Abstract
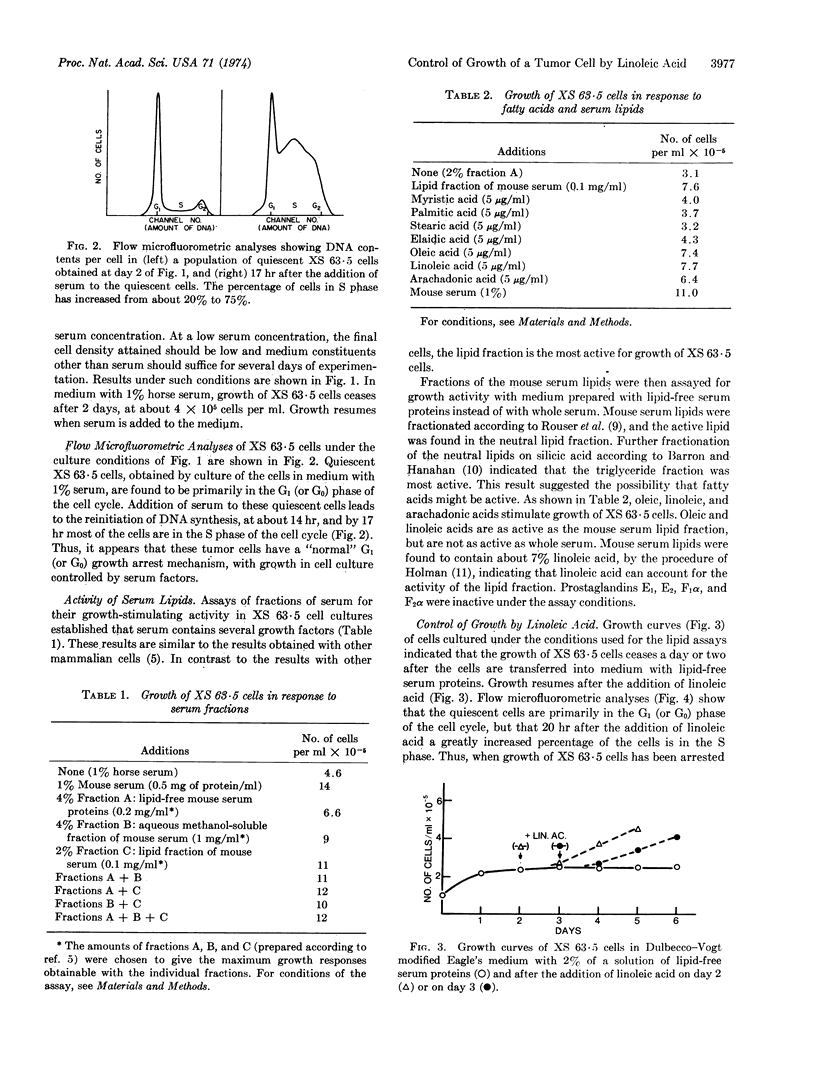
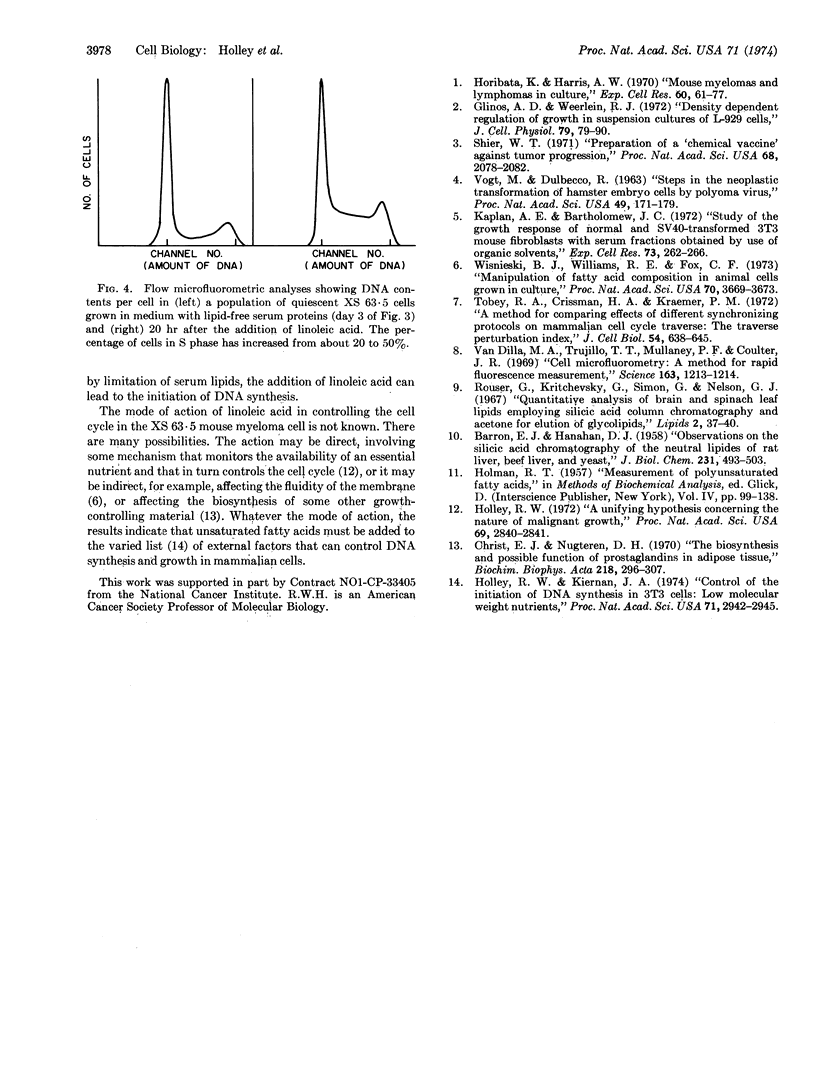
The growth of mouse myeloma XS 63·5 cells in cell culture is dependent on serum. Among the several growth factors present in serum, the lipid fraction is highly active. The growth factor(s) provided by the serum lipid fraction can be replaced by unsaturated fatty acids. If XS 63·5 cells are cultured in medium that is low in serum lipids, the cells become quiescent, with most of the cells in the G1 (or G0) phase of the cell cycle. Addition of linoleic acid to such quiescent cells leads to reinitiation of DNA synthesis and growth.
Keywords: cell cycle, control of DNA synthesis, serum lipids, unsaturated fatty acids
Full text
PDF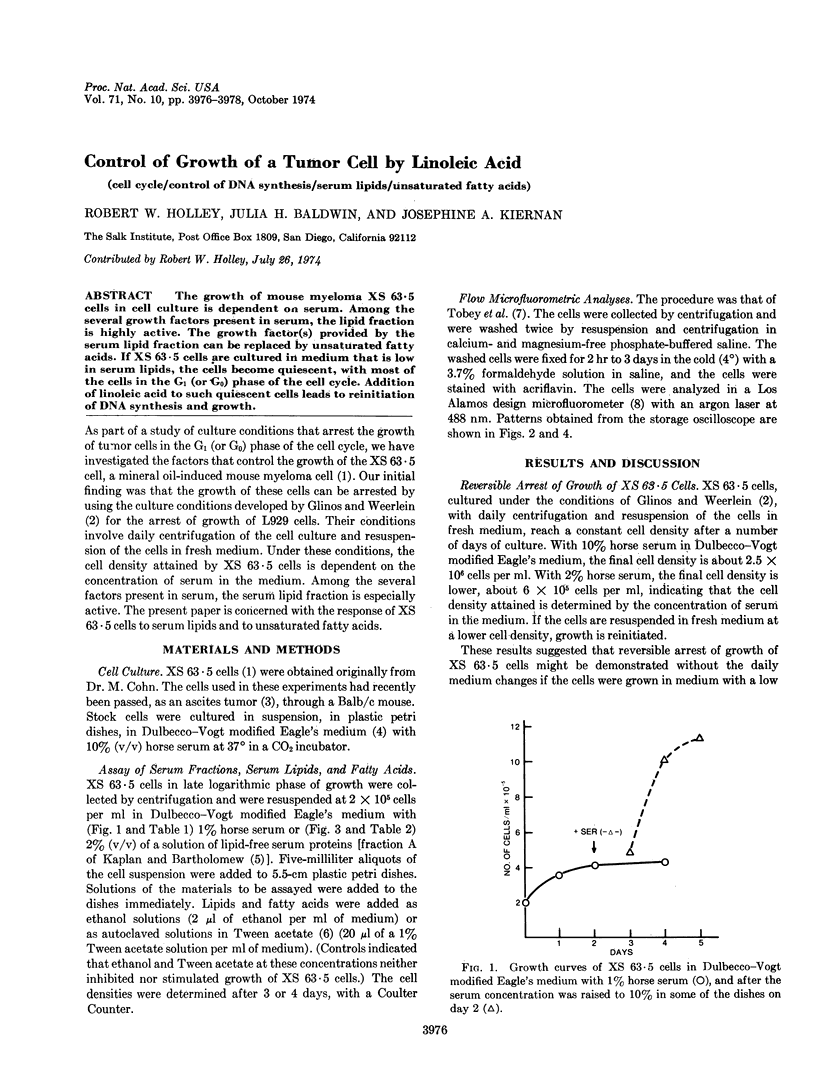
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRON E. J., HANAHAN D. J. Observations on the silicic acid chromatography of the neutral lipides of rat liver, beef liver, and yeast. J Biol Chem. 1958 Mar;231(1):493–503. [PubMed] [Google Scholar]
- Glinos A. D., Werrlein R. J. Density dependent regulation of growth in suspension cultures of L-929 cells. J Cell Physiol. 1972 Feb;79(1):79–90. doi: 10.1002/jcp.1040790109. [DOI] [PubMed] [Google Scholar]
- HOLMAN R. T. Measurement of polyunsaturated fatty acids. Methods Biochem Anal. 1957;4:99–138. doi: 10.1002/9780470110201.ch3. [DOI] [PubMed] [Google Scholar]
- Holley R. W. A unifying hypothesis concerning the nature of malignant growth. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2840–2841. doi: 10.1073/pnas.69.10.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. Control of the initiation of DNA synthesis in 3T3 cells: low-molecular weight nutrients. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2942–2945. doi: 10.1073/pnas.71.8.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Kaplan A. E., Bartholomew J. C. Study of the growth response of normal and SV40-transformed 3T3 mouse fibroblasts with serum fractions obtained by use of organic solvents. Exp Cell Res. 1972 Jul;73(1):262–266. doi: 10.1016/0014-4827(72)90133-4. [DOI] [PubMed] [Google Scholar]
- Rouser G., Kritchevsky G., Simon G., Nelson G. J. Quantitative analysis of brain and spinach leaf lipids employing silicic acid column chromatography and acetone for elution of glycolipids. Lipids. 1967 Jan;2(1):37–40. doi: 10.1007/BF02531998. [DOI] [PubMed] [Google Scholar]
- Shier W. T. Preparation of a "chemical vaccine" against tumor progression. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2078–2082. doi: 10.1073/pnas.68.9.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey R. A., Crissman H. A., Kraemer P. M. A method for comparing effects of different synchronizing protocols on mammalian cell cycle traverse. The traverse perturbation index. J Cell Biol. 1972 Sep;54(3):638–645. doi: 10.1083/jcb.54.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Steps in the neoplastic transformation of hamster embryo cells by polyoma virus. Proc Natl Acad Sci U S A. 1963 Feb 15;49:171–179. doi: 10.1073/pnas.49.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dilla M. A., Trujillo T. T., Mullaney P. F., Coulter J. R. Cell microfluorometry: a method for rapid fluorescence measurement. Science. 1969 Mar 14;163(3872):1213–1214. doi: 10.1126/science.163.3872.1213. [DOI] [PubMed] [Google Scholar]
- Wisnieski B. J., Williams R. E., Fox C. F. Manipulation of fatty acid composition in animal cells grown in culture. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3669–3673. doi: 10.1073/pnas.70.12.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]



