-Ser727 and STAT3-Tyr705 phosphorylation. Protein phosphorylation levels were analyzed by densitometry and are displayed as a bar histogram. Results shown are representative of two to three independent experiments.
To determine the role of an AMPK/PI3K/mTORC1 pathway in the regulation of STAT3 activation, we generated BMDM from C57BL/6J mice and pretreated the cells with LY294002 or rapamycin. IL-10-induced STAT3 activation was assessed in these cells. As shown in Fig. 2C and Fig. 5C, pretreatment of LY294002 and rapamycin efficiently blocked Akt and p70 S6K phosphorylation (left panels) whereas AMPK activation was not affected by those inhibitors (right panels). Pretreatment with these two inhibitors resulted in similar effects on STAT3 phosphorylation in that they both suppressed IL-10-induced serine phosphorylation on STAT3 (Fig. 5D left panels) whereas the tyrosine phosphorylation of STAT3 was only modestly reduced (Fig. 5D right panels). These data indicate an upstream role of AMPK in the activation of both the tyrosine kinase (JAK1) and serine kinase (mTORC1) activities necessary for optimal activation of STAT3 via IL-10 stimulation in macrophages.
AMPK contributes to IL-10 suppression of LPS-induced proinflammatory cytokine production
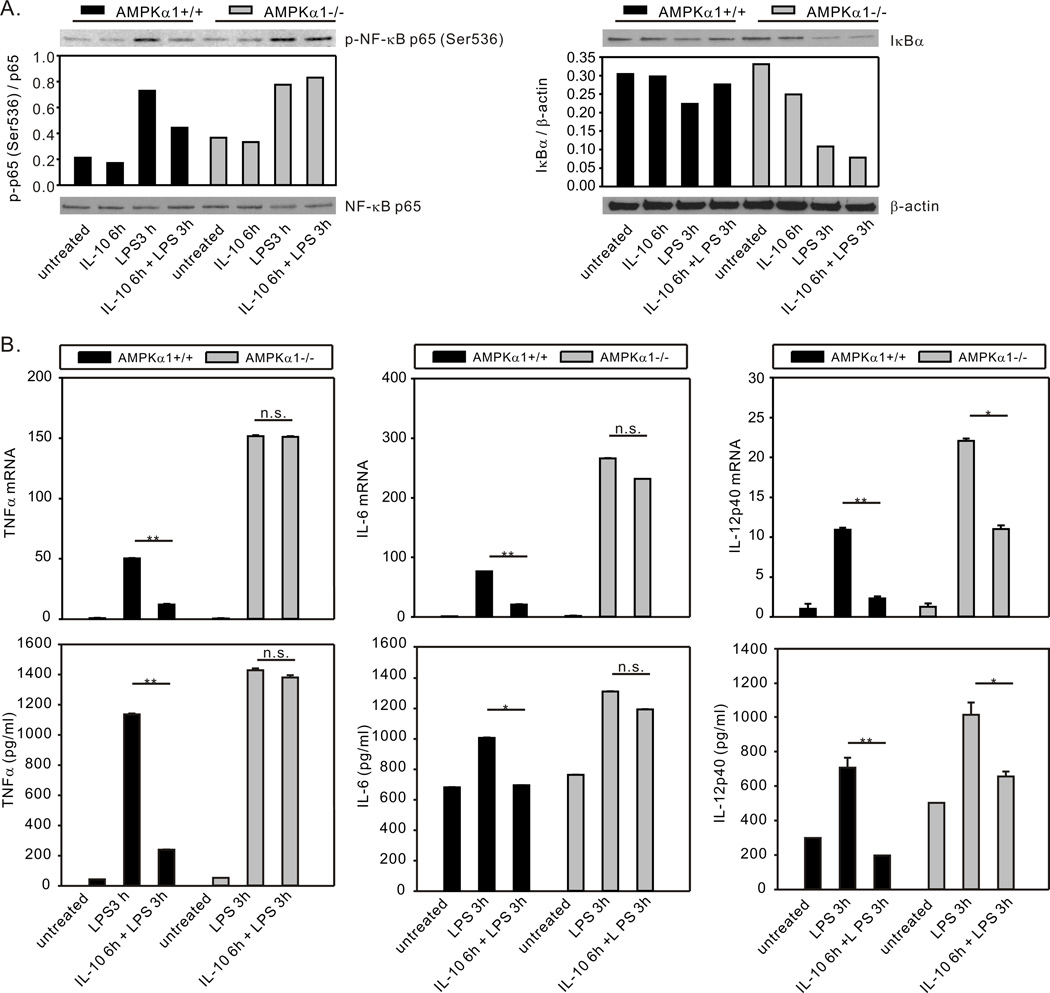
A number of previous publications have suggested a role of the STAT3/SOCS3 pathway in IL-10 suppression of LPS-induced inflammation. For example, overexpression of a constitutively active form of STAT3 (56) or forced constitutive expression of SOCS3 (53) mimicked IL-10 suppression of LPS-induced TNFα and IL-6 production in macrophages. In contrast, deficient expression of STAT3 (30) or SOCS3 (53, 54) in macrophages resulted in an inability of IL-10 to inhibit LPS-induced TNFα production. The ability of IL-10 to limit LPS-induced TNFα and IL-6 production is greatly reduced in macrophages generated from mice with myeloid specific STAT3 deletion (57). Myeloid specific deletion of STAT3 (57) or SOCS3 (58) results in increased NF-κB activation and elevated TNFα, IL-6, and IL-12 production in response to LPS challenge both in vivo and in vitro. Our data demonstrating AMPK’s role in IL-10-mediated activation of STAT3 and expression of SOCS3 suggested that AMPK activity should impact IL-10’s suppressive influence on TLR responses in macrophages. To address this possibility, we examined the influence of IL-10 on LPS-induced NF-κB activation in BMDM generated from AMPKα1+/+ and AMPKα1−/− mice. As shown in Fig. 6A, stimulation with LPS (10 ng/ml) induced IκB degradation and NF-κB p65 phosphorylation in AMPKα1+/+ BMDM and this effect was blocked by IL-10 pretreatment. However, IL-10 pretreatment was unable to inhibit LPS-induced IκB degradation or NF-κB p65 phosphorylation in AMPKα1−/− BMDM (Fig. 6A). Stimulation with LPS induced expression of the proinflammatory cytokines TNFα, IL-6, and IL-12p40 in both AMPKα1+/+ and AMPKα1−/− BMDM at the mRNA and protein levels (Fig. 6B). As we have shown previously, AMPKα1−/− BMDM produced higher levels of TNFα and IL-6 (4, 6) and, in addition, higher levels of IL-12p40 (Fig. 6B). Pre-treatment with IL-10 resulted in strong suppression (~80%) of LPS-induced TNFα, IL-6, and IL-12p40 mRNA transcription in AMPK−/− BMDM (Fig. 6B). However, in AMPKα1−/− BMDM, IL-10 suppression of LPS-induced expression of TNFα and IL-6 was nearly absent, and suppression of IL-12p40 expression was substantially reduced as compared to that observed in AMPKα1+/+ BMDM (Fig. 6B).
Figure 6.
AMPK contributes to IL-10-mediated suppression of LPS-induced NF-κB activation and proinflammatory cytokine production. (A), BMDM generated from AMPKα1+/+ and AMPKα1−/− mice were treated with rm-IL-10 (20 ng/ml) for 6h, then exposed to LPS (10 ng/ml) for 3 h. Total cellular lysates were collected for Western blot assessment of IκB degradation and NF-κB p65 phosphorylation. Protein expression or phosphorylation levels were analyzed by densitometry and are displayed as a bar histogram. (B), BMDM generated from AMPKα1+/+ and AMPKα1−/− mice were treated with rm-IL-10 (20 ng/ml) for 6h, then exposed to LPS (10 ng/ml) for 3 h. Total cellular lysates were collected for real-time PCR analysis of TNFα, IL-6 and IL-12p40 mRNA expression (top panels). The mRNA expression of each gene was normalized to β-actin and compared to the AMPKα1+/+ untreated group. Supernatants were collected for analysis by ELISA (bottom panels). The production level of each cytokine was compared to the AMPKα1+/+ untreated group. RT-PCR data shown are mean ± SD of triplicate determinations. ELISA data shown are mean ± SEM of triplicate determinations. Statistical significance between groups was calculated with an unpaired Student’s t test, with a value of p < 0.050 considered statistically significant. (**, p < 0.001. *, p < 0.050. n.s., p > 0.050). The data shown are representative of two or three independent experiments.
Discussion
Macrophages display profound phenotypic and functional heterogeneity due to their ability to adapt to the tissue microenvironment (59). This functional plasticity is crucial to their response to tissue damage, clearance of pathogens, contribution to adaptive immune responses, and wound resolution. Macrophages can respond rapidly and reversibly to cytokine stimulus and in doing so can change functional phenotype (60). The ability of macrophages to be polarized to inflammatory and anti-inflammatory states, e.g., via treatment with LPS + IFNγ or IL-4, respectively, has been studied extensively for over a decade, including attempts to elucidate the intracellular signaling pathways controlling the polarization process (61, 62). We identified AMPK as a negative regulator of LPS-induced inflammatory responses in macrophages and dendritic cells via induction of a PI3K/Akt/GSK3β/CREB pathway and inhibition of NF-κB activity (4, 6). Likewise, Yang et al., reported that AMPK activity inhibited fatty acid-induced inflammation in macrophages in a SIRT1-dependent manner (8). In the current report we follow-up on the finding that antiinflammatory cytokines such as IL-10, IL-4 and TGFβ are efficient activators of AMPK (4) and examined the role of AMPK in IL-10 signal transduction and antiinflammatory function.
IL-10 induces expression of a number of genes encoding proteins with antiinflammatory and atheroprotective function. We began by asking the simple question of whether or not AMPKα1 contributes to IL-10-induced gene expression. Our data provide direct evidence that IL-10 induces mRNA transcription of important atheroprotective genes in macrophages including ApoE, LXRα, and ABCA1, as well as the SOCS family proteins in an AMPK-dependent manner (Fig. 1). The influence of AMPKα1 on ApoE expression matched our earlier observations wherein macrophages transfected with constitutively active-AMPKα1 expressed ~ 60 fold higher ApoE mRNA as compared to empty-vector transfected macrophages, while macrophages transfected with AMPKα1 siRNA did not express ApoE in response to IL-10 (unpublished data). Interestingly, as is the case for AMPK (4), ApoE has been recognized as a regulator of macrophage plasticity that promotes an antiinflammatory phenotype (63).
SOCS3 is rapidly induced by IL-10 and has been shown to inhibit inflammatory responses in a variety of cell types including macrophages (53, 54, 58). Our data demonstrate a requirement for AMPKα1 expression for IL-10 induced SOCS3 expression at both the mRNA (Fig. 1) and protein (Fig. 4D) level. This positive regulatory role of AMPKα1 in SOCS3 expression is also supported by our unpublished data showing elevated SOCS3 protein expression in constitutively active-AMPKα1 transfected macrophages as compared to empty-vector transfected macrophages (not shown). In our investigation of the mechanisms underlying AMPK’s role in IL-10-mediated SOCS3 expression we found that IL-10 induced rapid PI3K activation at 5–15 min (Fig. 2A) and phosphorylation of both Akt Thr308 and Akt Ser473 (Fig. 2B). The Akt downstream target mTORC1 was likewise activated as indicated by both mTOR phosphorylation and phosphorylation of the mTORC1 target p70 S6K (Fig. 3A, B). Each of these IL-10-induced phosphorylation events were reduced in AMPKα1−/− BMDM as compared to BMDM derived from AMPKα1+/+ mice (Figs. 2 and 3). Neither the PI3K inhibitor LY294002 nor the mTORC1 inhibitor rapamycin affected IL-10 induced AMPK activity (Fig. 2C and Fig. 5C), indicating an upstream regulatory role of AMPK IL-10 activation of the PI3K/Akt/mTOR pathway. The intermediate steps, i.e., kinases/adapters responsible for the influence of AMPK, a serine/threonine kinase, on the tyrosine phosphorylation of PI3K p55 have not been identified. AMPK phosphorylation of insulin response substrate (IRS)-1 leading to enhanced PI3K activity has been demonstrated (64) and, interestingly, it has been reported that IL-10 stimulation of a pro-myeloid cell line resulted in phosphorylation of IRS-2 (16). However, our attempts to demonstrate involvement of IRS proteins in IL-10 signaling in macrophages have not yielded conclusive results.
Phosphorylation of Akt at Thr308 is mediated by PDK1, downstream of PI3K. Thus, the upstream role of AMPK can explain its impact on phosphorylation of Akt on this site. The Rictor-mTOR complex (mTORC2) has been identified as a mediator of phosphorylation of Akt Ser473 (65). However, the regulatory mechanism of mTORC2 activation is still poorly understood. The demonstration that IL-10 induces phosphorylation of Akt at Ser473 in an AMPK-dependent manner suggests that AMPK influences mTORC2 activity in macrophages via as yet undescribed mechanism.
Studies of non-immune cells have shown that mTORC1 activity is negatively regulated via phosphorylation/activation of TSC2 on by AMPK (on residues Ser1387 and Thr1227) under conditions of energy stress, and is positively regulated via phosphorylation/inactivation of TSC2 (Ser929) by Akt in response to insulin stimulation (37, 51). Additionally, Akt positively regulates mTORC1 activity via direct phosphorylation of mTOR-Ser2448 residue (22). In our study we observed decreased mTOR-Ser2448 in AMPKα1−/− BMDM but did not observe changes in phosphorylation of either TSC2 Ser1387 or TSC2 Ser939 levels in AMPKα1+/+ or AMPKα1−/− BMDM in response to IL-10 (Fig. 3C). These data suggest that the AMPK’s influence on mTORC1 in macrophages in response to IL-10 stimulation is Akt-dependent, but independent of TSC2. It is noted that phosphorylation of mTOR is reduced but not completely abrogated in AMPK−/− BMDM, yet there is complete abrogation of phosphorylation of p70 S6K. We suggest two possible explanations for this observation: 1) it is possible that other AMPK substrates, in addition to mTOR, contribute to p70 S6K activation, and 2) although IL-10-induced phosphorylation of mTOR is not totally abrogated in AMPK-deficient macrophages, it is reduced to a level below a threshold needed for full activity of the kinase.
Activation of the PI3K/Akt/mTORC1 pathway in macrophages elicits an antiinflammatory phenotype including induction of IL-10 expression (36). Activated mTORC1 can also influence STAT3 activity via phosphorylation of STAT3 on Ser727. We found diminished STAT3 phosphorylation on both Tyr705 and Ser727 sites in AMPKα1−/− macrophages as well as AMPKα1+/+ macrophages treated with an inhibitor of CaMKKβ, an upstream activator of AMPK (Figs. 4A and 5A). These data suggest that STAT3 activation in response to IL-10 is a two-step mechanism involving AMPK dependent regulation of both JAK kinase and PI3K/mTORC1-mediated STAT3 phosphorylation. This conclusion is supported by our observation that the activation of JAK1, PI3K, and mTORC1 were impaired in AMPKα1−/− BMDM in response to IL-10 stimulation (Figs. 3A and 4B), and that pre-treatment with the JAK inhibitor (CP-690550), PI3K inhibitor (LY294002), or the mTORC1 inhibitor (rapamycin) did not affect IL-10 induced AMPK activation (Figs. 4C, 2C, and 5C). The impaired STAT3 activity in AMPKα1−/− BMDM is accompanied by diminished levels of SOCS3 protein (Fig. 4D), a result that is consistent with the reduced SOCS3 mRNA level observed in AMPKα1−/− BMDM (Fig. 1).
Although SOCS3 expression in macrophages has been reported to be associated with an anti-inflammatory phenotype, there have been conflicting reports on its role in IL-10 function. For example, in a study using rat BMDM, IL-10 only modestly induced SOCS3 expression and siRNA knockdown of SOCS3 resulted in reduced production of IL-6 and nitrate concurrent with elevated mannose receptor and arginase expression (66). In contrast, we observed rapid and robust expression of SOCS3 in mouse BMDM in response to IL-10 (Fig. 1 and Fig. 4D), and the lack of SOCS3 expression in response to IL-10 stimulation in AMPKα1−/− BMDM is accompanied with reduced ability of IL-10 to suppress LPS-induced NF-κB activation and proinflammatory cytokine production in these macrophages (Fig. 6).
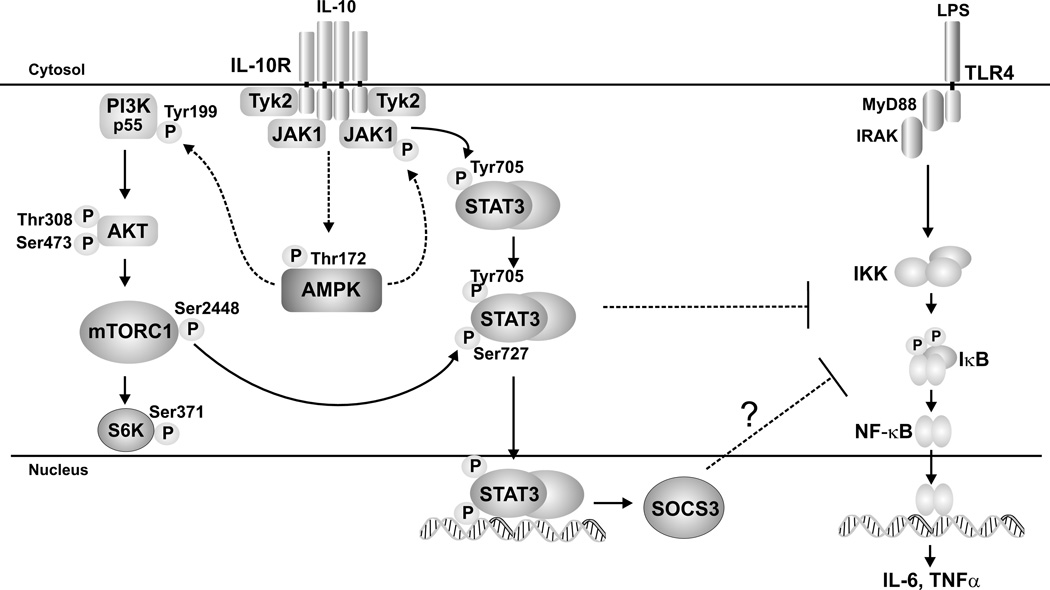
The contribution of AMPK to the signaling events culminating in STAT3 activation and SOCS3 expression, as suggested by our data, are summarized in Fig. 7. Although we show the association of AMPK-deficiency with absence of SOCS3, the role of SOCS3 in IL-10 suppressive activity has not been directly assessed herein. In addition to activation of the STAT3/SOCS3 pathway, a number of other mechanisms have been shown to mediate IL-10’s suppressive function. For example, prolonged IL-10 pre-incubation is able to suppress LPS-induced TNFα production in the absence of SOCS3 expression in macrophages (54). Proposed mechanisms for IL-10 suppression of LPS-induced IL-12p40 production include reduced RNA polymerase II recruitment to the p40 promoter (67), promoter histone deacetylation (68), and STAT3-dependent induction of NFIL3, which targets and inhibits an upstream enhancer of the Il12b promoter (69). In addition to SOCS3, other STAT3-dependent proteins induced by IL-10 including Etv3, Bcl3 (35) are reported to mediate IL-10’s suppressive function. A role for both STAT3/SOCS3 dependent and independent mechanism of IL-10-medicated suppression could account for the partial effect of AMPKα1-deficiency on IL-10 suppression of LPS-induced IL-12p40 expression (Fig. 6B). Interestingly, although IL-6 is a strong inducer of STAT3 phosphorylation, it does not share IL-10’s anti-inflammatory properties and our preliminary data (not shown) demonstrate that IL-6 activation of STAT3 does not require AMPK. IL-6-induced STAT3 phosphorylation was intact and robust in AMPK−/− BMDM. Thus, activation of STAT3 is not universally linked to the activity of AMPK.
Figure 7.
AMPK activation promotes the anti-inflammatory properties of IL-10 through bifurcated activation of the Akt/mTORC1 and JAK/STAT signaling pathways. IL-10 signaling promotes the rapid phosphorylation of JAK1 in an AMPK-dependent manner. AMPK’s influence on JAK1 phosphorylation is indirect, as indicated by the dotted line. Activation of JAK1 subsequently leads to the phosphorylation and activation of STAT3 (Tyr 705), which positively regulates STAT3 (Ser727) phosphorylation and is critical for SOCS3 production. In addition to its role in JAK/STAT signaling, AMPK also simultaneously promotes the activation of PI3K by enhancing the phosphorylation of the p55 subunit (indirectly, as indicated by the dotted line). Phosphorylation/ activation of Akt follows leading to an increase in mTORC1 activity, reflected by an increase in S6K phosphorylation (Ser371). Activation of mTORC1 leads to an increase in phosphorylation of STAT3 (Ser727), which further enhances STAT3 transcriptional activity leading to SOCS3 gene expression. STAT3-regulated genes, possibly including SOCS3, in turn, suppresses TLR-activated inflammatory cytokine production.
Thus far, our evaluation of myeloid-expressed AMPKα1 reveals its function as a counter-regulator of inflammatory signaling pathways induced, for example, via TLR and CD40 stimulation (4, 6) and as a mediator of the suppressive function of the anti-inflammatory cytokine, IL-10. Given the ability of numerous antiinflammatory mediators to rapidly activate AMPK in myeloid cells (4, and our unpublished data), it is likely that AMPK is a common upstream component of multiple signaling pathways, independent of metabolic stress. Although IL-10 activation of AMPK may impact downstream metabolic pathways, the AMPK-dependent antiinflammatory function induced by IL-10 appears to be independent of this effect. IL-10 is well-established as a critical mediator of immune homeostasis, in mice and humans, with the ability to dampen the destructive effects of inflammation in numerous pathologies (10). Thus, the identification of AMPK as a mediator of IL-10 action supports continued exploration of therapies directed towards the modulation of AMPK action.
Abbreviations used in this paper
- AMPKα1
AMP-activated protein kinase α1
- TIM-4
T cell immunoglobulin- and mucin domain-containing molecule-4
- CaM-KKβ
Ca2+/calmodulin-dependent protein kinase kinase β
- CaMKIα
Ca2+/calmodulin-dependent protein kinase I α
- DC
dendritic cell
- PDK1
phosphoinositide-dependent kinase 1
- Akt
protein kinase B
- mTORC1
mammalian target of rapamycin complex 1
- p70 S6K
p70 S6 Kinase
- TSC2
tuberous sclerosis complex protein 2
- SOCS3
suppressor of cytokine signaling 3
- Arg 1
arginase 1
- MMP-9
matrix metallopeptidase 9
- ApoE
apolipoprotein E
- LXRα
liver X receptor α
- ABCA1
ATP-binding cassette transporter A1
Footnotes
This research is funded by National Institutes of Health Grant R01 AI48850 (to J.S.) and by an American Heart Association Great Rivers Affiliate Predoctoral Fellowship, AHA Award #10PRE3030060 (to Y.Z.).
Conflict-of-Interest Disclosure:
The authors declare no competing financial interests
References
- 1.Hawley SA, Da vison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 2.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS lett. 2003;546:113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 3.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Bio. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5’-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J. Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, Pearce EJ. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll KC, Viollet B, Suttles J. AMPKalpha1 deficiency amplifies proinflammatory myeloid APC activity and CD40 signaling. J. Leukoc. Bio. 2013;94:1113–1121. doi: 10.1189/jlb.0313157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baghdadi M, Yoneda A, Yamashina T, Nagao H, Komohara Y, Nagai S, Akiba H, Foretz M, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Takeya M, Viollet B, Yagita H, Jinushi M. TIM-4 glycoprotein-mediated degradation of dying tumor cells by autophagy leads to reduced antigen presentation and increased immune tolerance. Immunity. 2013;39:1070–81. doi: 10.1016/j.immuni.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J. Biol. Chem. 2010;285:19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 10.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 12.Croxford JL, Feldmann M, Chernajovsky Y, Baker D. Different therapeutic outcomes in experimental allergic encephalomyelitis dependent upon the mode of delivery of IL-10: a comparison of the effects of protein, adenoviral or retroviral IL-10 delivery into the central nervous system. J. Immunol. 2001;166:4124–4130. doi: 10.4049/jimmunol.166.6.4124. [DOI] [PubMed] [Google Scholar]
- 13.Caligiuri G, Rudling M, Ollivier V, Jacob MP, Michel JB, Hansson GK, Nicoletti A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol. Med. 2003;9:10–17. [PMC free article] [PubMed] [Google Scholar]
- 14.Antoniv TT, Ivashkiv LB. Interleukin-10-induced gene expression and suppressive function are selectively modulated by the PI3K-Akt-GSK3 pathway. Immunology. 2011;132:567–577. doi: 10.1111/j.1365-2567.2010.03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J. Immunol. 2004;172:567–576. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]
- 16.Zhou JH, Broussard SR, Strle K, Freund GG, Johnson RW, Dantzer R, Kelley KW. IL-10 inhibits apoptosis of promyeloid cells by activating insulin receptor substrate-2 and phosphatidylinositol 3’-kinase. J. Immunol. 2001;167:4436–4442. doi: 10.4049/jimmunol.167.8.4436. [DOI] [PubMed] [Google Scholar]
- 17.Inukai K, Anai M, Van Breda E, Hosaka T, Katagiri H, Funaki M, Fukushima Y, Ogihara T, Yazaki Y, Kikuchi , Oka Y, Asano T. A novel 55-kDa regulatory subunit for phosphatidylinositol 3-kinase structurally similar to p55PIK Is generated by alternative splicing of the p85alpha gene. J. Biol. Chem. 1996;271:5317–5320. doi: 10.1074/jbc.271.10.5317. [DOI] [PubMed] [Google Scholar]
- 18.Xia X, Cheng A, Akinmade D, Hamburger AW. The N-terminal 24 amino acids of the p55 gamma regulatory subunit of phosphoinositide 3-kinase binds Rb and induces cell cycle arrest. Mol. Cell. Bio. 2003;23:1717–1725. doi: 10.1128/MCB.23.5.1717-1725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang GH, Luo XL, Sun L, Deng Y, Li XL, Tao DD, Hu JB, Gong JP. [Inhibitory effect of N-terminal 24 amino acids of the p55 gamma, a regulatory subunit of phosphoinositide 3-kinase, on proliferation of colon carcinoma cell line HT29] Ai zheng = Aizheng = Chinese Journal of Cancer. 2008;27:1034–1038. [PubMed] [Google Scholar]
- 20.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 21.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 22.Nave BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem. J. 1999;344:427–431. [PMC free article] [PubMed] [Google Scholar]
- 23.Crawley JB, Williams LM, Mander T, Brennan FM, Foxwell BM. Interleukin-10 stimulation of phosphatidylinositol 3-kinase and p70 S6 kinase is required for the proliferative but not the antiinflammatory effects of the cytokine. J. Biol. Chem. 1996;271:16357–16362. doi: 10.1074/jbc.271.27.16357. [DOI] [PubMed] [Google Scholar]
- 24.Williams LM, Ricchetti G, Sarma U, Smallie T, Foxwell BM. Interleukin-10 suppression of myeloid cell activation--a continuing puzzle. Immunology. 2004;113:281–292. doi: 10.1111/j.1365-2567.2004.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J. Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 26.Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J. Immunol. 1995;155:1079–1090. [PubMed] [Google Scholar]
- 27.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Blenis J, Li HC, Schindler C, Chen-Kiang S. Requirement of serine phosphorylation for formation of STAT-promoter complexes. Science. 1995;267:1990–1994. doi: 10.1126/science.7701321. [DOI] [PubMed] [Google Scholar]
- 29.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 30.Riley JK, Takeda K, Akira S, Schreiber RD. Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action. J. Biol. Chem. 1999;274:16513–16521. doi: 10.1074/jbc.274.23.16513. [DOI] [PubMed] [Google Scholar]
- 31.Yokogami K, Wakisaka S, Avruch J, Reeves SA. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr. Biol. 2000;10:47–50. doi: 10.1016/s0960-9822(99)00268-7. [DOI] [PubMed] [Google Scholar]
- 32.Kim JH, Kim JE, Liu HY, Cao W, Chen J. Regulation of interleukin-6-induced hepatic insulin resistance by mammalian target of rapamycin through the STAT3-SOCS3 pathway. J. Biol. Chem. 2008;283:708–715. doi: 10.1074/jbc.M708568200. [DOI] [PubMed] [Google Scholar]
- 33.Kim JH, Yoon MS, Chen J. Signal transducer and activator of transcription 3 (STAT3) mediates amino acid inhibition of insulin signaling through serine 727 phosphorylation. J. Biol. Chem. 2009;284:35425–35432. doi: 10.1074/jbc.M109.051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J. Interferon Cytokine Res. 1999;19:563–573. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- 35.Hutchins AP, Diez D, Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief Funct. Genomics. 2013;12:489–498. doi: 10.1093/bfgp/elt028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weichhart T, Haidinger M, Katholnig K, Kopecky C, Poglitsch M, Lassnig C, Rosner M, Zlabinger GJ, Hengstschlager M, Muller M, Horl WH, Saemann MD. Inhibition of mTOR blocks the anti-inflammatory effects of glucocorticoids in myeloid immune cells. Blood. 2011;117:4273–4283. doi: 10.1182/blood-2010-09-310888. [DOI] [PubMed] [Google Scholar]
- 37.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 38.Guo L, Stripay JL, Zhang X, Collage RD, Hulver M, Carchman EH, Howell GM, Zuckerbraun BS, Lee JS, Rosengart MR. CaMKIalpha regulates AMP kinase-dependent, TORC-1-independent autophagy during lipopolysaccharide-induced acute lung neutrophilic inflammation. J. Immunol. 2013;190:3620–3628. doi: 10.4049/jimmunol.1102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viollet B, Andreelli F, Jorgensen SB, Perrin C, Flamez D, Mu J, Wojtaszewski JF, Schuit FC, Birnbaum M, Richter E, Burcelin R, Vaulont S. Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem. Soc. Trans. 2003;31:216–219. doi: 10.1042/bst0310216. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds JM, Liu Q, Brittingham KC, Liu Y, Gruenthal M, Gorgun CZ, Hotamisligil GS, Stout RD, Suttles J. Deficiency of fatty acid-binding proteins in mice confers protection from development of experimental autoimmune encephalomyelitis. J. Immunol. 2007;179:313–321. doi: 10.4049/jimmunol.179.1.313. [DOI] [PubMed] [Google Scholar]
- 41.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Montovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Shultze JL, Shirey KA, Sica A, Suttles J, Udalova I, Ginderachter JAvan, Vogel SN, Wynn TA. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J. Immunol. 2002;169:2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 44.Han X, Kitamoto S, Wang H, Boisvert WA. Interleukin-10 overexpression in macrophages suppresses atherosclerosis in hyperlipidemic mice. FASEB J. 2010;24:2869–2880. doi: 10.1096/fj.09-148155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ewart MA, Kennedy S. AMPK and vasculoprotection. Pharmacol. Ther. 2011;131:242–253. doi: 10.1016/j.pharmthera.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Plump AS, Breslow JL. Apolipoprotein E and the apolipoprotein E-deficient mouse. Annu. Rev. Nutr. 1995;15:495–518. doi: 10.1146/annurev.nu.15.070195.002431. [DOI] [PubMed] [Google Scholar]
- 47.Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr. Opin. Pharmacol. 2006;6:379–386. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Tolloczko B, Turkewitsch P, Al-Chalabi M, Martin JG. LY-294002 [2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one] affects calcium signaling in airway smooth muscle cells independently of phosphoinositide 3-kinase inhibition. J. Pharmacol. Exp. Ther. 2004;311:787–793. doi: 10.1124/jpet.104.069013. [DOI] [PubMed] [Google Scholar]
- 49.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Wang X, Yang H, Liu H, Lu Y, Han L, Liu G. Kinase AKT controls innate immune cell development and function. Immunology. 2013;140:143–152. doi: 10.1111/imm.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 52.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 53.Berlato C, Cassatella MA, Kinjyo I, Gatto L, Yoshimura A, Bazzoni F. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J. Immunol. 2002;168:6404–6411. doi: 10.4049/jimmunol.168.12.6404. [DOI] [PubMed] [Google Scholar]
- 54.Qasimi P, Ming-Lum A, Ghanipour A, Ong CJ, Cox ME, Ihle J, Cacalano N, Yoshimura A, Mui AL. Divergent mechanisms utilized by SOCS3 to mediate interleukin-10 inhibition of tumor necrosis factor alpha and nitric oxide production by macrophages. J. Biol. Chem. 2006;281:6316–6324. doi: 10.1074/jbc.M508608200. [DOI] [PubMed] [Google Scholar]
- 55.Tokumitsu H, Inuzuka H, Ishikawa Y, Ikeda M, Saji I, Kobayashi R. STO-609, a specific inhibitor of the Ca2+/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 2002;277:15813–15818. doi: 10.1074/jbc.M201075200. [DOI] [PubMed] [Google Scholar]
- 56.Williams LM, Sarma U, Willets K, Smallie T, Brennan F, Foxwell BM. Expression of constitutively active STAT3 can replicate the cytokine-suppressive activity of interleukin-10 in human primary macrophages. J. Biol. Chem. 2007;282:6965–6975. doi: 10.1074/jbc.M609101200. [DOI] [PubMed] [Google Scholar]
- 57.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 58.Qin H, Holdbrooks AT, Liu Y, Reynolds SL, Yanagisawa LL, Benveniste EN. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J. Immunol. 2012;189:3439–3448. doi: 10.4049/jimmunol.1201168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J. Leukoc. Biol. 2004;76:509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 61.Locati M, Mantovani A, Sica A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv. Immunol. 2013;120:163–184. doi: 10.1016/B978-0-12-417028-5.00006-5. [DOI] [PubMed] [Google Scholar]
- 62.Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, Li J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell. Signal. 2014;26:192–197. doi: 10.1016/j.cellsig.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Raffai RL. Apolipoprotein E regulation of myeloid cell plasticity in atherosclerosis. Curr. Opin. Lipidol. 2012;23:471–478. doi: 10.1097/MOL.0b013e328356f967. [DOI] [PubMed] [Google Scholar]
- 64.Longnus SL, Segalen C, Giudicelli J, Sajan MP, Farese RV, Obberghen EVan. Insulin signalling downstream of protein kinase B is potentiated by 5’AMP-activated protein kinase in rat hearts in vivo. Diabetologia. 2005;48:2591–2601. doi: 10.1007/s00125-005-0016-3. [DOI] [PubMed] [Google Scholar]
- 65.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Stewart KN, Bishop E, Marek CJ, Kluth DC, Rees AJ, Wilson HM. Unique expression of suppressor of cytokine signaling 3 is essential for classical macrophage activation in rodents in vitro and in vivo. J. Immunol. 2008;175:342–349. doi: 10.4049/jimmunol.180.9.6270. [DOI] [PubMed] [Google Scholar]
- 67.Zhou L, Nazarian AA, Smale ST. Interleukin-10 inhibits interleukin-12 p40 gene transcription by targeting a late event in the activation pathway. Mol. Cell. Biol. 2004;24:2385–2396. doi: 10.1128/MCB.24.6.2385-2396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kobayashi T, Matsuoka K, Sheikh SZ, Russo SM, Mishima Y, Collins C, deZoeten EF, Karp CL, Ting JP, Sartor RB, Plevy SE. IL-10 regulates Il12b expression via histone deacetylation: implications for intestinal macrophage homeostasis. J. Immunol. 2012;189:1792–1799. doi: 10.4049/jimmunol.1200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith AM, Qualls JE, O’Brien K, Balouzian L, Johnson PF, Schultz-Cherry S, Smale ST, Murray PJ. A distal enhancer in Il12b is the target of transcriptional repression by the STAT3 pathway and requires the basic leucine zipper (B-ZIP) protein NFIL3. J. Biol. Chem. 2011;286:23582–23590. doi: 10.1074/jbc.M111.249235. [DOI] [PMC free article] [PubMed] [Google Scholar]