Abstract
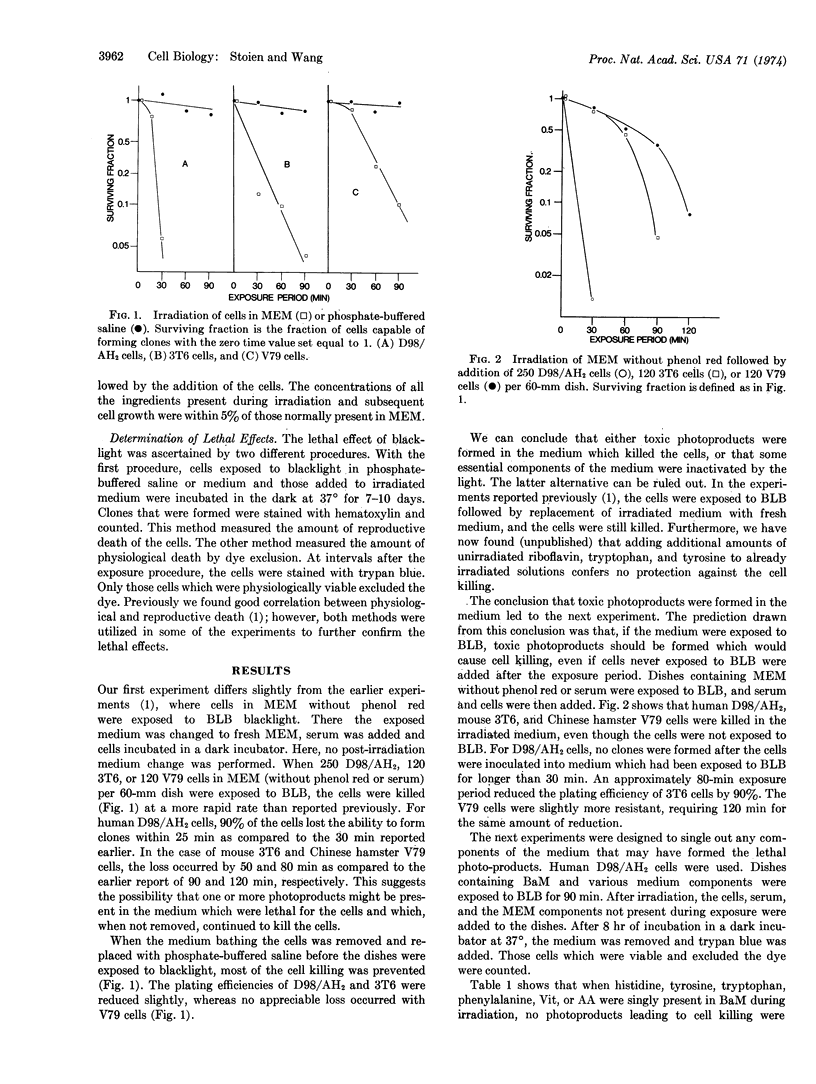
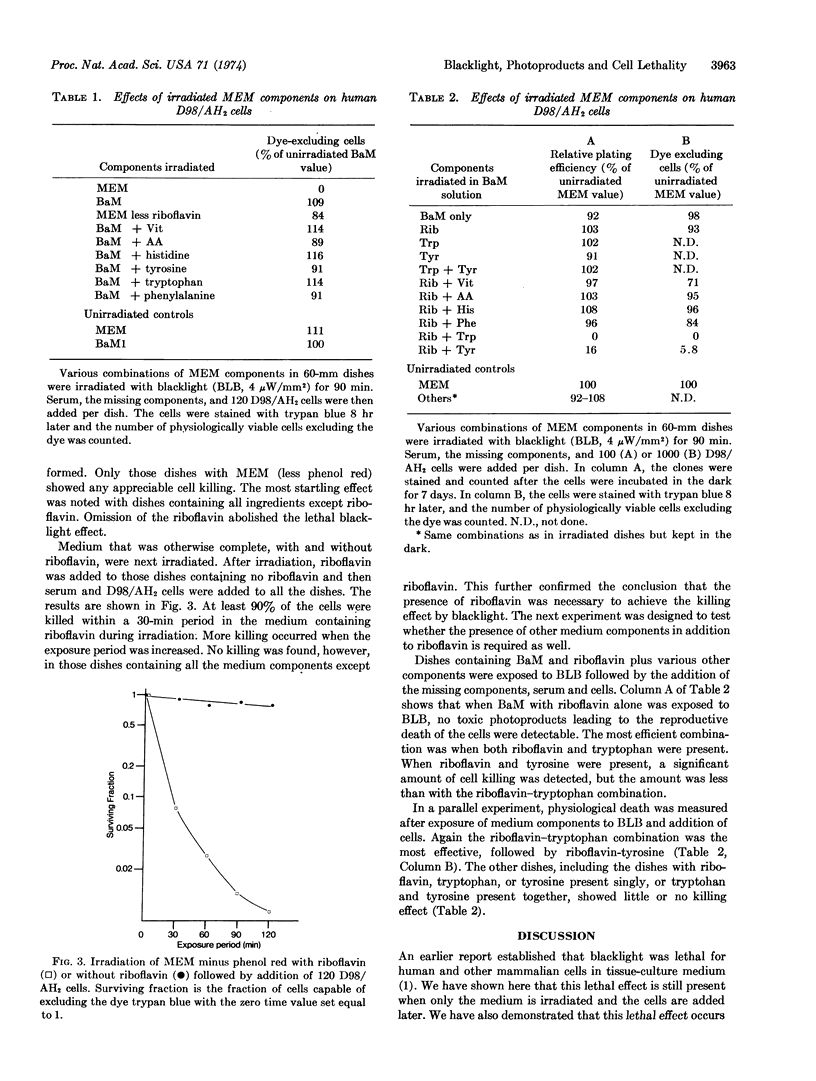
Near-ultraviolet radiation was found to be lethal for mammalian cells in Dulbecco's modified Eagle's medium without serum or phenol red. Irradiation of the cells with near-ultraviolet light while the cells were in phosphate-buffered-saline abolished the lethal effect. When only the medium was irradiated followed by the addition of unirradiated cells and serum, the cells were still killed. The photoactive components of the medium for this effect were riboflavin, tryptophan, and tyrosine. When riboflavin was deleted from the medium being irradiated and added later, almost no killing was detected. Irradiation of salt solution of riboflavin and tryptophan or riboflavin and tyrosine, resulted in cell killing. Little or no killing resulted when riboflavin, tryptophan, or tyrosine was irradiated singly.
The formation of photoproducts toxic for mammalian cells appears to involve photodynamic action. Experiments utilizing Dulbecco's or similar media without proper controls may produce anomalous results from light illuminating the laboratory.
Keywords: human cells, cell lethality, photodynamic action, riboflavin-tryptophan-tyrosine
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Hur E., Elkind M. M. Survival response of asynchronous and synchronous Chinese hamster cells exposed to fluorescent light following 5-bromodeoxyuridine incorporation. Mutat Res. 1972 Feb;14(2):237–245. doi: 10.1016/0027-5107(72)90050-4. [DOI] [PubMed] [Google Scholar]
- CABRERA JUAREZ E. "BLACK LIGHT" INACTIVATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID FROM HAEMOPHILUS INFLUENZAE. J Bacteriol. 1964 Apr;87:771–778. doi: 10.1128/jb.87.4.771-778.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E. H., Sun N. C., Chang C. C. Induction of auxotrophic mutations by treatment of Chinese hamster cells with 5-bromodeoxyuridine and black light. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3459–3463. doi: 10.1073/pnas.69.11.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., FREEMAN G. Plaque production by the polyoma virus. Virology. 1959 Jul;8(3):396–397. doi: 10.1016/0042-6822(59)90043-1. [DOI] [PubMed] [Google Scholar]
- Fogel M. Induction of polyoma virus by fluorescent (visible) light in polyoma-transformed cells pretreated with 5-bromodeoxyuridine. Nat New Biol. 1973 Feb 7;241(110):182–184. doi: 10.1038/newbio241182a0. [DOI] [PubMed] [Google Scholar]
- Foote C. S. Mechanisms of photosensitized oxidation. There are several different types of photosensitized oxidation which may be important in biological systems. Science. 1968 Nov 29;162(3857):963–970. doi: 10.1126/science.162.3857.963. [DOI] [PubMed] [Google Scholar]
- GALSTON A. W. Riboflavin, light, and the growth of plants. Science. 1950 Jun 9;111(2893):619–624. doi: 10.1126/science.111.2893.619. [DOI] [PubMed] [Google Scholar]
- Gailani S., Murphy G., Kenny G., Nussbaum A., Silvernail P. Studies on tryptophen metabolism in patients with bladder cancer. Cancer Res. 1973 May;33(5):1071–1077. [PubMed] [Google Scholar]
- Galston A. W., Baker R. S. Inactivation of Enzymes by Visible Light in the Presence of Riboflavin. Science. 1949 May 13;109(2837):485–486. doi: 10.1126/science.109.2837.485. [DOI] [PubMed] [Google Scholar]
- Galston A. W. Riboflavin-Sensitized Photoöxidation of Indoleacetic Acid and Related Compounds. Proc Natl Acad Sci U S A. 1949 Jan;35(1):10–17. [PMC free article] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. M., Edsall P. C. Interference by near ultraviolet and green light with growth of animal and plant cell cultures. Photochem Photobiol. 1967 Nov;6(11):841–850. doi: 10.1111/j.1751-1097.1967.tb08897.x. [DOI] [PubMed] [Google Scholar]
- Kostenbauder H. B., Sanvordeker D. R. Riboflavin enhancement of bilirubin photocatabolism in vivo. Experientia. 1973 Mar 15;29(3):282–283. doi: 10.1007/BF01926476. [DOI] [PubMed] [Google Scholar]
- Kubitschek H. E. Mutagenesis by near-visible light. Science. 1967 Mar 24;155(3769):1545–1546. doi: 10.1126/science.155.3769.1545. [DOI] [PubMed] [Google Scholar]
- Lichtenstein N. S., Goldman I. D. Riboflavin-methotrexate interactions. Photochemical reaction and competition for transport in the L1210 mouse leukemia cell. Biochem Pharmacol. 1970 Apr;19(4):1229–1239. doi: 10.1016/0006-2952(70)90038-9. [DOI] [PubMed] [Google Scholar]
- Nilsson R., Merkel P. B., Kearns D. R. Unambiguous evidence for the participation of singlet oxygen ( 1 ) in photodynamic oxidation of amino acids. Photochem Photobiol. 1972 Aug;16(2):117–124. doi: 10.1111/j.1751-1097.1972.tb07343.x. [DOI] [PubMed] [Google Scholar]
- Peak M. J., Peak J. G., Webb R. B. Inactivation of transforming DNA by ultraviolet light. I. Near-UV action spectrum for marker inactivation. Mutat Res. 1973 Nov;20(2):129–135. doi: 10.1016/0027-5107(73)90182-6. [DOI] [PubMed] [Google Scholar]
- Penzer G. R. The chemistry of flavins and flavoproteins: aerobic photochemistry. Biochem J. 1970 Feb;116(4):733–743. doi: 10.1042/bj1160733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puck T. T., Kao F. T. Genetics of somatic mammalian cells. V. Treatment with 5-bromodeoxyuridine and visible light for isolation of nutritionally deficient mutants. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1227–1234. doi: 10.1073/pnas.58.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivlin R. S. Riboflavin and cancer: a review. Cancer Res. 1973 Sep;33(9):1977–1986. [PubMed] [Google Scholar]
- SLUYTERMAN L. A. Photo-oxidation, sensitized by proflavine, of a number of protein constituents. Biochim Biophys Acta. 1962 Jul 16;60:557–561. doi: 10.1016/0006-3002(62)90874-0. [DOI] [PubMed] [Google Scholar]
- Shipley W. U., Elkind M. M., Prather W. B. Potentiation of x-ray killing by 5-bromodeoxyuridine in Chinese hamster cells: a reduction in capacity for sublethal damage accumulation. Radiat Res. 1971 Aug;47(2):437–449. [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd P., Schroy C. B., Lebed M. R. Post-irradiation effects of photoreactivating light and caffeine on cultured marsupial cells exposed to ultraviolet light. Photochem Photobiol. 1973 Nov;18(5):433–436. doi: 10.1111/j.1751-1097.1973.tb06445.x. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M., Webb R. B., Brown M. S. Destruction of photoreactivating enzyme by 365 nm radiation. Photochem Photobiol. 1973 Oct;18(4):249–254. doi: 10.1111/j.1751-1097.1973.tb06420.x. [DOI] [PubMed] [Google Scholar]
- Wang R. J., Stoien J. D., Landa F. Lethal effect of near-ultraviolet irradiation on mammalian cells in culture. Nature. 1974 Jan 4;247(5435):43–45. doi: 10.1038/247043a0. [DOI] [PubMed] [Google Scholar]
- Warburg O., Geissler A. W., Lorenz S. Wirkung von Riboflavin und Luminoflavin auf wachsende Krebszellen. Z Klin Chem Klin Biochem. 1968 Sep;6(5):467–468. [PubMed] [Google Scholar]
- Warburg O., Geissler A. W., Lorenz S. Wirkung von Riboflavin und von delta-Amino-lävulinsäure auf wachsende Krebszellen in vitro. Hoppe Seylers Z Physiol Chem. 1967 Dec;348(12):1683–1685. [PubMed] [Google Scholar]
- Webb R. B., Lorenz J. R. Toxicity of irradiated medium for repair-deficient strains of Escherichia coli. J Bacteriol. 1972 Oct;112(1):649–652. doi: 10.1128/jb.112.1.649-652.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb R. B., Malina M. M. Mutagenesis in Escherichia coli by visible light. Science. 1967 May 26;156(3778):1104–1105. doi: 10.1126/science.156.3778.1104. [DOI] [PubMed] [Google Scholar]
- Webb S. J., Tai C. C. Physiological and genetic implications of selective mutation by light at 320-400 nm. Nature. 1969 Dec 13;224(5224):1123–1125. doi: 10.1038/2241123a0. [DOI] [PubMed] [Google Scholar]
- Yang S. J., Hahn G. M., van Kersen-Bax I. Effects of light on viability and DNA synthesis of mammalian cells preincubated in media containing brominated pyrimidines. Photochem Photobiol. 1970 Feb;11(2):131–136. doi: 10.1111/j.1751-1097.1970.tb05979.x. [DOI] [PubMed] [Google Scholar]
- Yoakum G., Eisenstark A. Toxicity of L-Tryptophan photoproduct on recombinationless (rec) mutants of Salmonella typhimurium. J Bacteriol. 1972 Oct;112(1):653–655. doi: 10.1128/jb.112.1.653-655.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman S., Schultz J. B., Yulo T., Grover D. Effects of near -UV irradiation on lens and aqueous humor proteins. Isr J Med Sci. 1972 Aug-Sep;8(8):1590–1595. [PubMed] [Google Scholar]



