Significance
Bacterial pathogens produce pore-forming toxins that damage eukaryotic membranes, whereas the pore-forming immune defense proteins produced by vertebrates can damage bacterial membranes. Despite the opposite functions of these proteins in pathogenesis or protection, many use a common pore-forming mechanism whereby membrane-bound monomers oligomerize into a circular structure, termed the prepore, which then assembles a β-barrel structure that punches a hole in the membrane. Here we show that once the prepore is assembled, an intermolecular electrostatic interaction is established that drives the formation of the pore. This mechanism is likely to be used by toxins and other pore-forming proteins that span the biological domains of life.
Keywords: hemolysin, streptolysin, sterol, toxin, alpha-hemolysin
Abstract
β-Barrel pore-forming toxins (βPFTs) form an obligatory oligomeric prepore intermediate before the formation of the β-barrel pore. The molecular components that control the critical prepore-to-pore transition remain unknown for βPFTs. Using the archetype βPFT perfringolysin O, we show that E183 of each monomer within the prepore complex forms an intermolecular electrostatic interaction with K336 of the adjacent monomer on completion of the prepore complex. The signal generated throughout the prepore complex by this interaction irrevocably commits it to the formation of the membrane-inserted giant β-barrel pore. This interaction supplies the free energy to overcome the energy barrier (determined here to be ∼19 kcal/mol) to the prepore-to-pore transition by the coordinated disruption of a critical interface within each monomer. These studies provide the first insight to our knowledge into the molecular mechanism that controls the prepore-to-pore transition for a βPFT.
The cholesterol-dependent cytolysins (CDCs) make up the largest class of bacterial β-barrel pore-forming toxins (βPFTs) and are present in nearly 50 Gram-positive opportunistic pathogens. A central paradigm of βPFTs is the formation of an obligatory intermediate termed the prepore (1–6). The prepore is a membrane-bound oligomerized ring-shaped complex in which the membrane-spanning β-barrel pore has not formed. Pore conversion, characterized by β-barrel insertion, only occurs after prepore completion. For all βPFTs, the molecular mechanism or mechanisms that control the transition from the prepore to the pore remains unknown.
The archetype CDC, perfringolysin O (PFO), coordinates the assembly of about 37 monomers into a prepore and then assembles and inserts into the membrane a β-barrel pore composed of 74 amphipathic hairpins (7–12). On prepore completion, a signal is generated within the oligomeric complex that triggers this transition. The nexus of the prepore-to-pore transition is the disruption of the interface that domain 3 (D3) forms with domains 1 and 2 (D1,2) (10) (Fig. 1). This interface is formed between D1,2 and one of the two D3 α-helical bundles. The D3 α-helical structures ultimately unfurl and refold into the two transmembrane β-hairpins (TMH1 and TMH2), which contribute to the formation of the giant β-barrel pore (Fig. 1) (11–13). Pore formation also requires that the prepore undergo a 40-Å vertical collapse that allows the TMHs to span the bilayer (7, 8, 14).
Fig. 1.
PFO structure and pore complex assembly. (A) The PFO ribbon structure (19) with magnified D3 (Right) with pertinent residues and structures identified. The D3 core β-sheet (β1–β4) is shown in cyan, and the β5α1 loop is shown in magenta. The α-helical bundles that refold into TMH1 and TMH2 are shown in yellow and purple, respectively (11, 12). The waters surrounding N197 are shown as red spheres. The membrane-binding interface (34–37) is circled. Structures were created and rendered using UCSF Chimera (38). (B) Schematic overview of the PFO prepore assembly and its conversion to the pore complex (39). The D3 counterparts (dashed circle) are colored the same as in A. Bound monomers oligomerize into the prepore structure, and then the D3 interface with domains 1 and 2 (D1,2) is disrupted and the α-helical bundles unfurl and refold into the TMHs to form the β-barrel pore.
Here we show that the prepore-to-pore transition is controlled by an intermolecular electrostatic interaction between E183 and K336 in PFO. Loss of either charged residue traps PFO in a stable prepore state, whereas pore formation can be restored by weakening the D3–D1,2 interface by a single mutation or by increasing the temperature. Molecular measurements with cross-linkers and molecular dynamics simulations suggest these two residues are positioned to form a strong electrostatic interaction or salt bridge. Our studies suggest the interactions that stabilize the D3–D1,2 interface are the primary barrier to the prepore-to-pore transition and further show that the E–K interaction provides the necessary free energy to overcome this transition state barrier.
Results
Conserved E–K pair Is Essential for PFO and Streptolysin O Activity.
A Cys scan of the PFO primary structure revealed that K336 and E183 mutation reduced pore-forming activity >10-fold. E183 is located in D3 at the end of β1, whereas K336 is located on the opposite side of D3 at the tip of a loop formed by β-strand 5 (β5) and α-helix 1 (α1) (Fig. 1A). An essentially identical arrangement is observed in the streptolysin O (SLO) crystal structure (15). Primary structure alignments of known CDCs show conservation of these two residues in about 50% of the CDCs, with all but one of these CDCs clustering into the same large phylogenetic clade (Fig. S1).
The contribution of these charged residues to PFO pore-forming activity was examined in several purified mutants (Table 1). In essence, these results show that uncharged substitutions for either residue eliminate or reduce pore-forming activity >99%, whereas conservative substitutions (Glu→Asp or Lys→Arg) or swapping the E–K pair positions were not well tolerated.
Table 1.
Features of the E and K mutants of PFO and SLO
| Toxin | Percentage activity | SDS-stable oligomer |
| PFO | 100 | Yes |
| E183A | ND | Yes† |
| E183C | ND* | Yes |
| E183D | ND* | Yes |
| K336A | ND* | Yes |
| K336C | ND* | Yes |
| K336G | ND | Yes |
| K336R | 19 ± 4 | Yes |
| E183A/K336A | ND* | Yes |
| E183C/K336C | ND | Yes |
| E183K | ND | Yes |
| K336E | ND | Yes |
| E183K/K336E | ND* | Yes |
| SLO | 100 | Yes |
| E245A | ND | No |
| K407A | ND | No |
The relative percentage pore-forming activity (and SD) for each mutant relative to PFO or SLO and formation of an SDS stable oligomer are shown. ND, pore formation not detected in the range of toxin concentration used. ND*, the EC50 could not be determined, as 100% activity was not achieved at the highest toxin concentration used.
Partially resistant to SDS dissociation.
Partial activity of the electrostatic Cys mutants could be restored in either PFOE183C or PFOK336C by chemical modification with the positively and negatively charged sulfhydryl-specific reagents MTS-EA(+) (2-aminoethyl methanethiosulfonate) or MTS-ET(+) [2-(trimethylammonium)ethyl MTS] and MTS-ES(−) (2-sulfonatoethyl MTS) (Table S1), but only when each Cys mutant was modified with the cognate charge. More than 90% of the sulfhydryl groups were modified with these reagents, yet only ∼20% activity was restored, suggesting the correct positioning of the charges is necessary for efficient pore formation.
E183 and K336 Mutants Trap PFO in a Prepore State.
The late-stage prepore of PFO is characterized by its resistance to dissociation by SDS (without heating to 95 °C for 5 min) when separated by SDS-agarose gel electrophoresis (AGE) (5, 10), whereas the pore complex resists dissociation by SDS and heat. Single or double mutants of E183 and K336 were able to form SDS-resistant or, in the case of PFOE183A, partially resistant prepore complexes (Table 1) but were not resistant to SDS and heat.
Chemical modification of PFOE183C and PFOK336C with the appropriately charged MTS reagents (described in Table S2) was equally effective at restoring partial activity, whether they were added before or after prepore formation (Fig. 2). Hence, neither E183 nor K336 are necessary for prepore formation, but they are necessary for the prepore-to-pore transition.
Fig. 2.
Chemical modification of PFOE183C and PFOK336C with sulfhydryl-specific charged reagents trigger pore conversion. PFOE183C and PFOK336C (1 μM) were modified with charged MTS-ES(−) or MTS-ET(+), respectively, before (solid lines) or after (dashed lines) prepore formation on CF liposomes. CF release was continuously monitored over time. Arrows indicate addition of the MTS reagents after prepore formation. Graphs are representative of three independent experiments.
E183 and K336 Undergo a Polar-to-Nonpolar Transition on PFO Prepore Formation.
If E183 and K336 formed an intermolecular electrostatic interaction at the interface between the monomers in the prepore, then the side-chains of both residues would be predicted to transition from a polar to a less polar environment at this interface. A sulfhydryl-specific environmentally sensitive probe N,N′-dimethyl-N-(iodoacetly)-N′-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) ethylenediamine [IANBD (12)], the emission of which is quenched by water, was coupled to the Cys sulfhydryl of PFOE183C and PFOK336C, and the changes in its fluorescence emission were monitored as these proteins move from the soluble monomer to the prepore complex (12). The probe entered a less polar environment in both cases during prepore assembly, as evidenced by a blue-shifted and increased fluorescence emission of the probe that likely results from the exclusion of water at the interface (Fig. S2).
Distance Measurements Suggest the Charged Centroids of E183 and K336 Are Juxtaposed in the PFO Prepore Complex.
The double Cys mutant PFOE183C:K336C was subjected to cross-linking analysis with various length homobifunctional cross-linkers to estimate the distance between the α-carbons of the Cys residues. This measurement was then compared with the estimated distance separating the α-carbons of an E–K pair that forms a strong electrostatic interaction or salt bridge (Fig. 3B). The PFOE183C:K336C prepore is dissociated by the combination of SDS and heat; hence, the Cys sulfhydryls alone are not sufficiently close to form disulfides and stabilize its prepore [Fig. 3A, the lane without added bismaleimidoethane (BMOE) cross-linker].
Fig. 3.
Distance separating the α-carbons of E183 and K336 before and after β5 disengages from β4. Prepore complexes of PFOE183C:K336C were treated with homobifunctional, sulfhydryl-specific cross-linkers (40). (A) SDS-AGE panel 1: lanes 1–3, PFO or its pore complex treated as shown (SDS+Heat, 95 °C for 5 min). Panels 2–4: PFOE183C:K336C prepore treated with a 0–5 M excess of BMOE, BMB, and DBB, and then heated (95 °C for 5 min) in SDS sample buffer before SDS-AGE. (B, Upper and Lower, respectively) Approximate distances separating the ɑ-carbons of Cys residues cross-linked with BMOE or of an E–K pair that form an electrostatic interaction or salt bridge. (C) PFOE183C:K336C:Y181A was incubated with BMOE and BMB as in A. Images are representative of three independent experiments. L, liposomes; M, monomer; O, oligomer; X-L, molar excess of cross-linker to protein.
Highly efficient (85–98%) stabilization of the PFOE183C:K336C prepore complex was achieved at a 1:1 and 5:1, but not a 1:4, molar ratio of the BMOE cross-linker to protein (Fig. 3A). The longer [bismaleimidobutane (BMB)] and the shorter [dibromobimane (DBB)] cross-linkers were less efficient and did not stabilize the prepore (Fig. 3A), although several intermediate-sized oligomers were evident. These results suggest that the Cys α-carbons are ∼14 Å apart in the prepore, similar to the estimated distance separating the α-carbons of a E–K pair that position their centroids of charge sufficiently close (≤4 Å) to form a strong electrostatic interaction or salt bridge (16, 17), as schematically shown in Fig. 3B.
Disengagement of β5 from β4 Is Necessary to Appose E183 and K336.
Restricting the movement of β5 away from β4 with an engineered disulfide results in an SDS-sensitive early-stage prepore (18). Mutation of Y181 to Ala also results in an SDS-sensitive prepore complex, suggesting the disengagement of β5 from β4 is also restricted in this mutant, which we indeed determined to be the case (Fig. S3). Thus, this mutation was introduced to the PFOE183C:K336C background (rather than introducing additional cysteines for a disulfide bridge) to determine whether β5 must disengage from β4 to be repositioned sufficiently near E183 to be cross-linked. Neither BMOE nor the longer cross-linker BMB stabilized the prepore of PFOE183C:K336C with Y181A mutation (Fig. 3C). Hence, restricting the disengagement of β5 from β4 prevents K336 from being positioned near E183 in the prepore complex.
Altering the D3–D1,2 Interface Stability Restores Pore Formation in PFOE183C and PFOK336C.
In the CDCs that maintain the E–K pair, an Asn is conserved at the D3–D1,2 interface (Fig. S1). PFO N197 occupies a hydrophilic pocket at this interface where its side chain forms hydrogen bonds with Ser-93 (water mediated) and Asp-91 and is surrounded by a network of hydrogen-bonded water molecules, which also contribute to the interface stability (19) (Fig. 1A). Therefore, could the pore-forming activity be restored in PFO mutants that lacked either E183 or K336 by replacing N197 with a residue that would disrupt these stabilizing interactions?
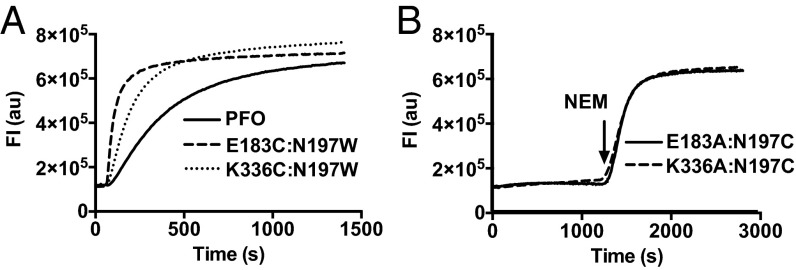
Tryptophan was substituted for N197, as modeling of this residue in the PFO structure suggested that its hydrophobic side chain would exhibit difficulty packing into the interface, be incapable of forming the N197 interactions, and disrupt the water-mediated interactions. This mutation restored pore-forming activity in both PFOE183C and PFOK336C to near PFO levels and increased the rate of pore formation over that of PFO (Fig. 4A). When Cys was substituted for N197, it did not restore activity in the cognate alanine mutants for E183 and K336. However, chemical modification of the Cys sulfhydryl with N-ethylmaleimide (NEM) after the assembly of the prepore triggered rapid and quantitative conversion to the pore (Fig. 4B).
Fig. 4.
Mutations for N197 restore pore-forming activity in PFOE183C and PFOK336C. (A) Trp was substituted for N197 in PFOE183C and PFOK336C, and then the rate and extent of pore formation on CF liposomes over time was compared with PFO. (B) PFOE183A:N197C and PFOK336A:N197C were incubated with CF-liposomes at RT to assemble the prepore complex while the fluorescence emission was continually recorded (20 min). NEM (20 molar excess over protein) was then injected (arrow) into the stirred cuvette, and CF release was monitored an additional 20 min. Results are representative of three independent experiments.
N197 Mutations Decrease the Melting Temperatures of PFO and Its Derivatives.
The introduction of Trp likely destabilized the D3–D1,2 interface, which allowed the prepore-to-pore transition. Consistent with this premise, the introduction of Trp at the D3–D1,2 interface decreased the melting temperatures (Tm) by 3.5 °C and 4.1 °C for PFOE183C and PFOK336C, respectively (Table 2). The N197C mutation also decreased the Tm of PFOE183A and PFOK336A by 4.3 °C and 2.8 °C, respectively, but it did not restore activity (Fig. 4). Modification with NEM decreased the Tm further and also restored activity. The stability of the D3–D1,2 interface is apparently the primary determinant of the Tm, as an engineered disulfide between D3 and D2 (PFOPP), which prevents the α-helical bundle of D3 from disengaging from this interface and traps PFO in a prepore (10), increased the Tm of oxidized PFOPP >4 °C over PFO, but decreased about 2 °C less than PFO when this disulfide was reduced (Table 2).
Table 2.
Tm of PFO and derivatives
| Toxin | Tm (°C) | ∆Tm (°C) |
| PFO | 49.0 ± 0.2 | — |
| PFO + NEM | 48.7 ± 0.5 | −0.3 |
| E183C | 47.6 ± 0.3 | −1.4 |
| E183C:N197W | 44.1 ± 0.5 | −4.9 (−3.5) |
| E183A:N197C | 44.7 ± 0.5 | −4.3 |
| E183A:N197CNEM | 43.9 ± 0.4 | −5.1 (−0.8) |
| K336C | 47.6 ± 0.3 | −1.4 |
| K336C:N197W | 43.5 ± 0.3 | −5.5 (−4.1) |
| K336A:N197C | 46.2 ± 0.2 | −2.8 |
| K336A:N197CNEM | 44.7 ± 0.3 | −4.3 (−1.5) |
| PFOPP (Ox) | 53.2 ± 0.4 | +4.2 (+6.0)* |
| PFOPP (Red) | 47.2 ± 0.2 | −1.8 |
The Tm values for PFO, PFOE183C, and PFOK336C with N197W or NEM-labeled N197C, and PFOPP under oxidizing (Ox) or reducing (Red) conditions. Experiments were performed in triplicate. ∆Tm, difference in Tm between PFO and mutant. Parenthesis indicates ∆Tm between indicated derivative and the cognate nonsubstituted or unlabeled counterpart.
∆Tm between the Ox and Red forms of PFOPP itself.
The Arrhenius Activation Energy for the Prepore-to-Pore Transition.
The Tm results showed that the D3–D1,2 interface stability is the primary determinant of the PFO Tm and suggested that elevating the temperature might drive prepore-to-pore conversion when the electrostatic interaction is absent. The PFOE183C pore-forming activity progressively increased as the temperature was increased (Fig. 5A), and it achieved similar activity to that exhibited by the PFOE183C:N197W mutant and PFO (Fig. 4A) when the temperature exceeded its Tm.
Fig. 5.
Arrhenius Ea of the prepore-to-pore transition. (A) Pore-forming activity of PFOE183C on CF-liposomes with increasing temperature. (B) The initial velocities of the CF release from A were used to calculate the Ea of pore formation by PFOE183C. (C) The Ea of PFOE183C pore formation was determined as in B, but only after it had first formed the prepore at RT for 20 min on CF liposomes, which eliminates the contribution of binding and oligomerization to the Ea. (D) The Ea for PFO was determined as in B, with PFO added directly to liposomes held at the indicated temperatures. The data are representative of two experiments.
These results allowed us to determine the activation energy (Ea) of the prepore-to-pore transition, as the Ea for binding and oligomerization (20) could be eliminated by first allowing PFOE183C to assemble into the prepore at room temperature. Using the initial rates (k0) of pore formation at 30–50 °C, the Ea was calculated to be ∼40 kcal/mol for pore formation when the PFOE183C prepore was not first formed on the liposomes, which reflects the total Ea required to bind, oligomerize, and form the pore (Fig. 5B). When the PFOE183C prepore was first formed at room temperature before determining the k0 at each temperature, the Ea for the prepore-to-pore transition was calculated to be ∼19 kcal/mol (Fig. 5C). Hence, the Ea for binding and oligomerization is ∼21 kcal/mol (i.e., 40–19 kcal/mol), which is similar to the Ea of about 19 kcal/mol determined previously for the binding and oligomerization of PFO on human erythrocytes (20).
The Ea for PFO was determined to be 24 kcal/mol (Fig. 5D). This value is consistent with the Ea of 22–23 kcal/mol for pore formation on erythrocytes by PFO (20, 21). Because the E–K pair remains intact in PFO, it provides the necessary free energy to disrupt the D3–D1,2 interface, so this component is largely absent from the Ea for PFO.
Molecular Dynamic Simulations of the E183 and K336 Interaction.
Molecular dynamic (MD) simulations of the PFO pore support the formation of an intermolecular salt bridge between the E183 and K336 residues of adjacent monomers. CDC monomers assume an upright position perpendicular to the membrane (Fig. 1) and then undergo a 40-Å collapse to insert the β-barrel pore (Fig. 1B) (7, 8, 14). These experimental data are consistent with a substantial tilt of the monomers with respect to the membrane surface (22). At the initiation of the MD simulation, when the prepore intermediate is not yet tilted, the charge centroids of E183 and K336 in adjacent monomers are separated by ∼16 Å, and the α-carbons by ∼19 Å (Fig. 6A). As the MD simulation proceeds, monomers within the prepore oligomer undergo a 30° tilt, K336 moves closer to E183 (Fig. 6B), and finally, the charge centroids come within ∼2 Å of each other and spontaneously form an intermolecular salt bridge (Fig. 6C). In addition, as the two residues move closer together, they concomitantly draw β-strands 1 and 4 closer together so they can form backbone hydrogen bonds.
Fig. 6.
MD simulations of the E183 and K336 interaction in the prepore complex. (A–C) Three images of two adjacent monomers are shown from simulations of the PFO prepore-to-pore transition depicting the distance between the E–K pair (shown as sticks inside the circles) as the monomers tilt within the prepore complex before spontaneously forming a salt bridge. (C) Includes a magnification of the E–K pair forming a salt bridge.
Discussion
The displacement of the β5α1 loop from its interaction with β4 (Fig. 1) is necessary for stable prepore formation (18, 23). We now show that after prepore assembly, the β5α1 loop plays a second critical role: it is repositioned so that K336 is placed sufficiently near E183 to form a strong intermolecular electrostatic interaction or salt bridge. This interaction then drives the disruption of the D3–D1,2 interface, thereby committing the prepore to undergo the transition to the pore complex. The loss of pore-forming activity with conservative substitutions or by swapping the charged pair positions suggests correct positioning of the charge centroids is critical for this interaction. It appears that this control system is also present in SLO, as knocking out either residue of its homologous E–K pair eliminates its pore-forming activity (Table 1). These studies reveal the first molecular mechanism to our knowledge by which a βPFT controls the critical prepore-to-pore transition.
Measurements using homobifunctional sulfhydryl-specific cross-linkers estimated the distance between the charged centroids for the E–K pair to be 2–4 Å (corresponding with 12–14 Å between their α-carbons) in the prepore complex, consistent with the formation of a strong intermolecular electrostatic interaction or a salt bridge (17, 24). The distance between these two residues increased when the movement of the β5α1 loop, which contains K336 at its tip (Fig. 1A), was restricted (22). BMOE cross-linking did not restore pore formation, which suggests the distance between the charged centroids of E183 and K397 is necessarily reduced to less than 4 Å (perhaps forming a salt bridge, as suggested by the MD simulation) to disrupt the D3–D1,2 interface and trigger the prepore-to-pore transition.
The loss of pore-forming activity was suppressed by mutation of the N197 to Trp, NEM modification of Cys-substituted N197, or increasing the temperature. The decreased Tm for the various mutants were consistent with this scenario and with the fact that we could restore pore-forming activity in the prepore trapped mutants by increasing the temperature to approximately the Tm of PFO. Interestingly, Cys substitution for N197 in the Ala-substituted mutants for E183 and K336 did not restore activity, even though the Tm of PFOE183A:N197C was decreased about 4 °C. In both cases, the chemical modification of its sulfhydryl group with NEM triggered rapid and quantitative conversion to the pore. The small difference (<1 °C) in Tm of the unmodified (inactive) and NEM-modified PFOE183A/N197C (active) suggests the stability of this interface is finely balanced between the prepore and pore states.
The soluble PFO monomer, the prepore, and the pore complex are intermediate structures that represent local minima in the energy landscape of pore formation. The first major transition state barrier for PFO-mediated pore formation is the assembly of the oligomeric prepore; the second major transition state barrier is the prepore-to-pore conversion. The Ea for the entire assembly mechanism (binding to pore formation) is about 40 kcal/mol. This Ea is consistent with the sum of the independently derived Ea of ∼19 kcal/mol for binding and oligomerization (20) and the Ea of ∼19 kcal determined here for the prepore-to-pore transition. The free energy contributed by the formation of a strong electrostatic interaction or salt bridge typically exceeds −1 kcal/mol (16); hence, the formation of one such interaction per monomer of the prepore oligomer (7) would easily exceed the Ea of 19 kcal/mol required to disrupt the D3–D1,2 interface and drive the prepore-to-pore transition. It also explains why Ea for pore formation by PFO is 24 kcal/mol, as the free energy provided by the intact E–K pair would eliminate the prepore-to-pore Ea.
We propose that once the prepore is established, the monomers tilt in a concerted fashion, a process that may be facilitated by the π-stacking of aromatic residues Y181 and F318 (18). This action positions the K336 side chain near the monomer–monomer interface, where E183 resides in the ring complex. The electrostatic interaction between these two residues likely progresses from a weak to strong interaction, which further decreases the distance between them and draws β1 closer to β4. Because β1 is contiguous with the α-helical bundle that resides at the D3–D1,2 interface, this interaction disrupts the remaining stabilizing interactions at the D3–D1,2 interface, which allows the α-helical bundle to unfold and refold into TMH1.
CDCs that exhibit a conserved E–K pair and the cognate Asn at the D3–D1,2 interface cluster in a single large clade when their primary structures are aligned (Fig. S1). Most other CDCs that lack the cognate E–K–N trio instead contain an E–G–G trio. For unknown reasons, these CDCs have apparently evolved a different approach to controlling the prepore-to-pore transition, but as with the electrostatic controlled mechanism, it is likely to be directed at disrupting the D3–D1,2 interface.
Compelling evidence suggests that the unrelated βPFT, Staphylococcus aureus α-hemolysin, may also use an electrostatic mechanism to control the prepore-to-pore transition. α-Hemolysin forms a heptameric pore complex; each monomer contributes a single TMH to the β-barrel (25). Bayley and colleagues (26, 27) showed that the mutation of K110 or D152, which are located at the beginning and end of its folded TMH structure in the monomer, respectively, trapped α-hemolysin in a stable prepore. Subsequently, the crystal structures for the α-hemolysin monomer (28) and pore complex (25) showed that K110 is displaced about 16 Å from its position in the monomer to form a salt bridge with D152 in the pore complex (Fig. S4). Hence, an electrostatically controlled prepore-to-pore transition, similar to that observed here for PFO, may take place in α-hemolysin. A similar arrangement may exist in the octameric hetero-oligomer of S. aureus γ-hemolysin (29) but remains to be shown experimentally.
In summary, the formation of a single intermolecular electrostatic interaction is a vital element that controls the prepore-to-pore transition by supplying the free energy necessary to disrupt a critical interface in the prepore complex, which commits it to inserting the β-barrel pore. These studies will form the basis for the investigation into the mechanisms that control prepore-to-pore conversion by other members of the large βPFT family of toxins (not just the CDCs), as well as the membrane attack complex/perforin family of pore-forming proteins, which are structurally and mechanistically related to the CDCs (30, 31).
Materials and Methods
Plasmids, Bacterial Strains, and Toxin Production and Purification.
The amino acid sequence for the PFO Cys-less derivative (PFOC459A) was codon optimized for E. coli expression and cloned into pET-15b (Novagen) by GenScript. Protein expressed from this plasmid (pRT30) is referred to as PFO here. QuikChange mutagenesis (Stratagene) was used to introduce mutations into PFO, using pRT30 as template. The DNA of each mutant was sequenced and then analyzed using Sequencher.
Hexahistidine-tagged, signal peptide-deficient PFO and derivatives were transformed into E. coli Tuner cells for expression (Novagen). Cells were cultured and the recombinant protein purified, assayed for concentration, and stored under conditions described previously (12), with the exception that cysteine-containing proteins were stored with 50 µM [Tris(2-carboxyethyl)phosphine] at −80 °C.
Liposome Preparation.
Liposomes containing a 45:55 mol % ratio of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (Avanti Polar Lipids) to cholesterol (Steraloids) with and without encapsulated 5 (6)-carboxyfluorescein (CF) (Sigma) were made as previously described (12, 32).
PFO Modification with IANBD and Charged MTS Reagents.
PFO derivatives with a Cys at various positions were labeled at the Cys sulfhydryl with the environmentally sensitive fluorescent dye N,N′-dimethyl-N-(iodoacetly)-N′-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) ethylenediamine (IANBD; Invitrogen) and the free dye removed by previously described methods (18).
Modification of PFO derivatives with the MTS reagents (Affymetrix) was carried out with ∼30,000 molar excess of MTS reagents to protein (per the manufacturers instructions). Modification of proteins with NEM (Invitrogen) was carried out using a 20 molar excess of NEM to protein.
Steady-State and Kinetic Fluorescence Spectroscopy.
All steady-state and kinetic fluorescence-based experiments were performed on a Fluorolog-3 Spectrofluorometer with the FluorEssence software. The pore-forming activity (i.e., EC50) of PFO and its derivatives on CF liposomes was determined as described (32). CF release kinetics before and after modification of the various PFO derivatives with NEM or the MTS reagents were examined by setting the excitation wavelength to 470 nm and following the changes in emission at 515 nm (1 nm bandpass) over time. Data were collected using an integration time of 1 s.
Cross-Linking and SDS-AGE.
Analysis of oligomer formation on liposomes was determined by SDS-AGE, as described previously (5). To ensure the sulfhydryl groups were reduced, PFOE183C:K336C was pretreated with 10 mM DTT for 30 min at room temperature (RT) before the DTT was removed with Sephadex G-50 (PBS with 50 mM EDTA). Toxin (3.5 μM, 7 μM Cys residues) was then incubated with liposomes (20 min at RT) to assemble the prepore before cross-linkers BMOE, BMB, and DBB (Santa Cruz Biotechnology) were added at 0.25, 1, or 5 times molar excess of the cysteines in the PFO derivatives and incubated for 60 min. The reactions were quenched with 10 mM DTT to prevent further cross-linking. SDS-AGE sample buffer (6 μL) and 6 μL of a 25% stock of SDS (in water) were added to each sample, which were then heated at 95 °C for 5 min and separated by SDS-AGE. Cross-linkers were suspended in DMSO or ethanol (DBB) and diluted (PBS-EDTA) to a working concentration of 0.1 mM.
Thermal Tm Measurements.
The Tms of the various PFO mutants (final concentration, 0.5 mg/mL in PBS) was determined using a 7500 Fast Real-Time PCR System using the Protein Thermal Shift Dye Kit (Applied Biosystems) according to the manufacturer’s protocol. The effect of NEM labeling on the Tm for PFO, PFOE183A/N197C, and PFOK336A/N197C was determined by incubating the proteins with NEM (20 molar excess) for 1 h at RT before Tm analysis. The PFOPP was incubated with 1 mM DTT (1 h on ice) to reduce the disulfide before Tm analysis (1 mM DTT had no effect on the Tm of PFO).
Arrhenius Ea Measurements.
PFO and PFOE183C (88 pmol/50 µL) were incubated with CF-liposomes (10 μL) in a final volume of 100 μL for 1 min on ice to minimize prepore formation. This mixture was then injected into 2.45 mL Hepes-buffered saline (HBS) in the fluorometer cuvette held at a specific temperature and CF emission measured over time. The same experiment was repeated with the exception that PFOE183C was first allowed to form the prepore on the CF liposomes at RT for 20 min before injection into HBS. The initial velocity (k0) of pore formation (i.e., the linear portion of the CF release curve) was determined from 20 °C to 50 °C. The Ea was then calculated from the equation Ea = −R*m, where m is the slope of the line derived by plotting ln(k0) versus 1/T(K) and R is the gas constant (8.314 J/molK).
MD Simulation.
A 36-monomer (1,384,727 total atoms) molecular model of the PFO prepore structure was assembled on the basis of the PFO monomer crystal structure (Protein Data Bank ID code 1PFO). The monomers were evenly rotated about a central axis and were solvated and ionized to be electrically neutral with 0.15 M NaCl. Cylindrical boundary conditions were used to restrict the simulation to a radius of 170 Å and a height of 140 Å. MD equilibrium simulations were performed at 310 K, using NAMD 2.9.51 (33) on an IBM BlueGene/Q for 5 ns. An additional model was built (36 monomers) tilted ∼30° vertically in a counterclockwise direction. To induce the tilting movement, weak harmonic constraints were applied (0.1 kcal/mol/Å2) to the monomer backbone in D1–D3 (residues 29–380) to the new template positions, an effective method for applying a bulk movement to large complexes without greatly interfering with side-chain conformations. This motion was repeated six times to sample conformational pathways and side-chain interactions, with each simulation on the order of 10 ns in length (with constraints applied). These were followed by 5–10 ns of simulations with constraints removed.
Supplementary Material
Acknowledgments
The technical assistance of P. Parrish and discussions with A. E. Johnson are appreciated. M.W.P. is a National Health and Medical Research Council of Australia Research Fellow. This work was supported by a grant from the NIH National Institute of Allergy and Infectious Diseases (1R01 AI037657), grants from the National Health and Medical Research Council of Australia, and a Victorian Life Sciences Computation Initiative Grant (VR0021) on its Peak Computing Facility at the University of Melbourne, an initiative of the Victorian Government. This work was partly carried out in the Australian Cancer Research Foundation Rational Drug Discovery Centre, with funding by the Victorian Government Operational Infrastructure Support Scheme to St Vincent’s Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423754112/-/DCSupplemental.
References
- 1.Walker B, Braha O, Cheley S, Bayley H. An intermediate in the assembly of a pore-forming protein trapped with a genetically-engineered switch. Chem Biol. 1995;2(2):99–105. doi: 10.1016/1074-5521(95)90282-1. [DOI] [PubMed] [Google Scholar]
- 2.Sellman BR, Kagan BL, Tweten RK. Generation of a membrane-bound, oligomerized pre-pore complex is necessary for pore formation by Clostridium septicum alpha toxin. Mol Microbiol. 1997;23(3):551–558. doi: 10.1046/j.1365-2958.1997.d01-1876.x. [DOI] [PubMed] [Google Scholar]
- 3.van der Goot FG, Pattus F, Wong KR, Buckley JT. Oligomerization of the channel-forming toxin aerolysin precedes insertion into lipid bilayers. Biochemistry. 1993;32(10):2636–2642. doi: 10.1021/bi00061a023. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen VT, Higuchi H, Kamio Y. Controlling pore assembly of staphylococcal gamma-haemolysin by low temperature and by disulphide bond formation in double-cysteine LukF mutants. Mol Microbiol. 2002;45(6):1485–1498. doi: 10.1046/j.1365-2958.2002.03125.x. [DOI] [PubMed] [Google Scholar]
- 5.Shepard LA, Shatursky O, Johnson AE, Tweten RK. The mechanism of pore assembly for a cholesterol-dependent cytolysin: Formation of a large prepore complex precedes the insertion of the transmembrane beta-hairpins. Biochemistry. 2000;39(33):10284–10293. doi: 10.1021/bi000436r. [DOI] [PubMed] [Google Scholar]
- 6.Löhner S, et al. Pore formation by Vibrio cholerae cytolysin follows the same archetypical mode as beta-barrel toxins from gram-positive organisms. FASEB J. 2009;23(8):2521–2528. doi: 10.1096/fj.08-127688. [DOI] [PubMed] [Google Scholar]
- 7.Czajkowsky DM, Hotze EM, Shao Z, Tweten RK. Vertical collapse of a cytolysin prepore moves its transmembrane β-hairpins to the membrane. EMBO J. 2004;23(16):3206–3215. doi: 10.1038/sj.emboj.7600350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tilley SJ, Orlova EV, Gilbert RJ, Andrew PW, Saibil HR. Structural basis of pore formation by the bacterial toxin pneumolysin. Cell. 2005;121(2):247–256. doi: 10.1016/j.cell.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 9.Hotze EM, et al. Monomer-monomer interactions drive the prepore to pore conversion of a beta-barrel-forming cholesterol-dependent cytolysin. J Biol Chem. 2002;277(13):11597–11605. doi: 10.1074/jbc.M111039200. [DOI] [PubMed] [Google Scholar]
- 10.Hotze EM, et al. Arresting pore formation of a cholesterol-dependent cytolysin by disulfide trapping synchronizes the insertion of the transmembrane beta-sheet from a prepore intermediate. J Biol Chem. 2001;276(11):8261–8268. doi: 10.1074/jbc.M009865200. [DOI] [PubMed] [Google Scholar]
- 11.Shatursky O, et al. The mechanism of membrane insertion for a cholesterol-dependent cytolysin: A novel paradigm for pore-forming toxins. Cell. 1999;99(3):293–299. doi: 10.1016/s0092-8674(00)81660-8. [DOI] [PubMed] [Google Scholar]
- 12.Shepard LA, et al. Identification of a membrane-spanning domain of the thiol-activated pore-forming toxin Clostridium perfringens perfringolysin O: An alpha-helical to beta-sheet transition identified by fluorescence spectroscopy. Biochemistry. 1998;37(41):14563–14574. doi: 10.1021/bi981452f. [DOI] [PubMed] [Google Scholar]
- 13.Sato TK, Tweten RK, Johnson AE. Disulfide-bond scanning reveals assembly state and β-strand tilt angle of the PFO β-barrel. Nat Chem Biol. 2013;9(6):383–389. doi: 10.1038/nchembio.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramachandran R, Tweten RK, Johnson AE. The domains of a cholesterol-dependent cytolysin undergo a major FRET-detected rearrangement during pore formation. Proc Natl Acad Sci USA. 2005;102(20):7139–7144. doi: 10.1073/pnas.0500556102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feil SC, Ascher DB, Kuiper MJ, Tweten RK, Parker MW. Structural studies of Streptococcus pyogenes streptolysin O provide insights into the early steps of membrane penetration. J Mol Biol. 2014;426(4):785–792. doi: 10.1016/j.jmb.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar S, Nussinov R. Relationship between ion pair geometries and electrostatic strengths in proteins. Biophys J. 2002;83(3):1595–1612. doi: 10.1016/S0006-3495(02)73929-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar S, Nussinov R. Close-range electrostatic interactions in proteins. ChemBioChem. 2002;3(7):604–617. doi: 10.1002/1439-7633(20020703)3:7<604::AID-CBIC604>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 18.Ramachandran R, Tweten RK, Johnson AE. Membrane-dependent conformational changes initiate cholesterol-dependent cytolysin oligomerization and intersubunit beta-strand alignment. Nat Struct Mol Biol. 2004;11(8):697–705. doi: 10.1038/nsmb793. [DOI] [PubMed] [Google Scholar]
- 19.Rossjohn J, Feil SC, McKinstry WJ, Tweten RK, Parker MW. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell. 1997;89(5):685–692. doi: 10.1016/s0092-8674(00)80251-2. [DOI] [PubMed] [Google Scholar]
- 20.Harris RW, Sims PJ, Tweten RK. Kinetic aspects of the aggregation of Clostridium perfringens theta-toxin on erythrocyte membranes. A fluorescence energy transfer study. J Biol Chem. 1991;266(11):6936–6941. [PubMed] [Google Scholar]
- 21.Bernheimer AW. Comparative kinetics of hemolysis induced by bacterial and other hemolysins. J Gen Physiol. 1947;30(4):337–353. doi: 10.1085/jgp.30.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reboul CF, Whisstock JC, Dunstone MA. A new model for pore formation by cholesterol-dependent cytolysins. PLOS Comput Biol. 2014;10(8):e1003791. doi: 10.1371/journal.pcbi.1003791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotze EM, et al. Monomer-monomer interactions propagate structural transitions necessary for pore formation by the cholesterol-dependent cytolysins. J Biol Chem. 2012;287(29):24534–24543. doi: 10.1074/jbc.M112.380139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donald JE, Kulp DW, DeGrado WF. Salt bridges: Geometrically specific, designable interactions. Proteins. 2011;79(3):898–915. doi: 10.1002/prot.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song L, et al. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 1996;274(5294):1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 26.Walker B, Bayley H. Key residues for membrane binding, oligomerization, and pore forming activity of staphylococcal alpha-hemolysin identified by cysteine scanning mutagenesis and targeted chemical modification. J Biol Chem. 1995;270(39):23065–23071. doi: 10.1074/jbc.270.39.23065. [DOI] [PubMed] [Google Scholar]
- 27.Panchal RG, Bayley H. Interactions between residues in staphylococcal alpha-hemolysin revealed by reversion mutagenesis. J Biol Chem. 1995;270(39):23072–23076. doi: 10.1074/jbc.270.39.23072. [DOI] [PubMed] [Google Scholar]
- 28.Foletti D, et al. Mechanism of action and in vivo efficacy of a human-derived antibody against Staphylococcus aureus α-hemolysin. J Mol Biol. 2013;425(10):1641–1654. doi: 10.1016/j.jmb.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita K, et al. Crystal structure of the octameric pore of staphylococcal γ-hemolysin reveals the β-barrel pore formation mechanism by two components. Proc Natl Acad Sci USA. 2011;108(42):17314–17319. doi: 10.1073/pnas.1110402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosado CJ, et al. The MACPF/CDC family of pore-forming toxins. Cell Microbiol. 2008;10(9):1765–1774. doi: 10.1111/j.1462-5822.2008.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunstone MA, Tweten RK. Packing a punch: The mechanism of pore formation by cholesterol dependent cytolysins and membrane attack complex/perforin-like proteins. Curr Opin Struct Biol. 2012;22(3):342–349. doi: 10.1016/j.sbi.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hotze EM, et al. Identification and characterization of the first cholesterol-dependent cytolysins from Gram-negative bacteria. Infect Immun. 2013;81(1):216–225. doi: 10.1128/IAI.00927-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips JC, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26(16):1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farrand AJ, LaChapelle S, Hotze EM, Johnson AE, Tweten RK. Only two amino acids are essential for cytolytic toxin recognition of cholesterol at the membrane surface. Proc Natl Acad Sci USA. 2010;107(9):4341–4346. doi: 10.1073/pnas.0911581107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramachandran R, Heuck AP, Tweten RK, Johnson AE. Structural insights into the membrane-anchoring mechanism of a cholesterol-dependent cytolysin. Nat Struct Biol. 2002;9(11):823–827. doi: 10.1038/nsb855. [DOI] [PubMed] [Google Scholar]
- 36.Soltani CE, Hotze EM, Johnson AE, Tweten RK. Specific protein-membrane contacts are required for prepore and pore assembly by a cholesterol-dependent cytolysin. J Biol Chem. 2007;282(21):15709–15716. doi: 10.1074/jbc.M701173200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soltani CE, Hotze EM, Johnson AE, Tweten RK. Structural elements of the cholesterol-dependent cytolysins that are responsible for their cholesterol-sensitive membrane interactions. Proc Natl Acad Sci USA. 2007;104(51):20226–20231. doi: 10.1073/pnas.0708104105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 39.Hotze EM, Tweten RK. Membrane assembly of the cholesterol-dependent cytolysin pore complex. Biochim Biophys Acta. 2012;1818(4):1028–1038. doi: 10.1016/j.bbamem.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green NS, Reisler E, Houk KN. Quantitative evaluation of the lengths of homobifunctional protein cross-linking reagents used as molecular rulers. Protein Sci. 2001;10(7):1293–1304. doi: 10.1110/ps.51201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.