Summary
Candida albicans is a dimorphic fungus responsible for chronic mucocutaneous and systemic infections. Mucocutaneous immunity to C. albicans requires T helper-17 (Th17) cell differentiation that is thought to depend on recognition of filamentous C. albicans. Systemic immunity is considered T cell independent. Using a murine skin infection model, we compared T helper cell responses to yeast and filamentous C. albicans, We found that only yeast induced Th17 cell responses through a mechanism that required Dectin-1 mediated expression of interleukin-6 (IL-6) by Langerhans cells. Filamentous forms induced Th1 without Th17 cell responses due to the absence of Dectin-1 ligation. Notably, Th17 cell responses provided protection against cutaneous infection while Th1 cell responses provided protection against systemic infection. Thus, C. albicans morphology drives distinct T helper cell responses that provide tissue specific protection. These findings provide insight into compartmentalization of Th responses, C. albicans pathogenesis and have critical implications for vaccine strategies.
Introduction
Candida albicans is a human commensal and a common mucosal and systemic pathogen in the setting of immunosuppression. Chronic mucocutaneous candidiasis (CMC) occurs in the absence of innate sources of interleukin-17 (IL-17) and IL-22 as well as in the setting of ineffective T helper-17 (Th17) cell-type immunity in both mice and in patients (e.g. HIV/AIDS or hyper IgE syndrome)(Hernández-Santos et al., 2013; McDonald, 2012; Murray et al., 1985). CMC is also associated with mutations in the fungal recognition C-type lectin receptor Dectin-1 or its down stream signaling molecules(Ferwerda et al., 2009; Glocker et al., 2009). In contrast, systemic candidiasis predominantly occurs in the clinical setting of neutropenia and in mice with innate immune defects (e.g. Il6−/− mice) resulting in ineffective neutrophil activation but does not occur in the absence of Th17 cell-associated or adaptive immunity(Bär et al., 2014; Horn et al., 2009; van de Veerdonk et al., 2010; Van Enckevort et al., 1999).
C. albicans is dimorphic and grows as yeast at 30°C and as a filamentous or hyphal form at 37°C. On the stratum corneum of the skin, C. albicans exist as budding yeasts. Pathogenic C. albicans in the dermis and systemic organs exists predominantly as hyphae(Gow et al., 2012). C. albicans mutants that are unable to form filaments, fail to establish robust infections suggesting that the yeast to hyphal transition is required for virulence(Lo et al., 1997). This transition has also been suggested to be required for the development of anti-Candida Th17 cell responses thereby allowing for discrimination between commensal and invasive C. albicans(Gow et al., 2012). In vitro, C. albicans hyphae induce Dectin-1 mediated Th17 cell differentiation(Cheng et al., 2011). C. albicans hyphae, however, have also been reported to promote Th1 and Th2 cell differentiation in vitro(d'Ostiani et al., 2000; van der Graaf et al., 2005). Importantly, T helper (Th) cell responses in vivo to yeast and filamentous forms of C. albicans and the ability of these adaptive responses to provide protection remain unclear.
In the skin, there are at least three well defined subsets of dendritic cells (DC) – epidermal Langerhans cells (LC), CD103+ dermal DC (dDC) and CD11b+ dDC(Kaplan, 2010). These DC subsets migrate from the skin into regional lymph nodes during infection where they present antigen to naïve T cells and secrete cytokines that determine Th cell differentiation(Reis e Sousa, 2004). During epicutaneous infection with C. albicans, CD103+ dDC generate IL-12 and are required for differentiation of Th1 cells (Igyártó et al., 2011). LC are required for Th17 cell differentiation and express high amounts of Th17 cell inducing cytokines IL-1β, transforming growth factor-β (TGF-β) and IL-6. The importance of individual cytokines in generating Th17 cells in vivo is controversial and varies by tissue. It has been demonstrated that IL-1β and IL-6 are both necessary for Th17 cell differentiation in peripheral tissues including skin (Hu et al., 2011). Th17 cell induction in the spleen is independent of IL-6 but not IL-1β. In the intestines, several studies have confirmed an IL-1β dependence for Th17 cell development while the requirement for IL-6 remains controversial (Hu et al., 2011; Persson et al., 2013; Shaw et al., 2012).
CD11b+ dDC make up the majority of the skin migratory DC and drive Th2 cell differentiation in the setting of dermal papain injection or parasitic infection (Gao et al., 2013; Kumamoto et al., 2013). In the setting of C. albicans infection, this DC subset generates high amounts of IL-1β and to a lesser extent IL-6 and IL-12 (Igyártó et al., 2011). Their ability, however, to promote Th cell differentiation other than Th2 cell differentiation remains unexplored (Kumamoto et al., 2013). Based on the functional differences between skin DC subsets and the transition of C. albicans from yeast to filamentous forms during epidermal invasion, we hypothesized that DC subsets and C. albicans morphology together determine Th cell differentiation.
Because of the requirement for immunosuppression to establish robust and consistent oropharyngeal C. albicans infection, we have focused on epicutaneous C. albicans infection. We found that infection with C. albicans yeast but not hyphae was capable of inducing Th17 cell responses through a mechanism that required interaction with LC, engagement of Dectin-1 and LC-derived IL-6. C. albicans in the dermis failed to induce Th17 cell differentiation despite the expression of Dectin-1 on CD11b+ dDC due to the absence of Dectin-1 ligation by hyphae that are the dominant morphology at that site. Finally, we demonstrated that Th17 but not Th1 cells were protective against secondary cutaneous infections while Th1 but not Th17 cells were protective against secondary systemic infections. Thus, C. albicans morphology and skin DC subsets drive distinct Th cell responses that provide protection from either cutaneous or systemic infections.
Results
Distinct T helper cell responses mediated by C. albicans morphology
We have previously generated recombinant C. albicans derived from the standard SC5314 strain that expresses the peptides 2W1S and Eα, under the ubiquitous Eno1 promoter (Eno1-Ag). Using a TEα CD4+ T cell adoptive transfer system, we showed that mice that express Diptheria toxin fragment A (DTA) under a human Langerin (huLangerin) promoter with a constitutive absence of LC (henceforth referred to as LC−) failed to generate antigen-specific Th17 cells (Igyártó et al., 2011). To exclude the possibility that this phenotype resulted from a chronic absence of LC, we repeated these experiments using mice in which express the Diptheria toxin receptor (DTR) within huLangerin (referred to has huLangerin-DTR mice) that allow for inducible ablation of LC. As expected, ablation of LC just prior to Eno1-Ag infection also prevented efficient Th17 cell differentiation (Figure S1A). We next examined the responses of endogenous antigen specific CD4+ T cells to C. albicans using I-Ab:p2W1S tetramer staining 8 days post infection. Expansion of p2W1S-specific cells was equivalent in control and LC deficient mice (Figure 1A). Expansion of cells producing IL-17A but not interferon-γ (IFN-γ), however, was significantly reduced in LC deficient mice (Figure 1B). Thus, consistent with our earlier observations using adoptive transfer of T cell receptor (TCR) transgenic T cells specific for Eα (TEα), LC are required for efficient differentiation of endogenous naïve CD4+ T cells into Th17 cells.
Figure 1. Candida albicans yeast drives Th17 cell differentiation in vivo.
(A) The number of 2W1S-specific CD4+ T cells in six skin draining lymph nodes (axillary, brachial, inguinal) and spleen 8 days after mock (Neg) or skin infection with Eno1-Ag in wildtype (WT) or LC deficient mice (LC− mice is shown. (B) Percentage of PMA and Ionomycin stimulated 2W1S-specific cells in WT (white) or LC− (black) mice expressing the indicated cytokines. (C) Antigen was targeted to huLangerin transgenic mice by i.p injection of 1.0 μg 2G3-Eα. Adjuvant was provided by skin infection with wild-type, cph1/cph1, or tup1/tup1 strains of C. albicans. TEα numbers were assessed in the skin draining lymph nodes of mice 4 days after infection. Neg refers to unimmunized mice. (D) The expression of IFN-γ and IL-17A in PMA + Ionomycin stimulated Eα-specific CD4+ TEα cells from (C) is shown. (E) GFP expression in Eno1-Ag or Hwp1-Ag C. albicans under yeast or filamentous growth conditions was determined by immunofluorescence. (F) TEα expansion and (G) cytokines production 4 days after infection of WT mice with Eno1-Ag (white) or Hwp1-Ag (black) or naïve (Neg). Each symbol represents data from an individual animal. Scales represents mean ± SEM. *p<0.05, **p<0.01, ***p<0.001.Data is representative of two to three independent experiments. See also Figure S1 and S2.
To determine whether recognition by LC of yeast or filamentous forms of C. albicans affects Th cell differentiation, we selectively targeted antigen to LC by i.p. injection of 1.0μg anti-huLangerin mAb-Eα conjugates (2G3-Eα) in human Langerin transgenic mice (huLangerin) (Figure S1B)(Bobr et al., 2010; Flamar et al., 2012; Igyártó et al., 2011; Kaplan et al., 2012). HuLangerin is expressed exclusively by LC in these mice resulting in efficient and LC-selective acquisition of 2G3-Eα. As a source of adjuvant, we epicutaneously infected 2G3-Eα targeted huLangerin mice with wild-type SC5314 as well as cph/cph and tup1/tup1 mutants derived from SC5314 that are locked into yeast or hyphal forms, respectively(Braun, 1997; Liu et al., 1994). Expansion of adoptively transferred TEα cells was equivalent in mice infected with all 3 strains (Figure 1C). Notably, Th17 cell differentiation was intact in the mice infected with the yeast-locked cph1 mutant but was absent in those infected with the filament locked tup1 mutant suggesting that LC-mediated Th17 cell differentiation requires the presence of C. albicans yeast forms (Figure 1D).
To determine the effect of C. albicans morphology under conditions in which antigen presentation is not limited to LC, we next generated recombinant C. albicans in which expression of a fusion protein of GFP with the antigens Eα and 2W1S was driven by the promoter for the hyphae specific gene Hwp1 (Hwp1-Ag)(Staab, 1999). Unlike Eno1-Ag where GFP expression was present in all C. albicans morphologies, expression of GFP by Hwp1-Ag was absent in yeast and observed only for filamentous forms (Figure 1E and S2A-E). Both Eno1-Ag and Hwp1- Ag strains demonstrated similar virulence in vivo after epicutaneous or intravenous infection (Figure S2F-G). Epicutaneous infection of WT mice with Eno1-Ag and Hwp1-Ag induced similar antigen specific CD4+ T cells expansion and Th1 cell differentiation (Figure 1F). Notably, mice infected with Hwp1-Ag failed to efficiently induce Th17 cell-associated responses (Figure 1G). Thus, LC and C. albicans yeast but not hyphae are required for optimal Th17 cell differentiation.
Th17 cell differentiation requires LC derived IL-6
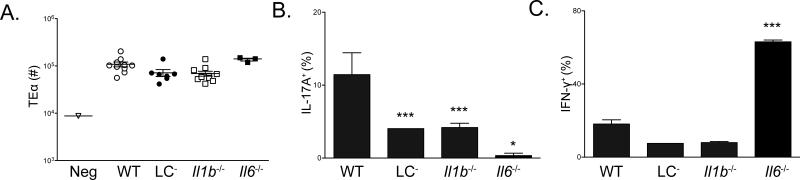
In vitro, Th17 cell differentiation of naïve cells requires combinations of IL-1β, IL-6, IL-23 and TGF-β (Chung et al., 2009; Ivanov et al., 2006; Zúñiga et al., 2013). The requirement for IL-1β and IL-6 in vivo differs based on the site of immunization(Hu et al., 2011; Shaw et al., 2012). To determine which cytokines are responsible for LC-mediated Th17 cell induction, we first compared responses in IL-1β and IL-6 deficient mice. We found that both IL-1β and IL-6 deficiency resulted in diminished Th17 cell responses (Figure 2A-B). Notably, Th17 cells were entirely absent in Il6−/− mice and was associated with a reciprocal increase in Th1 cells(Figure 2B-C).
Figure 2. IL-1β and IL-6 are required for Th17 cell differentiation during C. albicans skin infection.
(A) Expansion of TEα cells isolated from skin draining lymph nodes 4 days after skin infection of the indicated mouse strains with Eno1-Ag C. albicans. Each symbol represents results from an individual animal. (B) The percent of ex vivo PMA and Ionomycin stimulated TEα cells in cutaneous lymph nodes that express IL-17A or (C) IFN-γ as determined intracellular flow cytometry is shown. Neg refers to mock infected WT mice. Scales represents mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. Data is representative of at least three independent experiments.
Since expression of IL-1β, TGF-β, and IL-6 was increased in LC during C. albicans infection(Igyártó et al., 2011), we next sought to determine which cytokines were required for Th17 cell differentiation. To generate LC-specific ablation of TGF-β1, we used huLangerin-creER-T2 Tgfb1f/f mice that efficiently and selectively excise TGF-β1 in LC after administration of tamoxifen(Bobr et al., 2012). We have previously reported that ablation of TGF-β1 in LC results in a gradual spontaneous migration of LC out of the epidermis over the course of weeks. To avoid this potential confounding factor, we infected mice on day 5 after tamoxifen administration, a time point when TGF-β has been efficiently excised but LCs have not begun to migrate in large numbers. As controls, we also infected tamoxifen treated huLangerin-creER-T2 YFP and huLangerin-creER-T2 Tgfbr2f/f mice which maintain TGF-β expression but also have increased homeostatic migration(Bobr et al., 2012). Antigen specific T cell expansion and Th cell phenotype differentiation was equivalent in all 3 strains demonstrating that LC-derived TGF-β is not required for Th17 cell differentiation (Figure 3A-B).
Figure 3. LC derived IL-6, but not TGF-β or IL-1β is required for Th17 cell differentiation.
(A) huLangerin-creERT2 Tgfb1f/f mice were treated with tamoxifen to generate LC-specific ablation of TGF-β1. These mice were skin infected with Eno1-Ag C. albicans along with tamoxifen treated huLangerin-creERT2 Tgfbr2f//f and YFP controls. Expansion of TEα at day 4 along with (B) cytokines expression is shown. (C) Expansion and (D) cytokine production by TEα cells in Eno1-Ag infected WT→WT (white) or WT→Il1b−/− (black) bone marrow chimeric mice is shown. (E) The expansion, (F) expression of IL-17A by TEα cells and (G) percent of TEα cells producing the indicated cytokines in WT→WT (white) or WT→Il6−/− (black) mice is shown. (H) Cytokine production by TEα cells in WT→WT (white) or Il6−/− WT (black) mice is shown. Neg refers to mock infected WT mice. Scales represents mean ± SEM. *P<0.05, **P<0.01, ***P<0.001. Data is representative of at least three independent experiments. See also Figure S3.
To generate LC-specific ablation of IL-1β and IL-6, we generated WT→Il1b−/− and WT→Il6−/− bone marrow chimeras. LC are the only radio-resistant antigen presenting cell (APC) in the skin and remained of cytokine deficient host origin while the hematopoietic system achieved 90-96% chimerism (Figure S3A-C). Infected WT→Il1b−/− chimeras developed cell proliferation and Th differentiation similar to control WT→WT mice indicating that LC-derived IL-1β is not required for Th17 induction (Figure 3C-D). In contrast, Th17 cell differentiation was reduced in WT→Il6−/− chimeras indicating that LC-derived IL-6 is required for Th17 (Figure 3E-F). Moreover, Th17 cell differentiation was intact in Il6−/− WT mice in which LC were the only IL-6 sufficient hematopoietic cell type (Figure 3H). Thus, IL-6 derived from LC is necessary for Th17 cell induction during skin infection.
LC-derived IL-6 requires Dectin-1 engagement
A subset of patients with chronic mucocutaneous candidiasis (CMC) have mutations in the transcription factor STAT3, that acts downstream of the IL-6 receptor (McDonald, 2012). Patients with Dectin-1 polymorphisms also suffer from CMC and have decreased IL-6 production by their peripheral blood mononuclear cells (PBMCs)(Ferwerda et al., 2009). In addition, budding yeast engages Dectin-1 while yeast but not hyphae trigger IL-6 secretion in human PBMCs(Armitage et al., 2011). Since Th17 cell differentiation depends on LC-derived IL-6, we next sought to determine whether Th17 cell differentiation required engagement of Dectin-1 on LC. We took advantage of a clinical isolate, s20175.016, that has similar virulence as SC5314 but its immune recognition is independent of Dectin-1 (Figure S4A-B)(Marakalala et al., 2013). Antigen was targeted to LC in huLangerin mice using 2G3-Eα followed by infection with SC5314 or s20175.016. As expected, CD4+ T cell expansion and Th1 cell differentiation were comparable between the two experimental groups but Th17 cell differentiation was reduced in mice infected with s20175.016 (Figure 4A-B). Analysis of LC isolated from the skin draining lymph nodes 3 days after infection revealed a large reduction in IL-6 production in mice infected with s20175.016 compared with SC5314 (Figure 4C and S5). Moreover, intradermal injection of 1μg recombinant IL-6 at the time of infection restored Th17 cell differentiation (Figure 4D). Finally, we directly explored the necessity of Dectin-1 signaling by comparing the host response to C. albicans infection in Dectin-1 deficient (Clec7a−/− ) mice. While WT and Clec7a−/− mice strains exhibited similar T cell expansion, Dectin-1 deficient host demonstrated diminished Th17 cell responses (Figure 4E-F). Thus Th17 cell differentiation requires engagement of Dectin-1 on LC that results in increased expression of IL-6.
Figure 4. Dectin-1 engagement on LC leads to IL-6 production and Th17 cell differentiation.
Antigen was directed to LC in huLangerin transgenic by i.p. injection of 1.0 μg 2G3-Eα followed by skin infection with SC5314 (white) or s20175.016 (black) strains of C. albicans. (A) TEα expansion and (B) cytokine production by TEα cells is shown. (C) LC were isolated from the skin draining lymph nodes by flow cytometry sorting on day 3 after skin infection with vehicle (white), SC5314 (gray) or s20175.016 (black). Expression of the indicated cytokines was determined by qPCR and is shown normalized to uninfected mice. (D) Antigen was targeted to LC (as in (A)) followed by infection with SC5314 or S20175.016. At the time of infection, PBS or 1.0 μg of rIL-6 was injected at the site of infection. CSFE dilution of TEα cells as well as expression of IL-17A is shown. (E) Expansion of TEα cells in the skin draining lymph nodes of WT and Dectin-1 deficient mice 4 days after infection with Eno1-Ag. (F) CSFE dilution and expression of IL-17A in PMA and Ionomycin stimulated TEα cells isolated from WT and Dectin-1 deficient mice on day 4 after infection. Neg refers to mock infected WT mice. Scales represents mean ± SEM. *P<0.05, **P<0.01, ***P<0.001. Data is representative of at least two independent experiments. See also Figure S4 and S5
Absence of Dectin-1 ligands prevent Th17 cell induction by dermal DC
To examine the function of dermal DC subsets in the induction of Th17 cell responses, we first compared expression of Dectin-1 by each subset. Dectin-1 is expressed by LC and CD11b+ dDC but not by CD103+ dDC (Figure 5A). The absence of Dectin-1 on CD103+ dDC is consistent with our previous observation that this DC subset was required for Th1 but not Th17 cell induction(Igyártó et al., 2011).
Figure 5. CD11b+ Dermal DC are not required for Th17 cell differentiation.
(A) Surface expression of Dectin-1 by the indicated skin DC subset isolated from the skin under steady-state conditions is shown. (B) Mgl2-DTR→WT chimeric mice were treated with vehicle (white circles) or diphtheria toxin (black circles) and skin infected with Eno1-Ag 1 day later. The expansion of TEα cells isolated from skin draining lymph nodes 4 days after infection is shown. “Neg” represents mock infected WT mice (triangles). (C) Expression of the indicated cytokines from PMA and Ionomycin TEa cells in (B) is shown. (D) Expansion and (E) cytokine production by TEα cells in WT (white) or LC−xBatf3−/− (black) mice 4 days after infection with Eno1-Ag is shown. Scales represents mean ± SEM. *P<0.05, **P<0.01, ***P<0.001. Data is representative of at least three independent experiments. See also Figure S6.
Since Th17 cell differentiation is reduced but not ablated in the absence of LC and since CD11b+ DC in the lung are required for Th17 cell responses during Aspergillus fumigatus infection(Schlitzer et al., 2013), we hypothesized that CD11b+ dDC could also contribute to Th17 cell during skin infection. Macrophage galactose C-type lectin type 2 (Mgl2) is expressed by most CD11b+ dDC. Administration of diphtheria toxin (DT) to the recently reported Mgl2-DTR mice results in ablation of CD11b+ dDC and LC (Figure S6A-C) (Kumamoto et al., 2013). DT treated Mgl2-DTR mice have reduced Th17 cell differentiation that could result from deletion of either subset (Figure S6D). To selectively ablate CD11b+ dDC, we generated Mgl2-DTR→WT chimeric mice (Figure S6C). Notably, antigen-specific T cells expansion and Th phenotype was unaltered in DT treated Mgl2-DTR→WT mice infected with Eno1-Ag indicating that CD11b+ dDC are not required for Th17 cell differentiation (Figure 5B-C). To determine whether this DC subset could be sufficient for Th17 cell induction, we generated LC−x Batf3−/− mice that have a genetic absence of both LC and CD103+ dDC. Eno1-Ag infection of these mice in which CD11b+ dDC are the dominant skin-resident DC subset demonstrated defective Th1 and Th17 cell differentiation (Figure 5D-E) that is consistent with the absence of Th17 cells generated by LC and Th1 cells induced by CD103+ dDC. Thus, CD11b+ dDC are neither necessary nor sufficient for Th17 cell generation during epicutaneous infection with C. albicans.
Despite the equivalent degree of T cells expansion in LC−xBatf3−/− mice, we considered the possibility that CD11b+ dDC have limited access to antigen during an epicutaneous infection. In wild-type mice, we compared epicutaneous with dermal infection using the same inoculum of Eno1-Ag. Though T cell expansion and Th1 cell differentiation was similar, Th17 cell differentiation was greatly reduced in response to intradermal infection (Figure 6A-B). Induction of Th17 cell was also absent in response to an i.v. systemic infection (Figure S7A-B). Notably, addition of the Dectin-1 ligand, curdlan, with the intradermal inoculation rescued the Th17 cell response suggesting that Dectin-1 ligation does not occur in the dermis during C. albicans infection but that CD11b+ dDC do have the capacity to induce Th17 cells(Figure 6C). During epicutaneous C. albicans infection, yeast are found on the epidermis and invasive filamentous forms in the dermis (Figure 6D)(Gow et al., 2012). To test whether hyphal forms of C. albicans are unable to induce Th17 cell due to the absence of Dectin-1 ligands, we infected mice both epicutaneously with Hwp1-Ag with the addition of exogenous curdlan or vehicle (Figure 6E). The addition of Dectin-1 ligands rescued the ability of antigen expressed by hyphal forms of C. albicans to generate Th17 cell responses.
Figure 6. Dermal infection and C. albicans hyphae do not induce Th17 cells without exogenous Dectin-1 ligands.
(A and B) WT mice were infected with an equivalent inoculum of Eno1-Ag C. albicans on the epidermis (white) or by intradermal injection (black). Expansion and cytokine production by PMA and Ionomycin stimulated TEα cells isolated from skin draining lymph nodes is shown. (C) As in (A), WT mice were infected epicutaneously or by dermal injection into which purified curdlan or vehicle has been added to the inoculum. CSFE dilution and expression of IL-17A by PMA and Ionomycin stimulated TEα cells is shown. (D) PAS stain of skin 2 days after C. albicans infection showing budding yeast (asterisk) restricted to the epidermis and numerous penetrating hyphae in the dermis (arrowhead) (bar=150uM). (E) The expression of IL-17A and IFNγ by PMA and Ionomycin stimulated TEα cells cells 4 days after epicutaneous infection of WT mice with Eno1-Ag or Hwp1-Ag is shown. Curdlan or vehicle alone was provided by dermal injection at the time of infection. “Neg” represents mock infected WT mice. Scales represents mean ± SEM. *P<0.05, **P<0.01, ***P<0.001. Data is representative of at least three independent experiments. See also Figure S7.
Compartmentalization of Th cell responses
The ability of C. albicans to induce Th17 cell in response to an epidermal infection and Th1 cells in response to an invasive infection raises the possibility that specific Th cell phenotypes could provide protection at distinct anatomical sites. To test this hypothesis, we epicutaneously infected WT, Il6−/− and Batf3−/− mice with Eno1-Ag. As expected, Il6−/− mice generated a robust Th1 cell response in the absence of Th17 cell and Batf3−/− mice developed a strong Th17 cell response in the absence of Th1 cells(Figure 7A). Separate cohorts of mice were re-challenged by skin infection 9 days after spontaneous clearance of the primary infection. The number of colony forming units (CFU) found in naïve mice challenged with SC5314 was similar in all 3 groups (open symbol) (Figure 7B). Notably, though IL-6 is an innate cytokine important for resistance to systemic C. albicans infection, naive Il6−/− mice did not develop an exaggerated cutaneous infection(Van Enckevort et al., 1999). Secondarily infected (closed symbols) WT mice had an approximate 10 fold increase in fungal resistance. In contrast, Batf3−/− mice had approximately 100 fold increase in pathogen resistance and Il6−/− mice showed no increased resistance to re-infection compared to naïve mice (Figure 7B). To test whether this was a CD4+ T cell dependent process, we purified CD4+ T cells from Il6−/− and Batf3−/− mice infected 7 days earlier and adoptively transferred them into naïve hosts that were then skin infected. Naïve recipients of CD4+ T cells from Batf3−/− but not Il6−/− mice were protected against a cutaneous infection. Thus, the Th17 cells that arise in response to a cutaneous C. albicans infection provide protection against subsequent skin challenges (Figure 7C).
Figure 7. Th1 and Th17 cells provide compartmentalized protection to C. albicans.
WT, Il6−/− and Batf3−/− mice were epicutaneously infected with Eno1-Ag. (A) The expression of IL-17A and IFN-γ by PMA and Ionomycin stimulated CD4+ TEα cells isolated from skin draining lymph nodes 4 days after infection is shown. (B) The indicated strains of mice were mock infected (white) or skin infected with SC5314. Mice were re-challenged in the skin with SC5314 9 days after spontaneous clearance of the initial infection. Fungal skin burdens of individual mice is shown as CFU/cm2. (C) CD4+ T cells were purified from skin draining LN and spleen of naïve WT, SC5314 infected Il6−/− or SC5314 infected Batf3−/− mice 7 days after infection and adoptively transferred into into naïve WT recipients. Recipients were skin infected with SC5314 1 day after transfer and CFU was assessed at the infected area 2 days later. (D) As in (B) except that mice were challenged by i.v. infection with 105 SC5314. (E) As in (C) except that mice were challenged by i.v. infection with 105 SC5314. Fungal burdens in the kidneys expressed as CFU/g is shown. Scales represents mean ± SEM. *P<0.05, **P<0.01, ***P<0.001.
In contrast, systemic challenge of previously skin infected mice by i.v. inoculation revealed protection in WT and Il6−/− mice but not in Batf3−/− mice (Figure 7D). Similarly, adoptive transfer of CD4+ T cells from skin infected Il6−/− mice but not Batf3−/− mice into naïve recipients conferred protection against systemic candidiasis (Figure 7E). Thus, mice with expanded numbers of Th17 cell effectors were more resistant to cutaneous C. albicans challenge while mice with expanded numbers of Th1 cells were more resistant to systemic challenge. We conclude that Th cell effector cells appear to be functionally compartmentalized with Th17 cells providing skin protection and Th1 cells providing systemic protection.
Discussion
Herein we have demonstrated that the differentiation of Th17 cells in response to C. albicans skin infection occurs during infection with C. albicans yeast but not hyphae. Th17 cell induction required the engagement of Dectin-1 and subsequent expression of IL-6 by Langerhans cells. CD11b+ dDC expressed Dectin-1 but were neither necessary nor sufficient for Th17 cell responses. The inability of C. albicans in the dermis to induce Th17 cells resulted from a morphologic change from yeast to hyphae that occurred during invasion and could be rescued by the addition of exogenous Dectin-1 ligand. Finally, we found that Th17 cell expansion provided protection against cutaneous but not systemic challenge while the Th1 cell expansion provided protection against systemic but not cutaneous challenge.
The ability of C. albicans morphology to dictate immune responses has been an area of considerable controversy. Our data using mutants that are locked into yeast (chp1/cph1) or hyphae (tup1/tup1) as well as a recombinant C. albicans strain in which model antigens are expressed exclusively by hyphae (Hwp1-Ag) demonstrate that, in vivo, C. albicans yeast but not hyphae induce Th17 cell differentiation. This is consistent with the finding that the ligand for Dectin-1 is largely accessible in the scars or patches of budding yeasts (Cabib and Bowers, 1971; Gantner et al., 2005; Gow et al., 2012). Using in vitro methods, others have found that C. albicans hyphae can induce Dectin-1 mediated Th17 cell differentiation through an IL-1β-dependent mechanism as well as induce Th2 or Tr1 cell responses (Cheng et al., 2011; d'Ostiani et al., 2000). These data can be reconciled by the differing availability in vitro vs. in vivo of Dectin-1 ligands on morphologic forms of C. albicans (Marakalala et al., 2013).
Differentiation of Th17 requires TGF-β as well an IL-1β and/or IL-6 depending on the site of immunization (Hu et al., 2011). Using total cytokine deficient mice, we found that Th17 cell development during skin infection is dependent on both IL-1β and IL-6. By generating mice with LC selective defects of TGF-β1, IL-1β, and IL-6 we found that LC were a non-redundant source of only IL-6. Though LC-derived IL-1β and TGF-β were not required, there are many other cellular sources of these cytokines that could be either be the primary source during infection or provide functionally sufficient quantities in the absence of LC.
Notably, specific ablation of MyD88 in LC resulted in reduced expression of IL-6 and defective Th17 differentiation during C. albicans infection suggesting that IL-1β could potentiate IL-6 expression by LC (Haley et al., 2012). It is important to note that Dectin-1 also cooperates with toll-like receptor-2 (TLR-2) and TLR-4 to mount pro-inflammtory responses(Ferwerda et al., 2008; Gantner et al., 2003). In addition to any possible role for IL-1β, we found that engagement of Dectin-1 on LC was required for optimal IL-6 expression. Infection with a Dectin-1 independent strain induced only low expression of IL-6 by LC that failed to induce Th17 cell differentiation. Addition of exogenous IL-6 was able to rescue the Th17 cell phenotype. Similarly, patients with Dectin-1 deficiency suffer from CMC and have defective IL-6 production in response to C. albicans (Ferwerda et al., 2009). Thus, we have defined a model in which interaction of LC with C. albicans yeast results in a Dectin-1 dependent expression of IL-6 that is required for Th17 cell differentiation during skin infection.
In the dermis, Dectin-1 is expressed by CD11b+ dDC but not CD103+ dDC. The absence of Dectin-1 on CD103+ dDC is consistent with our prior observations that these cells were required for induction of Th1 cell differentiation. Despite expressing Dectin-1, CD11b+ dDC were neither necessary nor sufficient for optimal induction of Th17 cell responses to either an epicutaneous or dermal infection with C. albicans. Importantly, during a dermal infection or an epicutaneous infection with Hwp1-Ag, situations in which antigen bypasses LC in the epidermis, efficient Th17 cell differentiation was restored by addition of the exogenous Dectin-1 ligand, curdlan. CD11b+ dDC have been reported to drive Th2 responses to papain immunization and nippostrongylus infection(Gao et al., 2013; Kumamoto et al., 2013). Our data suggests that in addition to Th2 cells, these cells may also have the capacity to induce Th17 cell differentiation given the appropriate adjuvant. Indeed, similar IRF4+CD11b+ DCs can produce Th17 cell responses in other tissues(Persson et al., 2013; Schlitzer et al., 2013). Thus, the inability of CD11b+ dDC to induce Th17 cells is not intrinsic to CD11b+ dDC but results from the morphologic change of C. albicans into hyphae as it invades into the dermis and likely represents an adaption of C. albicans to evade immune responses.
Humans are chronically exposed to C. albicans and circulating anti-Candida CD4+ Th1 and Th17 cells have been identified (Zielinski et al., 2012). The importance of adaptive responses to C. albicans has been demonstrated by the high prevalence of mucocutaneous candidiasis in patients with Th17 cell defects (e.g. hyper IgE syndrome) and some immunodeficiencies (e.g. HIV) (McDonald, 2012). Our results as well as the work of others has found that adaptive responses to C. albicans infection in mice are also dominated by Th1 and Th17 cells and provide protection from secondary infection thus mimicking the response seen in humans (Conti and Gaffen, 2010; Eyerich et al., 2008; Huppler et al., 2012; Puel et al., 2011; Zielinski et al., 2012). We found that Batf3−/− mice lacking CD103+ dDC and Il6−/− mice developed either greatly exaggerated or absent Th17 cell development, respectively, without any alteration in skin innate responses to C. albicans. Secondary skin infection of mice with exaggerated Th17 cell expansion resulted in significant increase in protection compared with mice lacking Th17 cells that showed no evidence of protection. Mice receiving exogenous C. albicans primed Th17 but not Th1 cells also were protected from skin infection. Thus, consistent with the well-documented role of Th17 cells in protection from CMC, Th17 cell expansion provided protection from secondary skin infection (Eyerich et al., 2008; McDonald, 2012). In addition, the ability of C. albicans yeast to drive Th17 cell responses and provide protection argues against the concept that the failure of yeast to generate Th17 cell is a mechanism that maintains C. albicans commensalism(Bonifazi et al., 2009; Romani, 2011).
Notably, we found that Th17 cell expansion failed to provide protection from systemic infection. Rather, enhanced protection from systemic challenge occurred in Il6/− mice that developed exaggerated Th1 cells as well as naïve recipients of primed CD4+ T cells isolated from infected Il6−/− mice. Notably, this protection developed despite an enhanced susceptible to primary disseminated C. albicans that occurs in Il6−/− mice(Van Enckevort et al., 1999). It was also independent of the phenomena of reprogrammed monocytes that can provide protection from systemic infection through an IL-6 dependent mechanism (Quintin et al., 2012). Notably, patients with mutations in STAT1 that reduce signaling of IFN-γ develop increased rates of systemic infection with C. albicans and other fungi (Puel et al., 2011; Sampaio et al., 2013). We speculate that IFN-γ or granulocyte-macrophage colony stimulating factor (GM-CSF) derived from Th1 cell effectors may enhance the anti-candida responses by promoting increased NK and/or macrophage activity (Bär et al., 2014).
In summary, these data reveal a role for C. albicans morphology and interaction with a single DC subset for the generation of Th17 cell responses. In addition, the tissue selective protection afforded by Th17 and Th1 cell effectors reveals a compartmentalization of Th function that suggests a rational basis for the development of Th phenotype-specific C. albicans vaccines.
Experimental Procedures
Mice
Mgl2eGFP-DTR/wt (referred to as Mgl2-DTR), human Langerin-DTA (LC−), human Langerin-DTAxBatf3−/− (referred to as LC−xBatf3−/−), huLangerin-DTR, muLangerineGFP, human Langerin-creERT2, YFP, Tgfb1f, Tgfbr2f, Clec7a−/− mice have been previously described (Bobr et al., 2012; Kaplan et al., 2005; Kissenpfennig et al., 2005; Kumamoto et al., 2013; Taylor et al., 2006; Welty et al., 2013). Il1b−/− and Il6−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 and B6-Ly5.2/Cr were purchased from the National Cancer Institute or Charles River laboratories (Frederick, MD). CD90.1 Rag1−/− CD4+ TEα TCR-transgenic mice that recognize Eα50-66 in I-Ab on C57BL/6 backgrounds were also used (Grubin et al., 1997). All experiments were performed with 6- to 12-week-old and sex-matched mice. Mice were housed in microisolator cages and fed irradiated food and acidified water. The University of Minnesota institutional care and use committee approved all mouse protocols.
Generation and Testing of Bone Marrow Chimeric Mice
Six weeks old cytokine deficient, wild type C57BL/6 or Ly5.2 congenically marked mice were irradiated using X-ray irradiator as previously described. The mice received two split dose at 500 cGy each. The following day, 5×106 bone marrow cells isolated from specified mice were injected intravenously. Mice were rested for at least 6 weeks prior to experiments. The efficiency of chimerism was determined flow cytometry of congenic markers on PBMC, lymph node and epidermis.
DC Depletion with Diphtheria Toxin
Mgl2-DTRmice were i.p. injected with 1 mg of diphtheria toxin (List Biological Laboratories, Campbell, CA) 2 days before infection, as previously described (Bobr et al., 2010; Igyártó et al., 2011).
Tamoxifen treatment
Tamoxifen (Sigma Aldrich) was dissolved in corn oil (Sigma Aldrich) and 10% ethanol and was administered 5 consecutive days by i.p. injection at 0.05 mg/g of mouse weight.
Antibodies
Fluorochrome-conjugated antibodies to CD4 (GK1.5), CD11b (M1/70), CD11c (N418), CD45.1(A20), CD45.2 (104), CD90.1 (OX-7), CD103 (2E7), I-A/I-E (M5/114.15.2), Dectin-1 (RH1), IFN-γ (XMG1.2), IL-10 (JES5-16E3) and IL-17A (TC11-18H10.1) were purchased from BioLegend (San Diego, CA). Anti-mouse Langerin (L31), IL-17F (18F10), IL-22 (1H8PWSR), Foxp3 (FJK-16S) were acquired from eBioscience (San Diego, CA).
Adoptive T Cell Transfer
T cells were adoptively transferred as previously described (Igyártó et al., 2011). Briefly, skin-draining lymph nodes, spleen and mesenteric lymph node of TEα mice were disrupted through a cell strainer and washed with sterile HBSS and labeled with CFSE (Invitrogen, Carlsbad, CA) in accordance with the manufacturer's instructions. The cells after 2 washes were resuspended in sterile PBS at a concentration of 1×106 cell/ml and 300 μl (3×105 cells) were injected intravenously into recipients.
In certain experiments, single cell suspension from secondary lymphoid organs from either naïve wildtype or skin infected IL-6 or Batf3 deficient mice were obtained. CD4+ T cells were magnetically isolated at >95% purity (STEMCELL Technologies, British Columbia, Canada ) according to manufactorers directions. A total of 5×106 million purified CD4+ T cells were transferred into naïve hosts in 300 μl PBS.
Flow Cytometry
Single-cell suspensions of epidermal cells were obtained as previously defined (Kaplan et al., 2005). Briefly skin from ear or trunk were obtained and incubated for 2 hours at 37°C in 0.3% trypsin (Sigma-Aldridge, St. Louis, MO) in 150 mM NaCl, 0.5 mM KCl, 0.5 mM glucose. The epidermis was physically separated from the dermis. Dermal cells were obtained from whole flank skin. Samples were minced and incubated for 2 hr at 37°C in collagenase XI (4830 U/ml, Sigma), hyaluronidase (260 U/ml, Sigma), DNase (0.1 mg/ml, ICN), 10 mM HEPES (Sigma) in RPMI (Invitrogen). The resulting cells were filtered though a 40 μm filter. Lymph node (axillary, brachial, and inguinal) and spleen cells were incubated in 400 and 150 U/ml Collagenase D for preparation of dendritic cells or smashed through a filter for lymphocytes (Roche Applied Science, Indianapolis, IN), respectively, for 90 min prior to erythroid cell lysis in ACK buffer (Biowhittaker, Walkersville, MD). Single-cell suspensions were pretreated for 10 min at 4°C with 2.4G2 except when anti-Rat secondaries were used, in which case cells were blocked with mouse Ig (Sigma). For evaluating cytokine expression, cells were incubated for 5 hours in complete IDMEM supplemented with PMA (50 ng/ml) and ionomycin (1.5 μM; Sigma-Aldrich, St. Louis, MO), with GolgiStop (BD PharMingen, San Jose, CA) added for the final 4 hr. The intracellular cytokine staining was performed with BD Bioscience Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA) in accordance with the manufacturer's instructions. Samples were analyzed on LSR-II flow cytometers (BD Biosciences). Data were analyzed with FlowJo software (TreeStar, Ashland, OR). Detection of 2W1S specific CD4+ T cells was performed as published with an I-Ab-2W1S tetramer (a kind gift of M. Jenkins) after ex vivo stimulation of secondary organs as previously described(Moon et al., 2007).
DC sorting by flow cytometry and qPCR
Single cell suspension of LN cells were enriched by CD11c MACS positive selection (Miltenyi Biotec, Auburn, CA). LCs were sorted using a FACSAria cell sorter based as GFP+, MHCII+, CD11c+, CD11b+, CD103−CD8−. RNA was isolated using an RNeasy Mini Kit (Qiagen, Valencia, CA) and quantified from Nanodrop readings (NanoDrop, Wilmington, DE). cDNA was generated using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA). TaqMan Gene Expression Master Mix, TaqMan Gene Expression Assays for IL-1, IL-6 and IL-12p40 was used. ABI Prism 7900HT(Applied Biosystems) were used to complete the qPCR. All kits were completed according the manufacturer's instructions. All Ct values were normalized to HPRT expression and are shown as 2−ΔCt to naïve.
Candida albicans strains
Recombinant C. albicans used in this study was derived from SC5314 and was designed as previously described (Fonzi and Irwin, 1993; Igyártó et al., 2011). They were grown at in rich medium at 30°C (YPAD), or synthetic complete medium. Escherichia coli strain XLI-blue (Stratagene, La Jolla, CA, USA), growth conditions, DNA manipulations and primer design and synthesis were essentially as described previously. Transformants were selected on YPAD medium containing 400μg/ml nourseothricin and were screened by PCR using oligonucleotide primers listed (Figure S2C).Briefly, strain Hwp1-eGFP-Ag was constructed as follows: plasmid pMG2268 was PCR with Primers 875 and 5266. PCR product was transformed into wild-type strain SC5314 using standard methods(Igyártó et al., 2011). Transformants were selected on YPAD medium containing 400μg/ml nourseothricin. Integration at the HWP1 locus was confirmed using 2 primers (877/3290) confirming integration of GFP-antigen at HWP1.
C. albicans strains s20175.016, yeast locked strain (JKC19) and filament locked strain (BCa2-9) were kindly provided by Dr. Neal Gow (University of Aberdeen) (Braun, 1997; Liu et al., 1994; MacCallum, 2012; Marakalala et al., 2013). Growth of fungi occurred after inoculation of a colony at 30°C in YPAD overnight and, the next day, diluted 1:10 and cultured in either 30°C in YPAD until OD600 reached 1.5 for yeast morphology or at 37°C in RPMI with 10% FBS for 3 hours for filamentous forms.
Infection Models
The skin infection was performed as described (Igyártó et al., 2011). Briefly, Mice were first anesthetized with a mixture of ketamine and xylazine (100/10 mg/kg body weight), shaved on the back with electric clipper, and chemically depilated with Nair hair remover (Church & Dwight, Princeton, NJ) per the manufacturer's instructions. The stratum corneum was removed with 15 strokes with 220 grit sandpaper (3M, St Paul, MN). After washing with sterile PBS, 2×108 C. albicans in 50 μl of sterile PBS was applied on to the skin. In some experiments, same dose of C. albicans or 50ug curdlan was injected intradermally spaced over 10 sites on the back . Where indicated, recombinant murine IL-6 (BioLegend) was injected into 4 different sites in the dorsum of the mice intradermally at a total dose of 1 μg per mice. IV infections with Eno1-Ag and Hwp1-Ag for assessment of T cell expansion were performed at 3×105 CFU in PBS.
In re-challenge experiments, previously mock or epicutaneously infected mice were challenged with 1×106 CFU C. albicans by intradermal injected into a single site or by 1×105 CFU intravenously. Skin and kidneys were harvest from mice 3 days later and homogenized and serially diluted onto YPAD plates and incubated at 30°C for 24-48 hours to assess the number of colony forming units.
LC Targeting with Anti-huLangerin
Anti human-Langerin antibodies (2G3) conjugated to antigen were generated and administered as previously described(Igyártó et al., 2011). Briefly, WT and huLangerin-DTR mice with were immunized by intraperitoneal injection of 1.0 μg of 2G3-Eα in 100 μl of sterile PBS. Six hours later, the mice were infected with the noted pathogen strains as above.
Histology
Immunofluorescence on ear epidermis was performed as previously described (Kaplan et al., 2005). Images were captured with a microscope (DM5500; Leica) with digital system and LAS AF software (version 1.5.1). Periodic Acid-Schiff (PAS) staining for fungal invasion was performed 2 days after infection with SC5314 on formaldehyde fixed frozen sections with commercial PAS staining kit (Sigma-Aldrich).
Supplementary Material
Highlights.
C. albicans yeast, not filamentous forms are required for Th17 cell responses
Th17 cell induction requires LC-derived IL-6 and Dectin-1 ligation
Absent Dectin-1 ligation by hyphae prevents Th17 cell induction by CD11b+ dDC
Th17 cells provide cutaneous protection and Th1 cells provide systemic protection
ACKNOWLEDGEMENTS
We thank M. Jenkins for assistance with 2W1S tetramers. B. Chicoine provided technical assistance. We thank Bruce Klein and Marcel Wuethrich for assistance with celc7a−/− mice. We also thank the University of Minnesota Research Animal Resources staff for expert animal care and P. Champoux and T. Martin of the Flow Cytometry Core Facility at the Center for Immunology for assistance flow cytometry experiments. This work was supported by grants from the National Institutes of Health (NIH) (AR056632 and AR060744) to D.H.K., a Dermatology Foundation research award (BZI), and an American Skin Association research award (BZI). D.H.K. is also supported by the Al Zelickson Family endowed professorship. S.W.K. was supported by the University of Minnesota NIH MSTP grant T32 GM008244.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contribution
SWK, BZI and DHK designed experiments. SWK and BZI performed experiments. MGN and JB helped in construction and validation of C. albicans strain Eno1-Ag and Hwp1-Ag. AI and GDB assisted with the development of experimental models. YK and AI developed and provided Mgl2-DTR mice. EJ, JAM and RAD assisted with experiments. SMZ and GZ developed and provided monoclonal antibody 2G3 against human Langerin. SWK, BZI and DHK wrote the manuscript. All authors participated in discussions of experimental results and edited the manuscript.
The authors have no conflicting financial interests.
References
- Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, Ho LP, Townsend ARM, Drakesmith H. Hepcidin regulation by innate immune and infectious stimuli. Blood. 2011;118:4129–4139. doi: 10.1182/blood-2011-04-351957. [DOI] [PubMed] [Google Scholar]
- Bär E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut-Landmann S. IL-17 Regulates Systemic Fungal Immunity by Controlling the Functional Competence of NK Cells. Immunity. 2014 doi: 10.1016/j.immuni.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Bobr A, Igyártó BZ, Haley KM, Li MO, Flavell RA, Kaplan DH. Autocrine/paracrine TGF-β1 inhibits Langerhans cell migration. Proc. Natl. Acad. Sci. U.S.a. 2012;109:10492–10497. doi: 10.1073/pnas.1119178109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobr A, Olvera-Gomez I, Igyártó BZ, Haley KM, Hogquist KA, Kaplan DH. Acute ablation of Langerhans cells enhances skin immune responses. J. Immunol. 2010;185:4724–4728. doi: 10.4049/jimmunol.1001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifazi P, Zelante T, D'Angelo C, De Luca A, Moretti S, Bozza S, Perruccio K, Iannitti RG, Giovannini G, Volpi C, et al. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol. 2009;2:362–374. doi: 10.1038/mi.2009.17. [DOI] [PubMed] [Google Scholar]
- Braun BR. Control of Filament Formation in Candida albicans by the Transcriptional Repressor TUP1. Science. 1997;277:105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- Cabib E, Bowers B. Chitin and yeast budding. Localization of chitin in yeast bud scars. J Biol Chem. 1971;246:152–159. [PubMed] [Google Scholar]
- Cheng S-C, van de Veerdonk FL, Lenardon M, Stoffels M, Plantinga T, Smeekens S, Rizzetto L, Mukaremera L, Preechasuth K, Cavalieri D, et al. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J. Leukoc. Biol. 2011;90:357–366. doi: 10.1189/jlb.1210702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, et al. Critical Regulation of Early Th17 Cell Differentiation by Interleukin-1 Signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Gaffen SL. Host responses to Candida albicans: Th17 cells and mucosal candidiasis. Microbes Infect. 2010;12:518–527. doi: 10.1016/j.micinf.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Ostiani CF, Del Sero G, Bacci A, Montagnoli C, Spreca A, Mencacci A, Ricciardi-Castagnoli P, Romani L. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 2000;191:1661–1674. doi: 10.1084/jem.191.10.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerich K, Foerster S, Rombold S, Seidl H-P, Behrendt H, Hofmann H, Ring J, Traidl-Hoffmann C. Patients with Chronic Mucocutaneous Candidiasis Exhibit Reduced Production of Th17-Associated Cytokines IL-17 and IL-22. J. Invest. Dermatol. 2008;128:2640–2645. doi: 10.1038/jid.2008.139. [DOI] [PubMed] [Google Scholar]
- Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, et al. Human Dectin-1 Deficiency and Mucocutaneous Fungal Infections. N. Engl. J. Med. 2009;361:1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell. Microbiol. 2008;10:2058–2066. doi: 10.1111/j.1462-5822.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- Flamar AL, Zurawski S, Scholz F, Gayet I, Ni L, Li XH, Klechevsky E, Quinn J, Oh S, Kaplan DH, et al. Noncovalent Assembly of Anti- Dendritic Cell Antibodies and Antigens for Evoking Immune Responses In Vitro and In Vivo. The Journal of Immunology. 2012;189:2645–2655. doi: 10.4049/jimmunol.1102390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative Induction of Inflammatory Responses by Dectin-1 and Toll- like Receptor 2. Journal of Experimental Medicine. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. Embo J. 2005;24:1277–1286. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Nish SA, Jiang R, Hou L, Licona-Limón P, Weinstein JS, Zhao H, Medzhitov R. Control of T Helper 2 Responses by Transcription Factor IRF4-Dependent Dendritic Cells. Immunity. 2013;39:722–732. doi: 10.1016/j.immuni.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker E-O, Hennigs A, Nabavi M, Schäffer AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami F, et al. A Homozygous CARD9Mutation in a Family with Susceptibility to Fungal Infections. N. Engl. J. Med. 2009;361:1727–1735. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow NAR, van de Veerdonk FL, Brown AJP, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat. Rev. Microbiol. 2012;10:112–122. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- Haley K, Igyártó BZ, Ortner D, Bobr A, Kashem S, Schenten D, Kaplan DH. Langerhans cells require MyD88-dependent signals for Candida albicans response but not for contact hypersensitivity or migration. J. Immunol. 2012;188:4334–4339. doi: 10.4049/jimmunol.1102759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Santos N, Huppler AR, Peterson AC, Khader SA, McKenna KC, Gaffen SL. Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol. 2013;6:900–910. doi: 10.1038/mi.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM. Epidemiology and Outcomes of Candidemia in 2019 Patients: Data from the Prospective Antifungal Therapy Alliance Registry. Clin. Infect. Dis. 2009;48:1695–1703. doi: 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- Hu W, Troutman TD, Edukulla R, Pasare C. Priming Microenvironments Dictate Cytokine Requirements for T Helper 17 Cell Lineage Commitment. Immunity. 2011;35:1010–1022. doi: 10.1016/j.immuni.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppler AR, Bishu S, Gaffen SL. Mucocutaneous candidiasis: the IL-17 pathway and implications for targeted immunotherapy. Arthritis Res. Ther. 2012;14:217. doi: 10.1186/ar3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igyártó BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen- specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010;31:446–451. doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH, Igyártó BZ, Gaspari AA. Early immune events in the induction of allergic contact dermatitis. Nat. Rev. Immunol. 2012;12:114–124. doi: 10.1038/nri3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhé C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, et al. Dynamics and Function of Langerhans Cells In Vivo. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Kumamoto Y, Linehan M, Weinstein JS, Laidlaw BJ, Craft JE, Iwasaki A. CD301b+ dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity. 2013;39:733–743. doi: 10.1016/j.immuni.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Köhler J, Fink GR. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- Lo HJ, Köhler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- MacCallum DM. Hosting Infection: Experimental Models to Assay Candida Virulence. International Journal of Microbiology. 2012;2012:1–12. doi: 10.1155/2012/363764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marakalala MJ, Vautier S, Potrykus J, Walker LA, Shepardson KM, Hopke A, Mora-Montes HM, Kerrigan A, Netea MG, Murray GI, et al. Differential Adaptation of Candida albicans In Vivo Modulates Immune Recognition by Dectin-1. PLoS Pathog. 2013;9:e1003315. doi: 10.1371/journal.ppat.1003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DR. TH17 deficiency in human disease. Journal of Allergy and Clinical Immunology. 2012;129:1429–1435. doi: 10.1016/j.jaci.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray HW, Hillman JK, Rubin BY, Kelly CD, Jacobs JL, Tyler LW, Donelly DM, Carriero SM, Godbold JH, Roberts RB. Patients at Risk for AIDS-Related Opportunistic Infections. N. Engl. J. Med. 1985;313:1504–1510. doi: 10.1056/NEJM198512123132403. [DOI] [PubMed] [Google Scholar]
- Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hägerbrand K, Marsal J, Gudjonsson S, Håkansson U, Reizis B, Kotarsky K, et al. IRF4 Transcription-Factor-Dependent CD103+CD11b+ Dendritic Cells Drive Mucosal T Helper 17 Cell Differentiation. Immunity. 2013;38:958–969. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, Jacobs L, Jansen T, Kullberg BJ, Wijmenga C, et al. Candida albicans Infection Affords Protection against Reinfection via Functional Reprogramming of Monocytes. Cell Host Microbe. 2012;12:223–232. doi: 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis e Sousa C. Activation of dendritic cells: translating innate into adaptive immunity. Curr. Opin. Immunol. 2004;16:21–25. doi: 10.1016/j.coi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Romani L. Immunity to fungal infections. Nat. Rev. Immunol. 2011;11:275–288.. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- Sampaio EP, Hsu AP, Pechacek J, Bax HI, Dias DL, Paulson ML, Chandrasekaran P, Rosen LB, Carvalho DS, Ding L, et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. Journal of Allergy and Clinical Immunology. 2013;131:1624–1634. e17. doi: 10.1016/j.jaci.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AWS, See P, Shin A, Wasan PS, et al. IRF4 Transcription Factor- Dependent CD11b+ Dendritic Cells in Human and Mouse Control Mucosal IL-17 Cytokine Responses. Immunity. 2013;38:970–983. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw MH, Kamada N, Kim Y-G, Nuñez G. Microbiota-induced IL-1β, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J. Exp. Med. 2012;209:251–258. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab JF. Adhesive and Mammalian Transglutaminase Substrate Properties of Candida albicans Hwp1. Science. 1999;283:1535–1538. doi: 10.1126/science.283.5407.1535. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, et al. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat. Immunol. 2006;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Kullberg BJ, Netea MG. Pathogenesis of invasive candidiasis. Current Opinion in Critical Care. 2010;16:453–459. doi: 10.1097/MCC.0b013e32833e046e. [DOI] [PubMed] [Google Scholar]
- van der Graaf CAA, Netea MG, Verschueren I, van der Meer JWM, Kullberg BJ. Differential cytokine production and Toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect. Immun. 2005;73:7458–7464. doi: 10.1128/IAI.73.11.7458-7464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Enckevort FHJ, Netea MG, Hermus ARMM, Sweep CGJ, Meis JFGM, Van Der Meer JWM, Jan Kullberg B. Increased susceptibility to systemic candidiasis in interleukin-6 deficient mice 1. Med Mycol. 1999;37:419–426. doi: 10.1046/j.1365-280x.1999.00247.x. [DOI] [PubMed] [Google Scholar]
- Welty NE, Staley C, Ghilardi N, Sadowsky MJ, Igyártó BZ, Kaplan DH. Intestinal lamina propria dendritic cells maintain T cell homeostasis but do not affect commensalism. Journal of Experimental Medicine. 2013;210:2011–2024. doi: 10.1084/jem.20130728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- Zúñiga LA, Jain R, Haines C, Cua DJ. Th17 cell development: from the cradle to the grave. Immunol Rev. 2013;252:78–88. doi: 10.1111/imr.12036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.