Significance
In many invertebrates, development depends on obligate bacterial symbionts that are confined to specialized host cells and are transmitted directly from mother to progeny. A primary model for this kind of symbiosis is the Buchnera/pea aphid association, which has been maintained for more than 100 million years through strict maternal transmission. The intimacy of this symbiosis has thwarted experiments aimed at dissecting how symbiont and host genotypes contribute to overall phenotype and ecological tolerances. Using a selectable Buchnera phenotype combined with microinjection, we successfully replaced Buchnera within a matriline, thus generating matrilines with identical aphid genotypes but distinct Buchnera genotypes. Buchnera replacement dramatically increased heat tolerance of the aphid matriline, demonstrating directly that symbiont genotype can affect host ecology.
Keywords: symbiosis, aphid, Buchnera, thermal tolerance, maternal transmission
Abstract
Symbiosis, the close association of unrelated organisms, has been pivotal in biological diversification. In the obligate symbioses found in many insect hosts, organisms that were once independent are permanently and intimately associated, resulting in expanded ecological capabilities. The primary model for this kind of symbiosis is the association between the bacterium Buchnera and the pea aphid (Acyrthosiphon pisum). A longstanding obstacle to efforts to illuminate genetic changes underlying obligate symbioses has been the inability to experimentally disrupt and reconstitute symbiont–host partnerships. Our experiments show that Buchnera can be experimentally transferred between aphid matrilines and, furthermore, that Buchnera replacement has a massive effect on host fitness. Using a recipient pea aphid matriline containing Buchnera that are heat sensitive because of an allele eliminating the heat shock response of a small chaperone, we reduced native Buchnera through heat exposure and introduced a genetically distinct Buchnera from another matriline, achieving complete replacement and stable inheritance. This transfer disrupted 100 million years (∼1 billion generations) of continuous maternal transmission of Buchnera in its host aphids. Furthermore, aphids with the Buchnera replacement enjoyed a dramatic increase in heat tolerance, directly demonstrating a strong effect of symbiont genotype on host ecology.
Symbiosis has been key in the ecological and evolutionary diversification of eukaryotes (1, 2). In many invertebrates, bacterial symbionts have been maternally transmitted for millions of years and are required for the growth and reproduction of hosts (3). These symbionts approach organelles in their degree of genetic and physiological integration with hosts and in their extreme genomic reduction. A model for obligate symbiosis is that of the pea aphid (Acyrthosiphon pisum) and its nutrient-provisioning bacterial symbiont, Buchnera aphidicola. Buchnera features a tiny genome (4), restriction to a small number of specialized host cells (bacteriocytes), host-controlled transmission (5), and regulated exchange of molecules with hosts (6, 7). This tight integration creates challenges for studies that aim to elucidate how symbiont variation affects host fitness and ecology.
The ability to transfer obligate symbionts between host matrilines could provide a tool for teasing apart the separate contributions of symbiotic partners. Facultative symbionts, such as those conferring defense against pathogens, have been transferred experimentally into novel host matrilines, where they are typically stably inherited, enabling direct measures of symbionts on hosts (8). The success of these transfers is presumably related to the fact that facultative symbionts possess their own machinery for invading host cells (9, 10) and typically persist in several locations in the insect body. In contrast, obligate symbionts such as Buchnera are packaged into specialized host cells during early development and do not survive in the hemocoel or in other cell types.
Buchnera colonizes developing aphids before birth, through a specialized transmission process that has been studied in detail in A. pisum (5, 11). Aphids are parthenogenetic for much of their life cycle, during which embryos develop within maternal ovarioles. Bacteriocytes and ovarioles containing developing embryos are located near one another within the mother’s abdomen, and transmission occurs when Buchnera cells are exocytosed from a maternal bacteriocyte in the vicinity of a blastula-stage embryo (5). The Buchnera cells become extracellular within the hemocoel, and some are endocytosed by the posterior syncytial cytoplasm of the embryo in which they are later packaged into the embryonic bacteriocytes. Buchnera cells released into the hemocoel quickly deteriorate if they do not enter an embryo. The molecular underpinnings of the transfer process are unknown. This specialized transmission process presents a challenge for the experimental transfer of symbionts between hosts.
We developed a strategy aimed at replacing resident Buchnera with genetically distinct Buchnera from a different host matriline (Fig. 1), using parthenogenetic (all-female) lines of aphids. We used heat tolerance as a selectable phenotype. A previous study showed that a single nucleotide deletion in the promoter of a small heat shock protein [inclusion body-associated protein A (IbpA)] of Buchnera results in a reduction in Buchnera numbers of >100-fold after exposure to 4 h of 35 °C heat (12). Buchnera lacking this mutation undergo only modest declines after heat exposure. Our strategy was to reduce the native Buchnera in recipient aphids using heat, and then to inject homogenate containing heat-tolerant Buchnera from another A. pisum matriline. Because transmission to embryos includes an extracellular stage in which Buchnera is free in the hemocoel, the injected Buchnera might colonize embryos in lieu of maternal Buchnera, which have been eliminated or depleted. After successful replacement of native Buchnera, we demonstrated a large effect on aphid ability to withstand heat exposure.
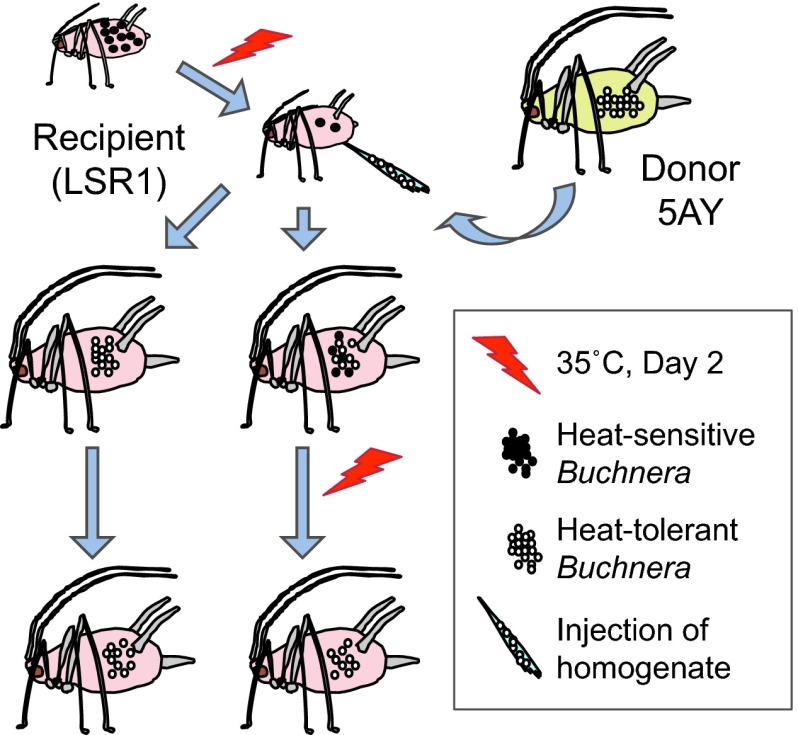
Fig. 1.
Experimental approach for replacement of the native Buchnera symbionts within an A. pisum matriline. The recipient line (LSR1) contains a heat-sensitive Buchnera genotype, and the donor (5AY) contains a heat-tolerant Buchnera genotype. Native Buchnera are depleted by heat in the recipient line, and microinjection is used to flood the hemocoel with donor Buchnera. Most embryos are successfully colonized by the donor symbionts. In some cases, complete replacement occurs in the progeny of injected females. In other cases, progeny have a mixed Buchnera population, which can be shifted completely to the donor type through further heat exposure.
Results
Replacement of Native Symbionts with Donor Symbionts.
We screened several parthenogenetic A. pisum lines for the Buchnera allele affecting heat tolerance. A. pisum LSR1 was chosen as the recipient matriline; this aphid clone was used for full-genome sequencing by the International Aphid Genome Consortium and had acquired the Buchnera mutation conferring heat sensitivity in our laboratory during the last 5 y. A. pisum 5AY, containing the heat-tolerant Buchnera allele, was selected as the donor. After heat exposure (4 h at 35 °C) on day 2 after birth, LSR1 juveniles were injected on day 3, using homogenate from 5AY. Two sets of injections were carried out, with 25–30 aphids per set. Most injected individuals died within 24 h from the combined trauma of the heat exposure and the injection. Those that survived the first 24 h usually became adult and reproduced, although ages at which they reached adulthood and first reproduced were considerably delayed. Under our conditions, normal A. pisum require 7 d from birth to adulthood and 8 d from birth to first reproduction, but heat-treated and injected females were as old as 15 d on reaching adulthood and as old as 22 d at first reproduction.
In set 1, four females survived to adulthood, and all four reproduced. In set 2, six females survived to adulthood and five reproduced. For each injected female that reproduced, progeny were sampled to check for the presence of the two Buchnera types. A genetic marker distinct from the locus affecting heat tolerance was used for screening.
Of the nine injected females that matured and reproduced, the donor (5AY) Buchnera was present in the F1 progeny of eight, either mixed with the LSR1 Buchnera or as the sole Buchnera type (Table 1). In set 1, all four females produced progeny containing a mixture of donor and recipient Buchnera types (Fig. 2). One of the four matrilines was propagated, and F2, F3, and F4 progeny also contained mixed Buchnera populations (Fig. 2). To achieve complete replacement with the 5AY Buchnera in this matriline, 2-d-old F5 nymphs were subjected to a further heat treatment, resulting in the exclusive presence of the 5AY Buchnera type in the F6 generation. Subsequent generations retained exclusively 5AY Buchnera (Fig. 2). In set 2, two mothers produced F1 progeny in which the donor had completely replaced the native Buchnera (Table 1, Fig. 2). Of the other three adults of set 2, two produced progeny with mixed Buchnera populations, as in set 1, and one produced progeny with only the native LSR1 Buchnera. Thus, although individual injections differed in outcome, this protocol gave a high frequency of transfected lines.
Table 1.
Experimental replacement of native Buchnera (LSR1 type) with donor Buchnera (5AY type)
| Set | Heat treatment | Number injected that survive to adulthood | Number injected that reproduce | Number surviving mothers with F1 having: | ||
| Mixed Buchnera | Only LSR1 Buchnera | Only 5AY Buchnera | ||||
| 1 | 4 h, 35 °C | 4 | 4 | 4 | 0 | 0 |
| 2 | 5 h, 35 °C | 6 | 5 | 2 | 1 | 2 |
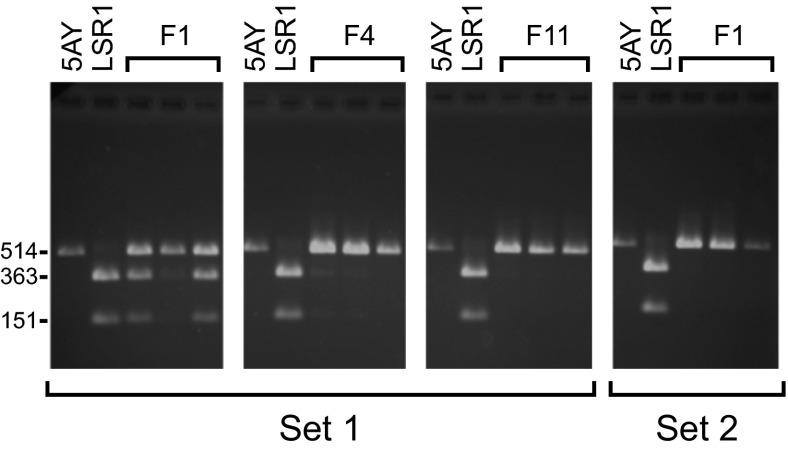
Fig. 2.
Screens for the presence of the LSR1 and 5AY Buchnera genotypes based on a PCR and diagnostic restriction digest. Each lane is from a screen of a single female aphid; lanes labeled 5AY and LSR1 are controls from stock aphids. LSR1 Buchnera contain a distinctive restriction site, yielding bands of 363 bp and 151 bp for LSR1 and a single band of 514 bp for 5AY. Results for set 1 are shown for the F1, F4, and F11 generations. Some LSR1 Buchnera remained in F4 females but were eliminated by a heat exposure in F5, and this was maintained in future generations, as shown by the assay in F11. In set 2, F1 individuals exhibited complete replacement of the original LSR1 Buchnera by the 5AY Buchnera.
Effects of Symbiont Replacement on Host Thermal Tolerance.
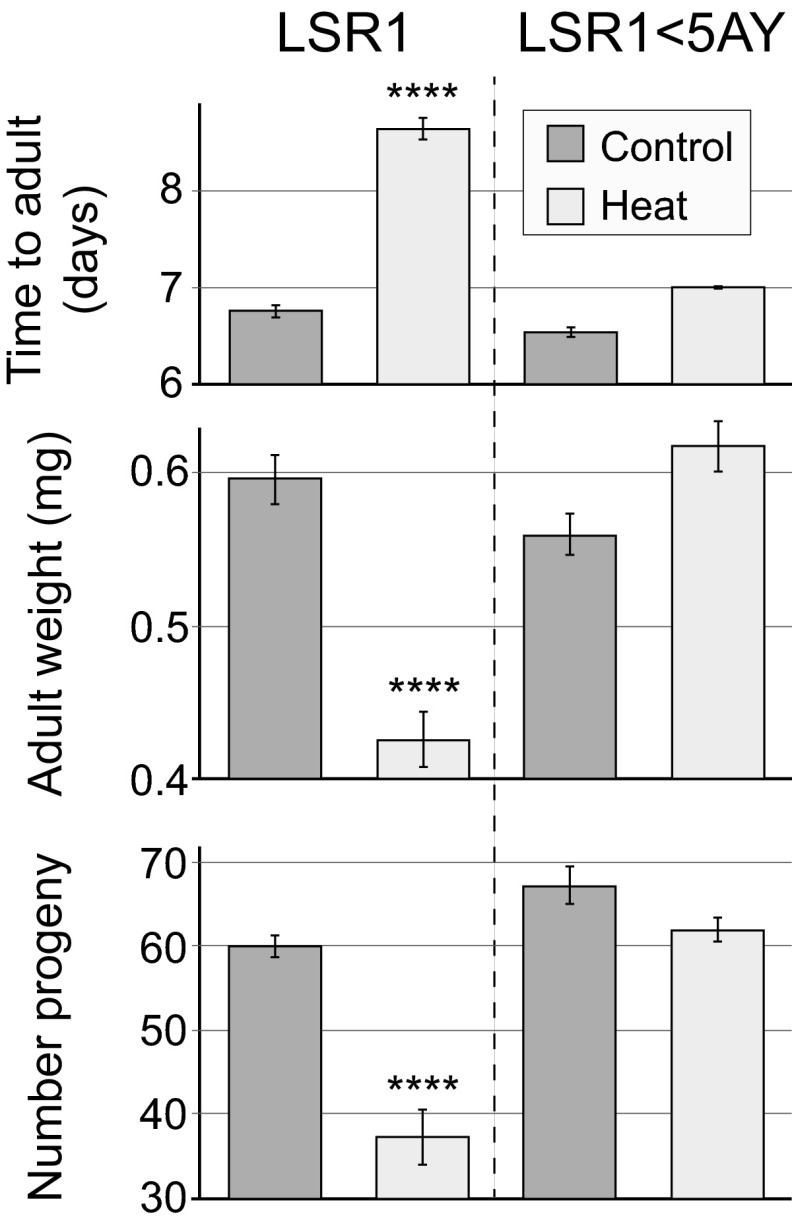
After the successful replacement of the heat-sensitive LSR1 Buchnera with heat-tolerant 5AY Buchnera, we examined the consequences for aphid growth and reproduction at different temperatures. We compared developmental time, adult mass, and fecundity of A. pisum LSR1 aphids retaining native Buchnera with those of A. pisum LSR1 aphids with the 5AY Buchnera under constant 20 °C and under 20 °C interrupted by exposure to 35 °C for 4 h on day 2 (SI Methods). Comparing across treatment categories, there were highly significant differences in all fitness measures: developmental time (Kruskal-Wallis, H = 59.7; df = 3; P < 0.0001), adult weight (Kruskal-Wallis, H = 111.3; df = 3; P < 0.0001), and fecundity (Kruskal-Wallis, H = 35.8; df = 3; P < 0.0001; Fig. 3). These differences were entirely attributable to lower fitness of A. pisum LSR1 with native Buchnera after heat: These aphids took longer to mature, were smaller, and had lower fecundity compared with other treatment categories. In contrast, A. pisum LSR1 with the replacement 5AY Buchnera were not significantly affected by heat exposure, for any of the measures, and the two lines had similar performance, with no significant differences between them, under constant 20 °C (Fig. 3). Thus, the replacement of its Buchnera greatly increased heat tolerance of the A. pisum LSR1 aphid matriline, demonstrating a major effect of symbiont genotype on host fitness.
Fig. 3.
Effect of symbiont replacement on aphid fitness under constant 20 °C and after heat exposure, measured as developmental time, adult weight, and fecundity (mean ± SE). The LSR1 matriline contains heat-sensitive Buchnera, whereas the LSR1 < 5AY matriline has a replacement Buchnera with normal heat tolerance. Replacement of Buchnera results in faster development, larger adult size, and increased fecundity after heat stress (****P < 0.0001, Kruskal-Wallis).
Discussion
In parthenogenetic aphid generations, embryos begin development before their mothers are mature, so embryos ready for colonization are present in the injected juvenile aphids. The success of the transfers indicates an ability of the donor Buchnera to invade the posterior syncytium of these developing embryos and to be packaged normally during subsequent development and inherited stably in subsequent generations. The heat-induced depletion of maternal Buchnera confers a numerical advantage to donor Buchnera. In many cases, the initial injection resulted in a mixed population of Buchnera, and further selection with heat resulted in complete replacement.
A limitation of this method is that the recipient matriline must contain Buchnera with the ibpA allele that confers heat intolerance. However, we have found that Buchnera in most A. pisum matrilines evolve to the heat-sensitive genotype after extended laboratory culture at 15–20 °C, reflecting a high mutation rate at this allele and selection favoring this genotype under cool conditions (12).
The persistence of both Buchnera types for four generations, before the replacement was completed through a second round of selection with heat, is somewhat unexpected; it suggests that the effective bottleneck size is large enough to enable persistence of polymorphism across generations. A previous estimate of the number of Buchnera infecting sexual progeny was 1,800 (13); if the number is similar for parthenogenetic generations, it would be consistent with the maintenance of polymorphism of equally fit Buchnera over numerous generations. This question of bottleneck size is complicated by several aspects of the symbiont life cycle: they divide rapidly around the time of inoculation, are packaged into bacteriocytes during development, and can colonize an embryo from more than one maternal bacteriocyte. The effective bottleneck size could be estimated by assaying the rate of loss of Buchnera polymorphism over aphid generations, which is a potential application of our method of Buchnera transfer.
In addition to obligate symbionts, exemplified by Buchnera, insects including aphids often contain facultative bacterial symbionts that are mostly maternally transmitted but undergo occasional horizontal transfer between host matrilines (14, 15). Many facultative symbionts are readily transferred between hosts in the laboratory, and such transfers have been a critical tool in the experimental identification of symbiont-based traits while controlling for host genetic background (8, 15–19). This ease of transferring facultative symbionts might be expected, as they undergo horizontal transfer in natural populations via several mechanisms (20–22), and their genomes encode machinery such as type III secretion systems, known to enable invasion of eukaryotic cells (9, 10). In contrast, exclusively maternally transmitted, obligate symbionts, such as Buchnera, are never faced with the challenge of invading new matrilines. These obligate symbionts have reduced genomes that do not encode recognizable mechanisms for host cell invasion (23). Microscopy studies suggest that Buchnera is much more dependent than facultative symbionts on host-based mechanisms for transmission from mother to progeny (5).
An alternative approach to assigning phenotypic traits to host versus symbiont is to create specific combinations of symbiont and host genotypes through genetic crosses that exploit the exclusive maternal transmission of symbionts (24, 25). However, genetic crosses are difficult or impossible in many host species, including aphids. Our method generates transfected insects with identical host genotypes within several weeks and enables examination of the effects of Buchnera genotype while controlling for aphid genetic background. Potentially, the approach could be combined with mutagenesis (26) to explore effects of Buchnera mutations on the symbiont–host interface.
The intimate interdependence of Buchnera and aphids approaches that of organelles and their host cells; for example, it involves exchange of molecules, including host-encoded proteins between the host and bacterial cytosols (7). An unanswered question is the extent of reciprocal coadaptation of host and symbiont lineages. Potentially, the rapid evolution of symbiont genes and genomes (23) is tracked by adaptive compensatory changes in host genes, accelerating the establishment of incompatibilities between symbiont and host genotypes from different host matrilines. If so, intimate symbioses might facilitate reproductive isolation and speciation. If beneficial symbionts such as Buchnera are generally interchangeable among related host species, this would suggest the lack of an evolutionary “arms race” in these mutualistic relationships. Transfers of Buchnera between aphids with different levels of divergence could be useful for exploring the extent of symbiont–host genetic matching and for illuminating the nature of the symbiotic interface.
Methods
Replacement of Native Buchnera.
After screening laboratory aphid lines for the Buchnera allele that governs heat sensitivity (SI Methods), we selected as recipient A. pisum LSR1, previously used for genome sequencing of A. pisum (27). This line had gained the heat-sensitive Buchnera mutation during long-term laboratory culture. As donor, we selected A. pisum 5AY, which was previously isolated as a subline of A. pisum 5A (28), for which the Buchnera genome has been sequenced (29). A. pisum 5AY contained Buchnera with the heat-tolerant allele. We developed an assay to distinguish the two Buchnera types, based on a polymorphism distinct from that affecting heat tolerance that introduced a unique restriction site into the LSR1 fragment. Thus, the assay consisted of PCR amplification and restriction digestion of a 514-base pair genomic fragment containing the polymorphism, followed by agarose gel electrophoresis (details in SI Methods).
To reduce Buchnera numbers in recipients, 2-d-old nymphs of A. pisum LSR1 were heat-exposed at 34–35 °C for 4 (set 1) or 5 (set 2) h. In each set, 25–30 individuals were injected the following day with homogenate consisting of freshly crushed abdomens of fourth instar or young (1–3 d postmolt) adults of the A. pisum 5AY donor line. Injected females were placed on plants to feed. Two different investigators performed the two sets of injections. Progeny of the injected females were allowed to mature and reproduce before being collected for screening, and screening was continued for up to 11 generations.
For cases of coexistence of both Buchnera genotypes within a matriline, 2-d-old females were again exposed to 4 h of 34–35 °C, and their descendants were allowed to mature and reproduce before being screened.
Measurement of Buchnera Effect on Aphid Thermal Tolerance.
Heat tolerance of a line of LSR1 < 5AY (A. pisum LSR1 in which the original Buchnera had been replaced with Buchnera of A. pisum 5AY) was compared with the heat tolerance of the original line of A. pisum LSR1. The LSR1 < 5AY line was from the set 1 transfer experiment and was compared using aphids from generation 12 after the transfer. Adults of LSR1 and of LSR1 < 5AY were placed on plants overnight at 20 °C and removed, resulting in even-aged nymphs on each plant. Eight colonies were established for each of the two matrilines. On the following day, four colonies of each line were placed at 35 °C for 4 h and then returned to 20 °C. Four control colonies of each line were kept at continuous 20 °C. Aphids were monitored daily, and dates of molting to adulthood and weights of new adults were recorded, with total sample sizes of 60, 48, 59, and 84 aphids for LSR1 heat-treated, LSR1 control, LSR1 < 5AY heat-treated, and LSR1 < 5AY control, respectively. For each of the 16 colonies, four newly eclosed adults were placed individually on fresh plants, and these were kept at 20 °C. Date of first reproduction was recorded, and total number of nymphs produced during the following 6 d was recorded as a measure of fecundity.
Supplementary Material
Acknowledgments
We thank Howard Ochman for running electrophoretic gels and Kim Hammond for help with growing plants and aphids. Y.Y. was supported by a fellowship from the China Scholarship Council, and other research funding was received from the University of Texas at Austin.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 1923.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1420037112/-/DCSupplemental.
References
- 1.Archibald J. One Plus One Equals One: Symbiosis and the Evolution of Complex Life. Oxford University Press; Oxford: 2014. [Google Scholar]
- 2.McFall-Ngai M, et al. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci USA. 2013;110(9):3229–3236. doi: 10.1073/pnas.1218525110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet. 2008;42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 4.Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature. 2000;407(6800):81–86. doi: 10.1038/35024074. [DOI] [PubMed] [Google Scholar]
- 5.Koga R, Meng X-Y, Tsuchida T, Fukatsu T. Cellular mechanism for selective vertical transmission of an obligate insect symbiont at the bacteriocyte-embryo interface. Proc Natl Acad Sci USA. 2012;109(20):E1230–E1237. doi: 10.1073/pnas.1119212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price DRG, et al. Aphid amino acid transporter regulates glutamine supply to intracellular bacterial symbionts. Proc Natl Acad Sci USA. 2014;111(1):320–325. doi: 10.1073/pnas.1306068111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakabachi A, Ishida K, Hongoh Y, Ohkuma M, Miyagishima SY. Aphid gene of bacterial origin encodes a protein transported to an obligate endosymbiont. Curr Biol. 2014;24(14):R640–R641. doi: 10.1016/j.cub.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 8.Oliver KM, Degnan PH, Burke GR, Moran NA. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol. 2010;55:247–266. doi: 10.1146/annurev-ento-112408-085305. [DOI] [PubMed] [Google Scholar]
- 9.Degnan PH, Yu Y, Sisneros N, Wing RA, Moran NA. Hamiltonella defensa, genome evolution of protective bacterial endosymbiont from pathogenic ancestors. Proc Natl Acad Sci USA. 2009;106(22):9063–9068. doi: 10.1073/pnas.0900194106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dale C, Plague GR, Wang B, Ochman H, Moran NA. Type III secretion systems and the evolution of mutualistic endosymbiosis. Proc Natl Acad Sci USA. 2002;99(19):12397–12402. doi: 10.1073/pnas.182213299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braendle C, et al. Developmental origin and evolution of bacteriocytes in the aphid-Buchnera symbiosis. PLoS Biol. 2003;1(1):E21. doi: 10.1371/journal.pbio.0000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunbar HE, Wilson ACC, Ferguson NR, Moran NA. Aphid thermal tolerance is governed by a point mutation in bacterial symbionts. PLoS Biol. 2007;5(5):e96. doi: 10.1371/journal.pbio.0050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mira A, Moran NA. Estimating population size and transmission bottlenecks in maternally transmitted endosymbiotic bacteria. Microb Ecol. 2002;44(2):137–144. doi: 10.1007/s00248-002-0012-9. [DOI] [PubMed] [Google Scholar]
- 14.Sandström JP, Russell JA, White JP, Moran NA. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol Ecol. 2001;10(1):217–228. doi: 10.1046/j.1365-294x.2001.01189.x. [DOI] [PubMed] [Google Scholar]
- 15.Boyle L, O’Neill SL, Robertson HM, Karr TL. Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science. 1993;260(5115):1796–1799. doi: 10.1126/science.8511587. [DOI] [PubMed] [Google Scholar]
- 16.Montllor CB, Maxmen A, Purcell AH. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol Entomol. 2002;27:189–195. [Google Scholar]
- 17.Oliver KM, Russell JA, Moran NA, Hunter MS. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci USA. 2003;100(4):1803–1807. doi: 10.1073/pnas.0335320100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouchet R, Vorburger C. Strong specificity in the interaction between parasitoids and symbiont-protected hosts. J Evol Biol. 2012;25(11):2369–2375. doi: 10.1111/j.1420-9101.2012.02608.x. [DOI] [PubMed] [Google Scholar]
- 19.Walker T, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476(7361):450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 20.Moran NA, Dunbar HE. Sexual acquisition of beneficial symbionts in aphids. Proc Natl Acad Sci USA. 2006;103(34):12803–12806. doi: 10.1073/pnas.0605772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gehrer L, Vorburger C. Parasitoids as vectors of facultative bacterial endosymbionts in aphids. Biol Lett. 2012 doi: 10.1098/rsbl.2012.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huigens ME, et al. Infectious parthenogenesis. Nature. 2000;405(6783):178–179. doi: 10.1038/35012066. [DOI] [PubMed] [Google Scholar]
- 23.McCutcheon JP, Moran NA. Extreme genome reduction in symbiotic bacteria. Nat Rev Microbiol. 2012;10(1):13–26. doi: 10.1038/nrmicro2670. [DOI] [PubMed] [Google Scholar]
- 24.Vogel KJ, Moran NA. Sources of variation in dietary requirements in an obligate nutritional symbiosis. Proc Biol Sci. 2011;278(1702):115–121. doi: 10.1098/rspb.2010.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaenike J. Spontaneous emergence of a new Wolbachia phenotype. Evolution. 2007;61(9):2244–2252. doi: 10.1111/j.1558-5646.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- 26.Tagu D, Le Trionnaire G, Tanguy S, Gauthier J-P, Huynh J-R. EMS mutagenesis in the pea aphid Acyrthosiphon pisum. G3 (Bethesda) 2014;4:657–667. doi: 10.1534/g3.113.009639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.International Aphid Genomics Consortium Genome sequence of the pea aphid Acyrthosiphon pisum. PLoS Biol. 2010;8(2):e1000313. doi: 10.1371/journal.pbio.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moran NA, Jarvik T. Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science. 2010;328(5978):624–627. doi: 10.1126/science.1187113. [DOI] [PubMed] [Google Scholar]
- 29.Moran NA, McLaughlin HJ, Sorek R. The dynamics and time scale of ongoing genomic erosion in symbiotic bacteria. Science. 2009;323(5912):379–382. doi: 10.1126/science.1167140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.