Abstract
Background
An optic disc hemorrhage (DH) has been associated with subsequent structural glaucoma progression but it is unknown if there is structural progression prior to a DH. We evaluated a cohort of patients to determine whether structural progression occurs before a DH, after a DH or is simply associated with a DH.
Methods
Eyes meeting inclusion criteria were placed into two groups. Group 1 included eyes that each had a baseline photograph of the optic nerve and a photograph with a DH at follow-up. Group 2 included eyes that each had a photograph of the optic nerve with a DH at baseline and a photograph at follow-up. Flicker images were created and graded by two ophthalmologists for structural glaucomatous change. We compared the proportion of structural progressors between Groups 1 and 2. Patient characteristics were also compared between the two groups.
Results
49 patients and 51 unique eyes were included. Group 1 and Group 2 had 28 and 38 sets of photographs respectively. The proportion of global progression in Groups 1 and 2 were 21.4% and 39.5% respectively (p=0.12). No significant differences in any structural progression feature and patient characteristics (besides age at time of DH (p=0.04) between the two groups were found.
Conclusions
Patients show structural glaucomatous progression both before and after the event of a disc hemorrhage without significant differences. This suggests that a DH is an ongoing structural progression in glaucoma and may not be a discrete event that leads to subsequent progression.
Keywords: glaucoma, optic nerve, disc hemorrhage, structural glaucoma progression, flicker chronoscopy
INTRODUCTION
Glaucoma affects approximately sixty million people worldwide and is the second leading cause of blindness, affecting an estimated 8.4 million people.1 Glaucomatous optic neuropathy is characterized by progressive retinal ganglion cell degeneration leading to neuroretinal rim loss, retinal nerve fiber layer (RNFL) defects, the development of visual field (VF) deficits, parapapillary atrophy (PPA) and optic disc hemorrhage (DH). Blindness can occur after significant functional visual loss as a result of glaucomatous optic neuropathy.
An optic DH is a splinter shaped region of bleeding in contact with the optic disc rim or RNFL and is a hallmark of glaucomatous optic neuropathy. Optic nerve hemorrhages have been demonstrated in several prospective randomized glaucoma investigations to be a risk factor for functional glaucoma progression. In the ocular hypertension treatment study, DHs occurred at a rate of 0.5% per year and increased the likelihood of developing glaucoma fivefold.2 In the early manifest glaucoma trial (EMGT), nearly half of participants developed DHs over the duration of the study and these hemorrhages were associated with an increased likelihood of progression and a 2% increase in relative risk of progression for every 1% of follow up visits in which a disc hemorrhage is noted (RR 1.02),3,4
Netland and Siegner documented that functional glaucoma progression occurs, on average, 16.8 +/−2.0 months after DH and that structural changes may occur, on average, 23.8+/−2.9 months after hemorrhage.5 Recently, De Moraes and colleagues evaluated VF progression both before and after an optic DH and demonstrated that progression indeed occurs prior to the hemorrhage though it may increase afterwards.6 While an optic DH has been associated with subsequent structural glaucoma progression, it is unknown if the optic nerve has progressed structurally prior to the development of an optic DH. We sought to evaluate a cohort of patients to determine whether structural progression occurs before or after the event of a DH, using the technique of flicker chronoscopy.
METHODS
Approval for the retrospective cohort study was obtained from the Institutional Review Board at Weill Cornell Medical College (New York, New York, USA).
Individuals aged eighteen years and above were considered for inclusion in this study. For inclusion, patients were required to have both a photographically documented DH and at least one additional optic nerve photograph and eye examination greater than twelve months before or after the occurrence of the DH at the Department of Ophthalmology at Weill Cornell Medical College. If a DH occurred bilaterally, both eyes were included. If an eye had recurring hemorrhages, only the baseline optic disc photograph and follow-up photograph of the first hemorrhage were included. Clinical patient data obtained from these patients included age, sex, central corneal thickness (CCT), Goldman-correlated intraocular pressure (IOP) values for each time a photograph was taken and documentation of IOP lowering medications. Eyes that had a history of amblyopia, traumatic eye injury, and conditions that compromised the morphology of the optic nerve head and parapapillary area (e.g., retinal detachment, giant cell arteritis, chorioretinitis) were excluded.
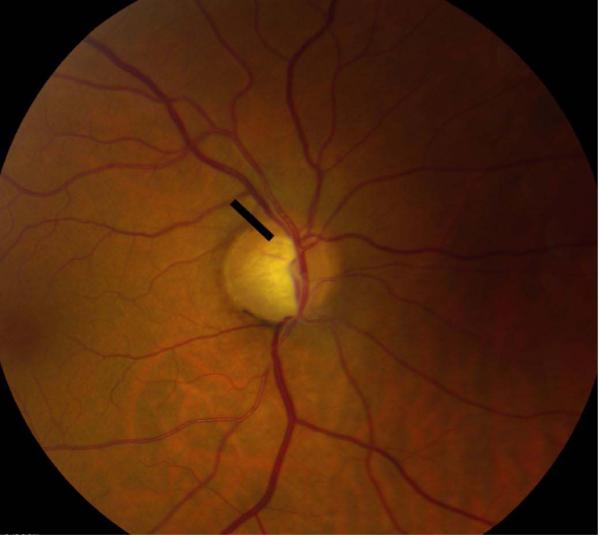
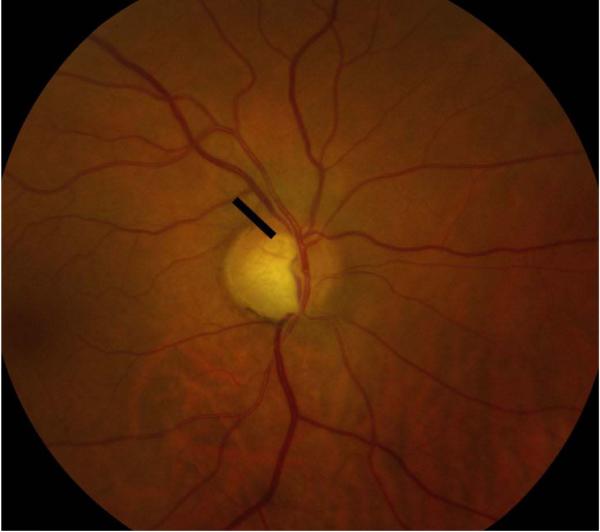
Eyes that were deemed suitable for our study were placed into two groups and each eye that was evaluated had a pair of photographs associated with it. Per the Department of Ophthalmology’s protocol, all patients with a glaucoma diagnosis (ocular hypertension, glaucoma, or suspected glaucoma) were photographed at baseline and optic nerve hemorrhages were photographed whenever presented. Group 1 (“hemorrhage at follow-up”) included eyes that each had a normal baseline disc image and a follow-up disc image containing a DH. Group 2 (“hemorrhage at baseline”) included eyes that each had a baseline disc image containing a DH and a follow-up disc image without a DH. A digital fundus camera (Topcon TRC50EX Retinal Camera; Topcon Co, Tokyo, Japan) operated by a single trained photographer was used to obtain optic nerve head photographs. Flicker images of a baseline and follow-up photograph were created from serial optic nerve head photographs using PerfectView software (Merge Eye Care Solutions Chicago, Illinois, USA) and full-screen images were viewed using a HP Compaq LA2405wg 24-inch widescreen LCD Monitor. The images within each pair were randomized with respect to the order in which they were displayed for viewing as alternating flicker images, masking graders to the actual temporal sequence of images. A small region of the optic nerve, the size of the DH, was deleted in both images of a given pair of photographs in order to mask the grader from the photograph containing the DH (Figure 1). Ten pairs of control images of optic discs without any DH were also included and similarly masked. Two glaucoma fellowship-trained ophthalmologists (N.R. and A.D.) independently graded 76 pairs of images for evidence of structural glaucomatous change. After determining the occurrence of progression, graders were asked to designate which photograph they believed to be the baseline photograph. Signs of structural progression assessed by the graders included neuroretinal rim loss, RNFL loss and PPA using previously defined criteria.7,8,9 Each progression feature was separately graded as having occurred in the superior optic nerve region, inferior optic nerve region or both. Patients were deemed to have no structural progression if morphologic changes were absent or insufficient to determine the temporal order of images (e.g., if the images appeared identical to each other). Both graders determined the temporal order of images only in cases of progression. Each grader remained masked to the other grader’s assessment prior to completing his or her assessment of the images. The two sets of results were then compared to determine the overall agreement between the subjective assessments of flicker images by the two masked, independent graders. For every set of analysis conducted, only cases in which both graders independently agreed on the presence of progression were included and analyzed to increase the specificity of the study.
Figure 1.
To evaluate whether the optic nerve was more likely to progress prior to or following the DH, we compared the proportion of progressing eyes between Groups 1 and 2 using the χ2 and Fischer’s exact tests. Mean IOP, number of IOP lowering medications, time between photographs, CCT, and age at time of DH (hereafter known as “patient characteristics”) were also compared between Groups 1 and 2 using χ2 and Fischer’s exact tests. Patient characteristics were also compared between eyes with and without progression using 2-sample t-tests. Univariate χ2 and Wilcoxon rank-sum tests were constructed to evaluate associations with increased odds of progression. Multivariable models were constructed from univariable predictors with P<0.2. All tests were run with a 0.05 level of significance.
RESULTS
Between January of 2009 and June 2013, 71 patients who had a disc hemorrhage diagnosis were identified from billing records and considered for inclusion. After the inclusion criteria were applied, 49 unique patients (mean age and standard deviation, 63.5 +/− 12.4 yrs) were identified. Of these patients, 47 patients had one of their eyes included in the study and two patients had both eyes included; 51 unique eyes with DHs were included in the analysis. Of these 51 eyes, 15 eyes had greater than one year of follow up both before and after the hemorrhage and qualified to be placed in both Groups 1 and 2. In total, 66 sets of photographs related to eyes that had DHs and ten sets of controls, were evaluated. There were 28 and 38 sets of photographs in Group 1 and Group 2, respectively. Of the entire group, 24 were women and 25 were men.
Table 1 illustrates the comparison of features of structural progression between Groups 1 and 2 using Fischer’s exact and χ2 tests. Global progression was defined as progression that included at least one feature of structural progression. PPA progression includes inferior or superior hemisphere PPA progression or both. RNFL progression included inferior or superior hemisphere RNFL progression or both. Rim progression included inferior or superior hemisphere rim progression or both. We did not find any significant differences between global, PPA, RNFL or neuroretinal rim progression between the two groups (p= 0.11 – 0.72). Subsequently, we compared patient characteristics including age at time of hemorrhage, average IOP, average number of IOP lowering medications, time between photographs, and CCT between the two groups. Patients in Group 2 were older at the time of DH (58.4 years vs. 64.8 years, p=0.04)(Table 2).
Table 1.
Comparison of Progression Features Between Patients with DH at Follow-Up (Group 1) and Patients with a DH at Baseline (Group 2)
| Progression | Group 1 (n=28)a |
Group 2 (n=38)a |
P valueb | 95% CI (Difference in Proportion) |
|---|---|---|---|---|
| Global Progression | 6 (21.4%) | 15 (39.5%) | 0.12 | −4.5% - 40.7% |
| PPA Progression | 2 (7.1%) | 8 (21.1%) | 0.17 | −3.7% - 31.7% |
| Superior PPA Progression | 1 (3.6%) | 7 (18.4%) | 0.13 | −1.5% - 31.1% |
| Inferior PPA Progression | 1 (3.6%) | 5 (13.2%) | 0.23 | −5.1% - 24.3% |
| RNFL Progression | 2 (7.1%) | 6 (15.8%) | 0.45 | −7.5% - 24.9% |
| Superior RNFL Progression | 1 (3.6%) | 5 (13.2%) | 0.23 | −5.1% - 24.3% |
| Inferior RNFL Progression | 1 (3.6%) | 3 (7.9%) | 0.63 | −8.3% - 16.9% |
| Rim Progression | 4 (14.3%) | 12 (31.6%) | 0.11 | −3.5% - 38.1% |
| Superior Rim Progression | 2 (7.1%) | 7 (18.4%) | 0.28 | −5.7% - 28.3% |
| Inferior Rim Progression | 3 (10.7%) | 6 (15.8%) | 0.72 | −11.4% - 21.6% |
Proportion of group represented as percentages of n
P values for Fisher's exact tests used for all categories of progression except global and rim progression. χ2 test used for global and rim progression
Table 2.
Comparison of Patient Characteristics Between Group 1 and Group 2
| Group 1 (n=28) |
Group 2 (n=38) |
P valuea | |
|---|---|---|---|
| Age at time of DH | 58.4 | 64.8 | 0.04 |
| Mean IOP (mmHg) | 15.3 | 15.6 | 0.72 |
| Central Corneal Thickness (μm) | 539.6 | 547.6 | 0.42 |
| Time between photographs (yrs) | 1.83 | 2.18 | 0.12 |
| Median number of IOP lowering medications | 1.0 | 1.0 | 0.73 |
P values for two sample t-test for age, mean IOP and central corneal thickness. Wilcoxon rank-sum test used for time between photographs and medications
We subsequently compared patient characteristics between progressors and non-progressors (Table 3). No significant characteristics besides time between photographs were found. Multivariate logistic regression analysis controlling for time showed that the variable did not remain significant (OR=1.96, CI = 0.6-6.9, P=0.30).
Table 3.
Comparison of Patient Characteristics between Progressors and Non-progressors
| Progressors (n=21) |
Non-Progressors (n=45) |
P valuea | |
|---|---|---|---|
| Age at time of DH | 61.2 | 62.5 | 0.72 |
| Mean IOP (mmHg) | 16.0 | 15.2 | 0.36 |
| Central Corneal Thickness (|im) | 541.9 | 545.2 | 0.76 |
| Time between photographs (yrs) | 3.1 | 1.42 | <0.0001 |
| Median no. of IOP lowering medications | 1.0 | 1.0 | 0.93 |
P values for two sample t-test for age, mean IOP and central corneal thickness. Wilcoxon rank-sum test used for time between photographs and medications
We also analyzed our data set to evaluate whether the location of a DH (i.e., superior or inferior) was associated with the location of global progression and the various features of progression (PPA, RNFL, and Rim). Using χ2 test (global and rim progression) and Fisher’s exact test (for all other categories), we found that the location of the DHs in our data set were not associated with any of features of progression (p=0.27-0.99).
The grading of the serial optic nerve photographs between the two masked graders showed fair to substantial interobserver agreement (kappa =0.31–0.61) per the grading scheme of Viera and Garrett.10 Superior RNFL, inferior RNFL and PPA progression showed the highest kappa values in our grading set (kappa=0.61, 0.45, and 46 respectively).
DISCUSSION
In this study, we evaluated structural progression both before and after the event of a DH in order to determine whether the optic nerve was more likely to progress prior to or after DH. We found that 21% of the patients with a DH at follow-up (Group 1) showed global progression whereas 39.5% of patients showed global progression after a DH (Group 2). This finding resulted in an insignificant difference between the two groups (p=0.12, 95% CI: -4.5% - 40.7%) although the proportion of progressors in Group 2 were nearly double that of Group 1. Our results also showed no significant differences for RNFL, PPA and rim progression between progressors before and after a DH (p=0.11-0.73). Our findings suggest that a DH is associated with progression but is not a discrete event that precedes progression. This is consistent with the hypothesis that a DH results from an ongoing structural degeneration of the neuroretinal rim, rather than a primary vasculopathic process that leads, de novo, to further marked structural progression.11, 12
To our knowledge, this is the first study to investigate RNFL, PPA and rim structural progression before a DH. In a different study that investigated optic disc characteristics prior to disc hemorrhage, Law et al. reported that focal rim notching, thin sloping rim, PPA presence and superior-inferior rim asymmetry were the characteristics that most antedated DHs and that specifically, focal rim notching may precede the occurrence of a DH at or adjacent to the notch.13 While the study by Law et al. showed that all eyes with pre-existing neural rim notches subsequently had DHs at or adjacent to the notches, our results showed that only 14.3% of patients had global rim progression before a DH. This suggests that the pre-existence of rim notching may lead to subsequent DH without rim progression. The existence of an underlying degenerative process or damaged tissue rather than recent progression may be the contributor to the occurrence of a future DH.
While there have been prior investigations on the evaluation of functional progression prior to and after a DH, to our knowledge, this is the first study to compare the prevalence of structural progression prior to and after a DH. In our investigation of structural glaucomatous progression, we compared our results to the findings of functional glaucoma progression before and after a DH by De Moraes et al.6 While we found no significant difference in structural progression before and after a DH, De Moraes et al. reported that the proportion of visual field progressors after a DH was 79% compared to before the DH at 58% (p=<0.01). This comparison is meaningful in that both structural progression and visual field progression seems to occur commonly before the event of a DH. While De Moraes’ results suggest that a DH may be a discrete event for more aggressive subsequent visual field loss, both studies suggest that in patients with DH, progression may be an ongoing, smoldering process. Indeed, in a study of functional progression after disc hemorrhage by Siegner and Netland, the investigators found that 63% of the eyes in their study showed progressive visual fields after a DH compared to 24% of control eyes.5 In a recent report that investigated the relationship between optic disc progression and rates of visual field, De Moraes et al. also showed that a DH was the single most significant predictor for VF deterioration.14
Our study not only compared progression before and after a DH but also compared clinical characteristics between progressors and non-progressors and found that time between photographs taken was significant. We hypothesize that increasing time between photographs increases sensitivity since with more time patients are more likely to exhibit progression, which occurs as a function of time but may not be clinically observable over short periods of time. Results from our study also demonstrated, interestingly, that RNFL, PPA, and rim progression were not associated with the location of the DH. This is in contrast to Radcliffe et al. who found that DHs tend to occur in the area with the greatest PPA width and are usually found within the region of its greatest width and the finding by Law et al. that focal rim notching may precede DH at or adjacent to the area of notching.13,15 Ahn et al. and Jonas et al. also showed that PPA area and neuroretinal rim area, respectively, predicted laterality of DH.16,17 To our knowledge, our study is the only study to have used flicker chronoscopy in investigating associations between progression and DH location. Syed et al. showed that automated alternation flicker had the highest sensitivity for DH detection compared to side-by-side comparisons and single photograph study, especially for hard to detect DHs.7 Consistent with this, it is possible that the use of flicker chronoscopy resulted in observing more hard to detect structural changes, provided a more robust data set to investigate associations between structural progression and DH location and caused the discrepancy between our results and previous findings on association between structural change and location of DHs.
LIMITATIONS
This study has several limitations. Even though we included 66 sets of photographs with documented DHs, it is possible that the number of patients included in this study was insufficient to determine some differences between the two groups. However, given that each patient had to have been photographed at least one year apart including the day the hemorrhage was identified, which required two separate high quality nerve photographs, this sample represents a reasonable size to evaluate this phenomenon. Our study did not include VF data since obtaining VF data requires five or more reliable, repeatable visual fields and this approach would have further limited the number of patients for this study. Our study was focused on the comparison between structural progression before and after a DH, eliminating the need for including VF data, and furthermore many previous works have evaluated the relationship between VF progression and DH. Excluding controls, this sample includes 132 photographs and represents over 200 years of patient monitoring. As part of our analysis, a power calculation was done. We determined that a two group chi-square test with a 0.05 two-sided significance level will have 80% power to detect the difference between a Group 1 proportion of 21.4% and a Group 2 proportion of 39.5% when the sample sizes are 88 and 120 patients, respectively.
An additional limitation in our study is the possibility that those with a DH would have been seen and examined more frequently, which could account for more progression detected in the DH at baseline group. However, it is also possible that those patients would be treated more aggressively, which would have the opposite effect. Only a prospective study could resolve these potential confounding variables.
This study was also limited by the treatment of a DH as a single event despite the potentially recurring nature of DHs. In the evaluation of IOP reducing treatment on the development of DHs in the EMGT, Bengtsson et al. found that in patients with at least one observed DH, 70% had two or more DHs recorded in clinical forms and 63% had two or more DHs recorded on photographs.4 Given the high frequency of recurring DHs, it is certainly possible that the patients in this study had a DH on days when no examinations or photographs were performed and that patients were misclassified into either Group 1 or 2. Indeed, this may explain why no differences were observed between Groups 1 and 2. The effect of recurrent DHs on functional and structural progression seem inconsistent in that increased frequency of DHs appear to have an effect on structural progression but none on functional progression. De Beaufort et al. reported no significant difference in mean global rate of progression and number of eyes reaching a progression end point between groups with a single DH and recurrent DHs.18 Similarly, Kim and Park demonstrated that patients with recurrent DH had no significant difference in VF progression but had a higher probability of progressive optic disc deterioration, including RNFL deterioration.19 On the other hand, in three case reports of primary-angle closure glaucoma patients with recurrent DHs, Hsieh and Lan describe progressive structural progression by way of enlarged optic disc cupping as well visual field defects in the form of increasing scotomas.20 Studies with larger numbers of patients and long follow-up periods are needed to gain further understanding on the effects of recurrent DHs on functional and structural progression. Notwithstanding this limitation, all patients in this study had photographs of both eyes taken at every visit and they were carefully inspected for a new or recurring DH.
Our methodology of evaluating structural progression by grading alternating optic nerve photographs is subjective versus using OCT which has been reported as a valuable tool for detection of progression.21-24 Sharma et al. report, however, that advantages of subjective assessment include a comprehensive evaluation of the optic nerve head including parameters that cannot be qualified such as disc hemorrhages and pallor.25 Our methodology was an event based technique that served to identify the occurrence of progression and not the rate nor extent of progression. It allowed us to study, for the first time, the structural progression history before a DH. We chose to use flicker chronoscopy as a tool to compare photographs based on findings by Chee et al. who demonstrated that flicker chronoscopy showed good interobserver agreement for global progression (k=0.7) and a low overall rate of incorrect temporal sequence determination (6.8%) whenever structural progression was judged to be present.26 As mentioned earlier, it has also been shown that automated alternation flicker had the highest sensitivity for DH detection compared to side-by-side comparisons and single photograph study especially for hard to detect DHs.7 Our study showed fair interobserver agreement for global progression (k=0.33) which may be due to the graders’ differing experience with using flicker chronoscopy, large number of variables studied or the subjective nature of structural progression.
For clinicians, our study suggests that the detection of a DH does not necessarily warrant more aggressive treatment of the glaucoma out of concern for subsequent structural progression but does warrant continuous vigilant monitoring of patients since structural progression is an ongoing process. Recent longitudinal studies have also shown a significant association between visual field progression and structural progression and thus structural progression needs to be carefully monitored. The temporal sequence between structural and visual field progression, however, needs to be studied in a prospective longitudinal study. 27,28
In conclusion, our study shows that structural glaucomatous progression does occur both before and after a DH. A DH is suggestive of ongoing structural progression in glaucoma but may not be a discrete event that leads to subsequent progression.
Figure 2.
Acknowledgments
Funding: Dr. Paul Christos was partially supported by the following grant: Clinical Translational Science Center (CTSC) (UL1-TR000457-06). Esther Chung was supported by a grant from the Jewish Foundation for the Education of Women.
Footnotes
Competing Interests: NMR: Reichert (Instrument support), Allergan, Inc. (Consultant and Speaker), Alcon Laboratories (C,S), Merge Healthcare (C,S), Carl Zeiss Meditec (C,S), Merck Pharmaceuticals (S), Iridex (C,S).
Contributor Information
Esther Chung, Weill Cornell Medical College, New York, NY, USA.
Anna M. Demetriades, Department of Ophthalmology, Weill Cornell Medical College, New York, NY, USA.
Paul J. Christos, Department of Public Health, Weill Cornell Medical College, New York, NY, USA
REFERENCES
- 1.Cook F, Foster P. Epidemiology of glaucoma: what’s new? Can J Ophthalmol. 2012 Jun;47(3):223–226. doi: 10.1016/j.jcjo.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Budenz DL, Anderson DR, Feur WJ, et al. Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology. 2006 Dec;113(12):2137–2143. doi: 10.1016/j.ophtha.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bengtsson B, Leske MC, Yang Z, et al. Disc hemorrhages and treatment in the early manifest glaucoma trial. Ophthalmology. 2008 Nov;115(11):2044–8. doi: 10.1016/j.ophtha.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Leske C, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007 Nov;114(11):1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Siegner SW, Netland PA. Optic disc hemorrhages and progression of glaucoma. Ophthalmology. 1996 Jul;103(7):1014–24. doi: 10.1016/s0161-6420(96)30572-1. [DOI] [PubMed] [Google Scholar]
- 6.De Moraes CG, Prata TS, Liebmann CA, et al. Spatially consistent, localized visual field loss before and after disc hemorrhage. Invest Ophthalmol Vis Sci. 2009 Oct;50(10):4727–4733. doi: 10.1167/iovs.09-3446. [DOI] [PubMed] [Google Scholar]
- 7.Syed ZA, Radcliffe NM, De Moraes CG, et al. Automated alternation flicker for the detection of optic disc haemorrhages. Acta Ophthalmol. 2012 Nov;90(7):645–650. doi: 10.1111/j.1755-3768.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- 8.Radcliffe NM, Sehi M, Wallace IB, et al. Comparison of stereo disc photographs and alternation flicker using a novel matching technology for detecting glaucoma progression. Ophthalmic Surg Lasers Imaging. 2010 Nov-Dec;41(6):629–34. doi: 10.3928/15428877-20100830-02. [DOI] [PubMed] [Google Scholar]
- 9.VanderBeek BL, Smith SD, Radcliffe NM. Comparing the detection and agreement of parapapillary atrophy progression using digital optic disk photographs and alternation flicker. Graefes Arch Clin Exp Ophthalmol. 2010 Sep;248(9):1313–1317. doi: 10.1007/s00417-010-1376-z. [DOI] [PubMed] [Google Scholar]
- 10.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005 May;37(5):360–3. [PubMed] [Google Scholar]
- 11.Begg IS, Drance SM, Sweeney VP. Ischaemic optic neuropathy in chronic simple glaucoma. Br J Ophthalmol. 1971 Feb;55(2):73–90. doi: 10.1136/bjo.55.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ssonjo B, Krakau CE. Arguments for a vascular etiology. Acta Ophthalmol (Copenh) 1993 Aug;71(4):433–444. doi: 10.1111/j.1755-3768.1993.tb04615.x. [DOI] [PubMed] [Google Scholar]
- 13.Law SK, Choe R, Caprioli J. Optic disk characteristics before the occurrence of disk hemorrhage in glaucoma patients. Am J Ophthalmol. 2001 Sep;132(3):411–413. doi: 10.1016/s0002-9394(01)01009-1. [DOI] [PubMed] [Google Scholar]
- 14.De Moraes CG, Liebmann JM, Park SC, et al. Optic disc progression and rates of visual field change in treated glaucoma. Acta Ophthalmol. 2013 Mar;91(2):e86–91. doi: 10.1111/j.1755-3768.2012.02577.x. [DOI] [PubMed] [Google Scholar]
- 15.Radcliffe NM, Leibmann JM, Rozenaum I, et al. Anatomic relationships between disc hemorrhage and parapapillary atrophy. Am J Ophthalmol. 2008 Nov;146(5):735–740. doi: 10.1016/j.ajo.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Ahn JK, Kang JK, Park KH. Correlation between a disc hemorrhage and peripapillary atrophy in glaucoma patients with a unilateral disc hemorrhage. J Glaucoma. 2004 Feb;13:9–14. doi: 10.1097/00061198-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Jonas JB, Martus P, Budde WM, et al. Morphologic predictive factors for development of optic disk hemorrhages in glaucoma. Invest Ophthalmol Vis Sci. 2002;43(9):2956–2961. [PubMed] [Google Scholar]
- 18.De Beaufort HC, De Moraes CG, Teng CC, et al. Recurrent disc hemorrhage does not increase the rate of visual field progression. Graefes Arch Clin Exp Ophthalmol. 2010 Jun;248(6):839–44. doi: 10.1007/s00417-010-1306-0. [DOI] [PubMed] [Google Scholar]
- 19.Kim SH, Park KH. The relationship between recurrent optic disc hemorrhage and glaucoma progression. Ophthalmology. 2006 Apr;113(4):598–602. doi: 10.1016/j.ophtha.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh JW, Lan YW. Progression of optic neuropathy after disc hemorrhage in primary angle-closure glaucoma. Jpn J Ophthalmol. 2009 Jul;53(4):380–3. doi: 10.1007/s10384-009-0676-5. [DOI] [PubMed] [Google Scholar]
- 21.Bussel II, Wollstein G, Schuman JS. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol Published Online First: Dec 19; doi: 10.1136/bjophthalmol-2013-304326. doi:10.1136/bjophthalmol-2013-304326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Na JH, Sung KR, Lee JR, et al. Detection of glaucomatous progression by spectral-domain optical coherence tomography. Ophthalmology. 2013 Jul;120(7):1388–95. doi: 10.1016/j.ophtha.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Wollstein G, Schuman JS, Price LL, et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol. 2005 Apr;123(4):464–470. doi: 10.1001/archopht.123.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medeiros FA, Zangwill LM, Alencar LM, et al. Detection of glaucoma progression with Stratus OCT retinal nerve fiber layer, optic nerve head, and macular thickness measurements. Invest Ophthalmol Vis Sci. 2009 Dec;50(12):5741–5748. doi: 10.1167/iovs.09-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma P, Sample PA, Zangwill LM, et al. Diagnostic tools for glaucoma detection and management. Surv Ophthalmol. 2008 Nov;53(SUPPL1):S17–S32. doi: 10.1016/j.survophthal.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chee R, Silva FQ, Ehrlich JR, et al. Agreement of flicker chronoscopy for structural glaucomatous progression detection and factors association with progression. Am J of Ophthalmol. 2013 Jun;155(6):983–990. doi: 10.1016/j.ajo.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Chauhan BC, Nicolela MT, Artes PH. Incidence and rates of visual field progression after longitudinally measured optic disc change in glaucoma. Ophthalmology. 2009 Nov;116(11):2110–2118. doi: 10.1016/j.ophtha.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 28.Medeiros FA, Alencar LM, Zangwill LM, et al. Prediction of functional loss in glaucoma from progressive optic disc damage. Arch Ophthalmol. 2009 Oct;127(10):1250–1256. doi: 10.1001/archophthalmol.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]