Significance
Ataxia telangiectasia (A-T) is a rare, autosomal recessive disorder characterized by progressive cerebellar degeneration. Oxidative stress is one cause of the symptoms of A-T disease. We report that inactivation of NADPH oxidase 4 (NOX4) reduced ROS, oxidative DNA damage, DNA double-strand breaks and replicative senescence in A-T primary cells. Analyses of A-T patients revealed elevated levels of NOX4 in the cerebellum that also correlated with increased levels of DNA damage and apoptosis. These observations were substantiated by the absence of abnormal NOX4 cerebellar expression in mouse models of A-T disease which do not display cerebellar degeneration. However, injecting A-T mice with NOX4 inhibitor decreased their elevated cancer incidence. Therefore, NOX4 appears as a critical mediator in A-T disease.
Keywords: ataxia telangiectasia, NOX4, ROS, neurodegeneration, DNA damage
Abstract
Ataxia telangiectasia (A-T), a rare autosomal recessive disorder characterized by progressive cerebellar degeneration and a greatly increased incidence of cancer among other symptoms, is caused by a defective or missing ataxia telangiectasia mutated (ATM) gene. The ATM protein has roles in DNA repair and in the regulation of reactive oxygen species (ROS). Here, we provide, to our knowledge, the first evidence that NADPH oxidase 4 (NOX4) is involved in manifesting A-T disease. We showed that NOX4 expression levels are higher in A-T cells, and that ATM inhibition leads to increased NOX4 expression in normal cells. A-T cells exhibit elevated levels of oxidative DNA damage, DNA double-strand breaks and replicative senescence, all of which are partially abrogated by down-regulation of NOX4 with siRNA. Sections of degenerating cerebelli from A-T patients revealed elevated NOX4 levels. ATM-null mice exhibit A-T disease but they die from cancer before the neurological symptoms are manifested. Injecting Atm-null mice with fulvene-5, a specific inhibitor of NOX4 and NADPH oxidase 2 (NOX2), decreased their elevated cancer incidence to that of the controls. We conclude that, in A-T disease in humans and mice, NOX4 may be critical mediator and targeting it will open up new avenues for therapeutic intervention in neurodegeneration.
Ataxia telangiectasia (A-T) is a fatal autosomal recessive disorder due to a defective ATM protein. In A-T patients, death usually occurs from progressive cerebellar degeneration (1, 2), whereas Atm-null mice usually die between 2 and 3 mo of age from lymphomas possibly before cerebellar degeneration has a chance to set in (3, 4). Many studies have suggested oxidative stress as a major factor for the progression of cerebellar degeneration (1, 5). Recently, ATM was found to regulate the levels of reactive oxygen species (ROS) in mammalian cells (6) but the source of this ROS remains unclear. Although mitochondrial leakage has been suggested as the primary ROS source, other work indicates that alternative uncharacterized sources may be important (7).
One alternative source of ROS is NADPH oxidase, an enzyme which transfers an electron from NADPH to molecular oxygen to generate superoxide anion, which in turn gives rise to the other forms of ROS (8–10). The NADPH oxidase (NOX/DUOX) family consists of seven homologs named NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1, and DUOX2. Although most NADPH oxidases are found at different cell locations and induced by specific functions (9), NADPH oxidase 4 (NOX4) constitutively produces ROS in close proximity of the nucleus in a large range of human cell types (11–13). Thus, NOX4 is a good candidate for ROS-induced DNA damage. In addition, NOX4 has been described as a key player in senescence, one of the features exhibited by A-T primary cells (12, 14). In this study, we provide, to our knowledge, the first evidence of a critical role for NADPH oxidase (NOX4) in phenotypes exhibited by A-T patient cells as well as in cerebellar degeneration associated with the disease.
Atm-null mice manifest the disease differently than humans, typically dying from lymphoma. ATM-deficient mice fed with antioxidants lived longer, exhibited a reduced incidence of lymphoma and showed mitigated loss of hematopoietic stem cells compared with controls (15, 16). We show here that Atm-null mice also exhibit a reduced incidence of lymphoma when given fulvene-5, a known inhibitor of NOX4 (17). Thus, down-regulation of NOX4 ameliorates the detrimental effects of ATM loss in both human cells and in mice.
Results and Discussion
NOX4 and ROS in A-T Fibroblasts.
The ataxia-telangiectasia mutated (ATM) protein kinase is a sensor for reactive oxygen species (ROS) levels among other functions (6, 18). Patients with A-T lack functional ATM protein and their cells exhibit higher ROS levels (6, 19). Although mitochondrial dysfunction has been an obvious potential source of oxidative stress in A-T cells, evidence is sparse. Another potential ROS source that has been previously unexplored is NADPH oxidase 4 (NOX4). Several findings make it a good candidate. First, NOX4 is constitutively expressed and active in a large range of cells (9); second, it is localized to the immediate environment surrounding the nucleus so that ROS generated there could be responsible for the increased levels of oxidative DNA damage found in A-T cells (11–13); third, NOX4 has been found to be involved in senescence, a major phenotype exhibited by primary A-T cells (12, 20, 21).
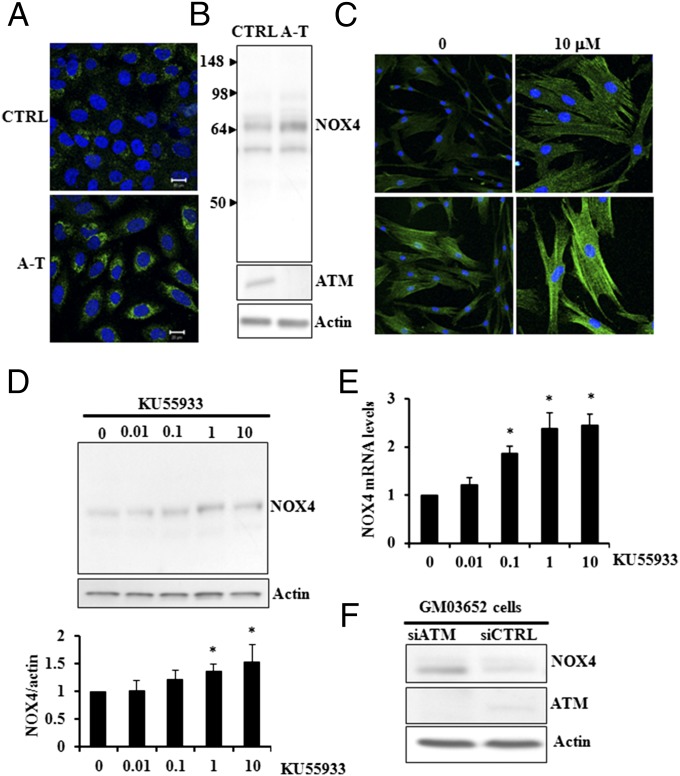
In line with these findings, we hypothesized that NOX4 may be at least in part responsible for the chronic oxidative stress and the subsequent phenotypes of A-T disease. When we measured NOX4 protein in fibroblasts from normal and A-T patients, the relative levels were found to be elevated in the A-T cell cultures by immunofluorescence staining and immunoblotting (Fig. 1 A and B). Additional experiments showing NOX4 antibody specificity are compiled in Fig. S1. To clarify whether the elevated NOX4 protein and/or mRNA levels were linked to ATM activity, normal fibroblasts were treated with the ATM-specific inhibitor (KU55933). Immunofluorescence (Fig. 1C), immunoblotting, (Fig. 1D), and RT-PCR (Fig. 1E) all confirmed that lowered ATM activity led to elevated NOX4 protein and mRNA levels. Furthermore, when ATM was down-regulated by siATM, NOX4 levels increased (Fig. 1F). Taken together, these results provide strong evidence that ATM down-regulation leads to NOX4 up-regulation.
Fig. 1.
ATM down-regulation and inhibition lead to NOX4 up-regulation. (A and B) Elevated levels of NOX4 were found in A-T (A-T: GM05849) compared with control fibroblasts (CTRL: GM00637) (Immunocytochemistry in A, immunoblot in B). (C–E) Inhibition of ATM activity in CTRL cells (CTRL: GM03652 primary fibroblasts) with increasing doses of KU55933 (µM) led to increased levels of NOX4 protein (C, Upper Left and Upper Right, 20× magnification; Lower Left and Lower Right; 40× magnification). (D, Upper) NOX4 detection by immunoblot. (D, Lower) Quantification. (E) NOX4 transcript levels. (F) Down-regulation of ATM in control cells (CTRL: GM03652 primary fibroblasts) with siRNA led to elevated NOX4 levels. *P < 0.05.
Along with their continuous state of oxidative stress, A-T fibroblasts exhibit genetic instability. In Atm-deficient mice, the antioxidant N-acetyl cysteine was shown to lower the amounts of carcinogenesis-associated DNA deletions and oxidative DNA damage (22). Similarly, a diet containing the antioxidant tempol resulted in reduced levels of tissue oxidative damage in these mice (23).
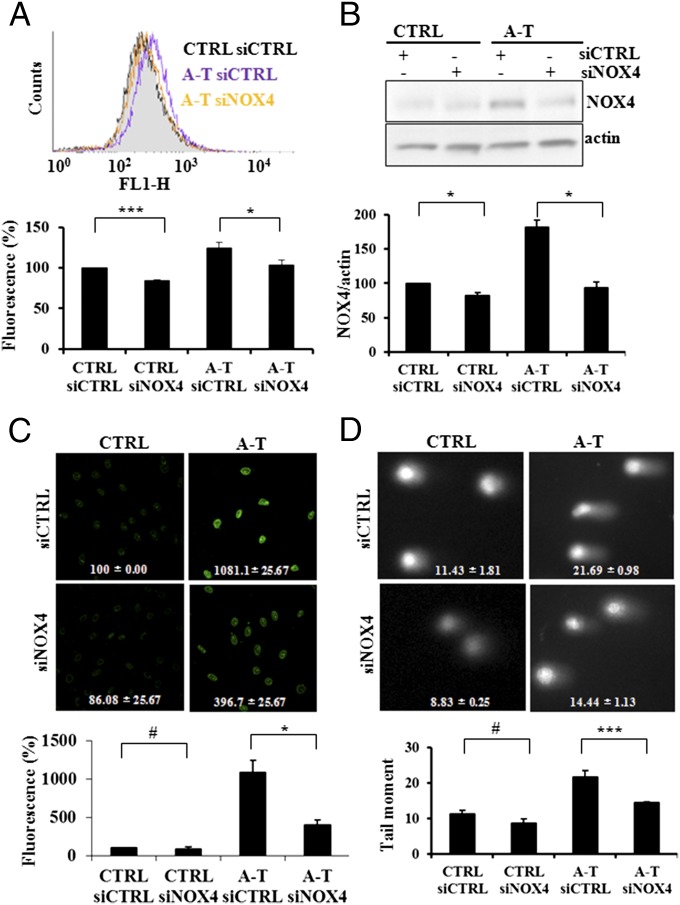
As NOX4 has been shown to constitutively generate ROS (9, 24, 25), we examined the effect of NOX4 down-regulation on the intracellular ROS levels in A-T fibroblasts using an oxidant sensitive indicator dye. As expected the ROS levels in the A-T fibroblasts were higher than in normal controls, but when NOX4 levels were lowered with siNOX4, the A-T cells exhibited a significant reduction of ROS levels (Fig. 2 A and B). Similar results were obtained using dihydroethidium (DHE) as indicator of superoxide anion, and by expression of an inactive dominant negative form of NOX4, NOX4 DN in cells (26) (Fig. S2). Next, to examine whether variations in NOX4 levels affected their DNA damage levels, we incubated A-T and control cells with siNOX4 (Fig. 2C). A-T cells exhibited approximately 10-fold elevations in 8-OH deoxyguanosine levels compared with control cells, and the silencing of NOX4 expression led to a 60% reduction in these levels, indicating that the elevated levels of oxidative DNA damage in A-T cells are at least partially due to the increased activity of NOX4. These observations were substantiated by increase of 8-OH deoxyguanosine levels in DNA from A-T cells using ELISA (Fig. S3A). To confirm this finding, we measured Fpg-specific DNA lesions, which form at 8-OH deoxyguanosine and other oxypurine residues, using the comet assay. A-T cells exhibited levels of oxypurines nearly 1.5–2 times higher than control cells, levels that were reduced by 40% when A-T cells were transfected with siNOX4 (Fig. 2D), confirming that NOX4-derived ROS led to increased levels of oxidative DNA lesions in A-T cells. Furthermore, NOX4 silencing was found to reduce the amount of the phosphorylated histone H2AX (γ-H2A.X) used as a marker for DNA double-strand breaks in A-T primary fibroblasts (Fig. S3B). These results suggest that the reduced levels of oxidatively induced DNA lesions resulting from NOX4 inactivation lead to decreases in DNA breaks including double-strand breaks as the cell’s DNA repair capacity is less overwhelmed (27).
Fig. 2.
NOX4 inactivation by siRNA reduces ROS and DNA damage. (A) A-T cells (A-T) exhibited elevated ROS levels compared with control cells (CTRL), levels which were reduced when the cells were treated with NOX4 siRNA (Upper, raw DCF fluorescence; Lower, quantification). (B) A-T cells (A-T) exhibited elevated NOX4 levels compared with controls cells (CTRL), levels which were reduced when the cells were treated with NOX4 siRNA (Upper, immunoblot; Lower, quantification). (C) NOX4 silencing by siRNA reduces 8-OH deoxyguanosine levels in A-T primary fibroblasts (A-T) (Upper, images of control (CTRL) and A-T cells treated with siCTRL and siNOX4 RNAs and stained for 8-OH deoxyguanosine; bottom, quantification). (D) Oxypurine levels were elevated in A-T cells (A-T) compared with controls (CTRL) and reduced in A-T cells (A-T) treated with siNOX4 (Upper). Fpg-specific lesions detection by comet assay (representative images). (Lower) Quantification. *P < 0.05; **P < 0.001; #, nonsignificant.
NOX4, Replication, and Senescence in A-T Fibroblasts.
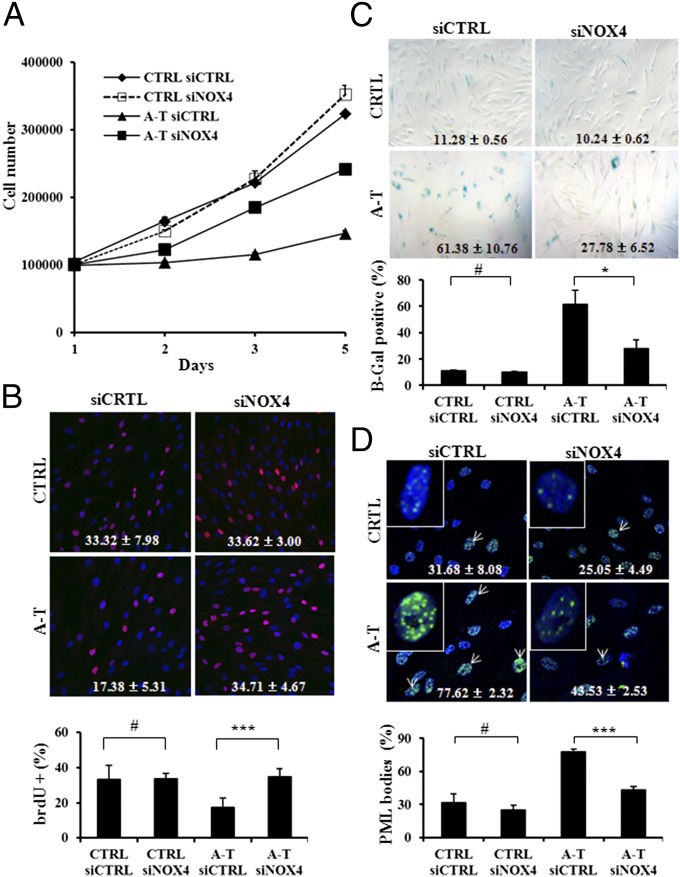
As previously reported, loss of ATM impairs proliferation of mouse astrocytes and embryonic fibroblasts (28, 29). In addition, ATM-deficient mouse T cells exhibit increased levels of apoptosis, which can be decreased by scavenging ROS with N-acetyl cysteine (30). Human primary A-T fibroblasts were also found to exhibit increased replicative senescence (31). A-T primary fibroblasts exhibited greatly decreased rates of proliferation relative to the controls, which were partially restored due to NOX4 inactivation by siRNA (Fig. 3A). Direct microscopic observations of the A-T cultures confirmed that cell density was increased in cultures incubated with siRNA against NOX4 (Fig. S4A), indicating that NOX4-dependent ROS generation participates in the growth defect exhibited by A-T cells. As a further confirmation, DNA replication was assessed by BrdU incorporation between the third and fourth day after NOX4 inactivation. The relative proportion of A-T cells incorporating BrdU doubled in the cells inactivated for NOX4 (Fig. 3B). Inactivation of NOX4 in A-T cells also resulted in decreased levels of SA β-galactosidase staining (Fig. 3C). Another senescence marker is the presence of PML bodies (12, 32). PML bodies become larger and more numerous as cells enter senescence. Our results revealed that A-T cells contained a nearly 50% greater fraction of cells with PML bodies compared with controls, and that NOX4 down-regulation prevented most of that increase (Fig. 3D). These observations were confirmed by the measurement of nuclear area occupied by PML bodies (Fig. S4B). These results indicate that scavenging NOX4-derived ROS alleviates replicative senescence, a hallmark of A-T cells (31). These findings are in line with previous observations that premature senescence in Atm-deficient mouse embryonic fibroblasts is prevented by the treatment of cells with antioxidant (29), and support the notion that ROS generation is causative for senescence (33). Taken together, our results suggest that the inability of A-T cells to respond properly to NOX4-derived ROS levels underlie at least in part their proliferation defect and subsequent senescence.
Fig. 3.
NOX4 inactivation by siRNA rescues A-T cells from proliferative deficiency and senescence. (A) A-T cells treated with NOX4 siRNA exhibit improved growth as evidenced by cell growth curves. (B) Cells were incubated with BrdU 3 d posttransfection with siNOX4. (Upper) BrdU-labeled cells stained by immunofluorescence. Red, cells positive for BrdU; blue, DAPI for nucleus counterstaining. (Lower) Quantification of BrdU-positive cells relative total cells (%). (C and D) A-T cells containing NOX4 siRNA exhibit lower levels of replicative senescence as evidenced by the extent of beta-gal staining (C, Upper, representative image; Lower, quantification) and PML bodies (D, Upper, representative image. Lower, quantification). Green, PML staining and blue, DAPI for nucleus counterstaining. White arrows indicate cells with larger and numerous PML bodies. *P < 0.05; ***P < 0.0001; #, nonsignificant.
NOX4 in A-T Patients.
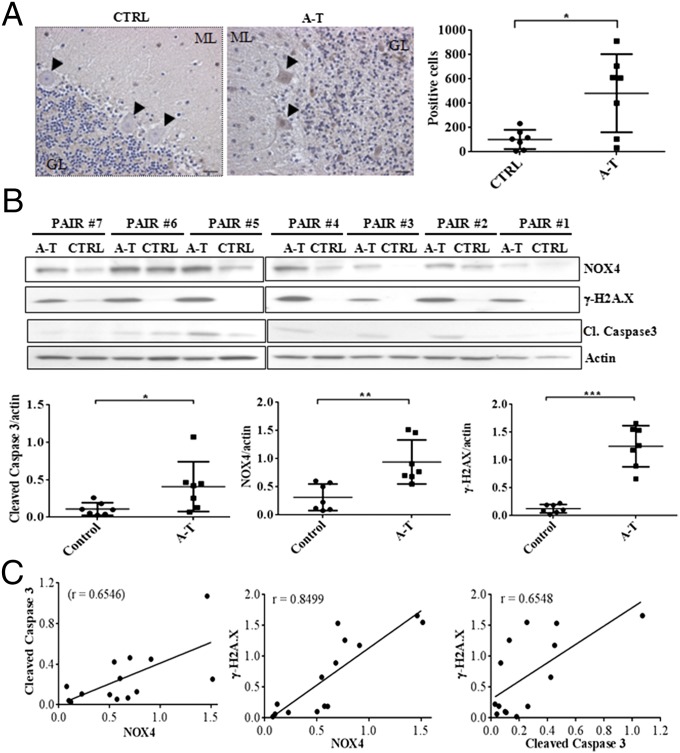
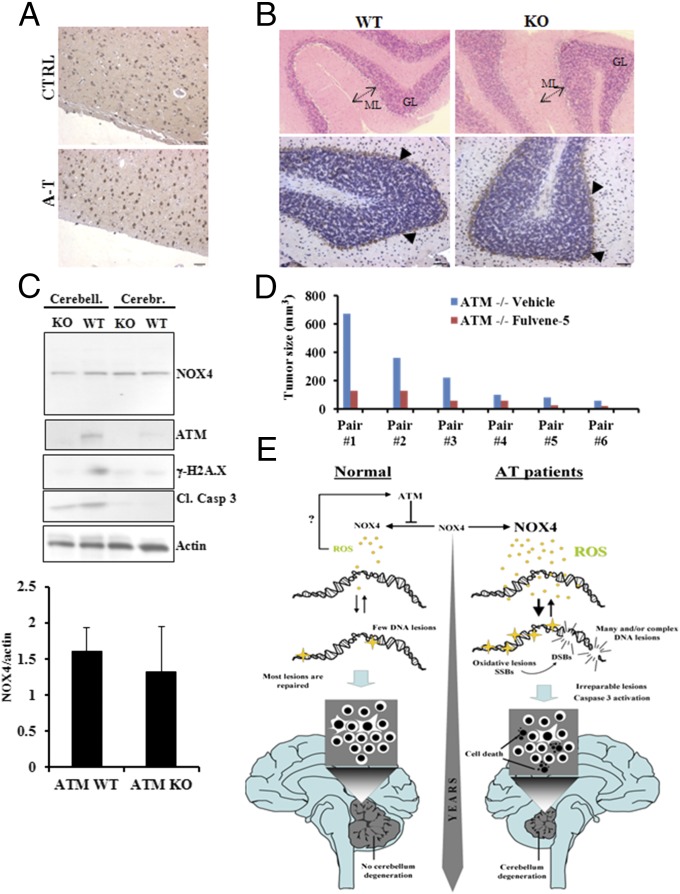
Clinically, A-T is a neurodegenerative disorder that prominently involves the progressive degeneration of Purkinje and granule cells in the cerebellum (34). Neurological deterioration is the major cause of death in A-T patients (2). Many studies have suggested a crucial role for oxidative stress in the symptoms of A-T disease, neuronal degeneration, and premature aging (1, 18, 23, 35, 36). An important question is whether NOX4 levels may be at least partially responsible for cerebellum degeneration in A-T patients. We analyzed NOX4 expression levels by immunohistochemistry in brain tissues from A-T patients. As expected, the density of molecular layer along with the number of Purkinje and granules cells were markedly reduced in A-T specimens compared with the controls. NOX4 exhibited higher expression levels in most of the seven A-T patients (Fig. 4A) with a nearly fivefold increase in the number of Purkinje and granules cells displaying NOX4 staining. These observations indicate that NOX4 is highly up-regulated in the cerebellum of A-T patients. Furthermore, NOX4 expression correlated with increased levels of DNA damage and apoptosis as evidenced by the extent of phosphorylated amounts of histone H2A.X (γ-H2A.X) and cleaved caspase 3 respectively (Fig. 4B). Most importantly, the NADPH oxidase 2 (NOX2), which has been described in the cerebellar granule neurons (37), did not exhibit differential expression between controls and A-T specimens (Fig. S5A). Using Pearson correlation coefficient, the expression intensity of NOX4, γ-H2A.X, and Cleaved Caspase 3 statistically correlated across all samples (Fig. 4C). These results were confirmed by immunohistochemistry, which revealed higher expression levels of these proteins in Purkinje and granules cells of A-T patients (Fig. S5B). In contrast, NOX4 expression remained unchanged in the cerebrum tissues from the same specimens (Fig. 5A and Fig. S5C), suggesting that NOX4 overexpression may lead to the persistent increase in DNA damage and apoptosis, mainly in the cerebellum of A-T patients. These observations strengthen the evidence that the cerebellum is the primary site of the degenerative process in A-T disease (34).
Fig. 4.
Correlation between NOX4 expression, DNA damage levels and apoptosis in A-T patients cerebelli. (A) NOX4 immunostaining in controls (CTRL) and A-T patients (A-T). GL, granular layer. ML, molecular layer. Arrows indicate Purkinje Cells. Blue staining corresponds to the nucleus counterstaining, whereas NOX4 staining is shown in brown. [Left, representative image; Right, quantification of NOX4 positive cells at the same magnificance (5×) of photomicrograph for controls and A-T samples]. (B) Immunoblot detection of NOX4, γ-H2A.X, and cleaved caspase 3 (Upper). A pair is age- and sex-matched specimen of control (CTRL) and A-T tissues (A-T). (Lower, quantification of proteins levels using the Mann–Whitney test). (C) Pearson correlation between cleaved caspase 3 and NOX4 (Left), γ-H2A.X and NOX4 (Center), and γ-H2A.X and cleaved caspase 3 (Right). *P < 0.05; **P < 0.001; ***P < 0.0001.
Fig. 5.
Lack of abnormal NOX4 expression in human cerebrum and in A-T mouse. (A) Immunohistostaining of NOX4 in human cerebral cortex. (B) NOX4 Immunohistostaining in ATM wild type (WT) and ATM knockout (KO) mice. GL, granular layer. ML, molecular layer. Arrows indicate Purkinje cells and blue staining corresponds to the nucleus counterstaining, whereas NOX4 staining is shown in brown. (C) Immunoblotting of NOX4, ATM, γ-H2A.X and cleaved caspase 3 in the cerebral cortex and the cerebellum of ATM wild type (WT) and ATM knockout (KO) mice [Upper, representative image; Lower, quantification of NOX4 expression level in ATM wild type (WT) and ATM knockout (KO) mice cerebelli. Cerebr, cerebrum. Cerebell, cerebellum]. (D) Measurement of thymic lymphomas in six pairs of ATM knockout (KO) mice injected with vehicle or Fulvene-5 for 14 d. (E) Model for the role of NOX4 in A-T neurodegeneration. DSB, DNA double-strand breaks; ROS, reactive oxygen species; SSB, DNA single-strand breaks.
The lack of ATM in mice results in a phenotype that differs from the human disease in significant ways. Although, in humans, the mortality of A-T patients is due to neurological disease, in mice it is due to cancer. It is widely established that there is no gross cerebellum degeneration in Atm-knockout mice (38, 39). Although NOX4 is prominently expressed in the Purkinje cells as previously described (40), our data revealed no difference in NOX4 protein levels in Atm-knockout mice compared with controls (Fig. 5B). These results were confirmed by immunoblot with the findings that NOX4 and Cleaved Caspase 3 remained unchanged in these two cohorts (Fig. 5C). The higher level of γ-H2A.X and Cleaved Caspase 3 in the cerebellum may be explained by increased transcriptional activity in this specific region of the brain (41), whereas the absence of γ-H2A.X in Atm-knockout tissues may be the consequence of the lack of Atm functional activity in these animals.
It is widely assumed that the lack of cerebellum degeneration in Atm-knockout mice is due to their early death at 3–4 mo from cancer (38, 42) so that cerebellar degeneration does not have time to occur. If this is the case and NOX4 levels are intimately involved in manifesting A-T disease, then inhibiting NOX4 may decrease the incidence of growth of tumors in the mice. To test this hypothesis, we compared ATM WT and KO mice that had been injected with fulvene-5. No behavioral changes were observed among the four cohorts, but the ATM KO mouse cohort showed higher incidence of thymic lymphoma, which was greatly reduced when ATM KO mice were given the NOX4/NOX2 inhibitor fulvene-5 (Fig. 5D).
Taken together, our results provide, to our knowledge, the first evidence that ATM deficiency leads to elevated NOX4 expression in human cerebellum. This increased NOX4 expression gives rise to chronic oxidative stress that induces accrued amounts of oxidative DNA lesions. The persistence of these high levels of oxidative lesions over years, coupled with the deficiency in the DNA repair due to the lack of ATM, may accelerate apoptosis and other degradative processes in human A-T patients (Fig. 5E). However, although NOX4 is critical for A-T phenotype, there may be other potential sources of oxidative stress in A-T disease.
These results suggest that the two major manifestations of A-T disease in humans and mice may both be due to at least in part to elevated levels of NOX4. This study shows that NOX4 may be a putative target for future therapeutic intervention in A-T neurodegeneration in humans, and opens up new lines of investigations toward the understanding of the specific mechanisms underlying the regulation of NOX4 by ATM.
Materials and Methods
In Vivo Studies (Mice).
Heterozygote ATM mice (SvEvTac-Atmtm1Awb/J) were purchased from Jackson Laboratory (stock no. 002753), and used to generate mice homogygous for Atm. All animal procedures were performed according to protocols approved by NCI-Frederick Animal Care and Use Committee (ACUC). Fulvene-5 treatment was performed as described (17). From day 1 to day 14, mice were treated with fulvene-5 by making a stock solution of 4 mg of fulvene-5 compound dissolved in 100 μL of 100% ethanol plus 1.1 mL of intralipid and then sonicated for 15 s. The treatment stock solution (330 μL) was injected intraperitoneally into each of six mice of the fulvene-5 treatment group. For control animals, a stock solution was made of 100 μL of 100% ethanol plus 1.1 mL of intralipid solution, and 330 μL of stock solution was injected intraperitoneally into each of six mice of the control group. Injections were continued for 2 wk. On day 14, tumors were measured in all animals.
Human Brain Specimens.
The human tissue used in this study was provided by the National Institute of Child Health and Human Development (NICHD) Brain and Tissue Bank for Developmental Disorders at the University of Maryland (Table 1). All tissues were harvested during autopsies, frozen and/or fixed in formalin before routine processing. The diagnosis of A-T was confirmed by a case identification file from the NICHD Brain and Tissue Bank. Microscopic evaluation of the A-T tissue after HE staining affirmed the A-T diagnoses as all seven cases displayed the neuropathologic hallmarks of the disease, namely loss of Purkinje cells, thinning of the granule cell layer, and decreased thickness of the molecular layer (Fig. 4A). Protocols for cells cultures, siRNAs transfection, real time PCR, comet assay, immunofluorescence, senescence assay, BrdU staining, ROS measurements, immunoblot, immunohistochemistry, and mouse study can be found in SI Materials and Methods.
Table 1.
Clinical data on patient cohorts for this study
| Pair number | Specimen | UMB no. | Sex | Age, years | PMI, h | Cause of death |
| 1 | CTRL | 5288 | M | 27.26 | 13 | Congestive heart failure |
| A-T | 4874 | M | 26.88 | 7 | Complications of disorder/pneumonia | |
| 2 | CTRL | 1101 | F | 19.18 | 7 | Accident, multiple injuries |
| A-T | 1459 | F | 19.93 | 2 | Complications of disorder | |
| 3 | CTRL | 1228 | M | 47.34 | 13 | ASCVD (arteriosclerotic cardiovascular disease) |
| A-T | 1236 | M | 47.26 | 13 | Edema/pneumonia | |
| 4* | CTRL | 4323 | M | 36.72 | 14 | Respiratory failure |
| A-T | 853 | M | 35.98 | 13 | Ataxia telangiectasia | |
| 4** | CTRL | 1226 | M | 23.24 | 21 | Drowning |
| A-T | 921 | M | 23.56 | 30 | Pneumonia | |
| 5 | CTRL | 1105 | M | 16.92 | 17 | Multiple injuries |
| A-T | 1722 | M | 16.81 | 15 | Ataxia telangiectasia | |
| 6 | CTRL | 1710 | F | 26.05 | 12 | Cardiac tamponade |
| A-T | 5512 | F | 26.76 | 3 | Complications of disorder | |
| 7 | CTRL | 1502 | M | 29.99 | 19 | Multiple injuries |
| A-T | 5485 | M | 29.48 | 12 | Complications of disorder |
PMI, estimated post mortem interval.
Only frozen tissues were available. Samples were used for Western blot.
Only fixed tissues were available. Samples were used for immunostaining.
Supplementary Material
Acknowledgments
Human tissue was obtained from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD. We thank Dr. Simone Difilippantonio, Dr. Diana Haines, Mrs. Christina Robinson, and the other members of the Laboratory of Animal Sciences Program and Pathology Histotechnology Laboratory staff (National Cancer Institute–Frederick) for help with animal maintenance and histological analysis. We also thank Dr. Lino Tessarollo of the Mouse Cancer Genetics Program (National Cancer Institute–Frederick) for help with motor coordination tests and Mrs. Jennifer E. Dwyer of the Laboratory of Cancer Biology and Genetics (National Cancer Institute) for help with immunohistochemistry slides scanning. This work was supported by the National Institute of Allergy and Infectious Diseases, Radiation/Nuclear Countermeasures Program and the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418139112/-/DCSupplemental.
References
- 1.Rotman G, Shiloh Y. The ATM gene and protein: Possible roles in genome surveillance, checkpoint controls and cellular defence against oxidative stress. Cancer Surv. 1997;29:285–304. [PubMed] [Google Scholar]
- 2.Chiesa N, Barlow C, Wynshaw-Boris A, Strata P, Tempia F. Atm-deficient mice Purkinje cells show age-dependent defects in calcium spike bursts and calcium currents. Neuroscience. 2000;96(3):575–583. doi: 10.1016/s0306-4522(99)00581-3. [DOI] [PubMed] [Google Scholar]
- 3.Puccini J, et al. Loss of caspase-2 augments lymphomagenesis and enhances genomic instability in Atm-deficient mice. Proc Natl Acad Sci USA. 2013;110(49):19920–19925. doi: 10.1073/pnas.1311947110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biton S, Barzilai A, Shiloh Y. The neurological phenotype of ataxia-telangiectasia: Solving a persistent puzzle. DNA Repair (Amst) 2008;7(7):1028–1038. doi: 10.1016/j.dnarep.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Kamsler A, et al. Increased oxidative stress in ataxia telangiectasia evidenced by alterations in redox state of brains from Atm-deficient mice. Cancer Res. 2001;61(5):1849–1854. [PubMed] [Google Scholar]
- 6.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330(6003):517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 7.Patel AY, McDonald TM, Spears LD, Ching JK, Fisher JS. Ataxia telangiectasia mutated influences cytochrome c oxidase activity. Biochem Biophys Res Commun. 2011;405(4):599–603. doi: 10.1016/j.bbrc.2011.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krause KH. Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn J Infect Dis. 2004;57(5):S28–S29. [PubMed] [Google Scholar]
- 9.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 10.Block K, Gorin Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nat Rev Cancer. 2012;12(9):627–637. doi: 10.1038/nrc3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spencer NY, et al. Control of hepatic nuclear superoxide production by glucose 6-phosphate dehydrogenase and NADPH oxidase-4. J Biol Chem. 2011;286(11):8977–8987. doi: 10.1074/jbc.M110.193821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weyemi U, et al. ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene. 2012;31(9):1117–1129. doi: 10.1038/onc.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsushima S, et al. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ Res. 2013;112(4):651–663. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geiszt M, Kopp JB, Várnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA. 2000;97(14):8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reliene R, Schiestl RH. Antioxidants suppress lymphoma and increase longevity in Atm-deficient mice. J Nutr. 2007;137(1) Suppl:229S–232S. doi: 10.1093/jn/137.1.229S. [DOI] [PubMed] [Google Scholar]
- 16.Ito K, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431(7011):997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 17.Bhandarkar SS, et al. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J Clin Invest. 2009;119(8):2359–2365. doi: 10.1172/JCI33877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosentino C, Grieco D, Costanzo V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011;30(3):546–555. doi: 10.1038/emboj.2010.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reliene R, Schiestl RH. Antioxidant N-acetyl cysteine reduces incidence and multiplicity of lymphoma in Atm deficient mice. DNA Repair (Amst) 2006;5(7):852–859. doi: 10.1016/j.dnarep.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Kodama R, et al. ROS-generating oxidases Nox1 and Nox4 contribute to oncogenic Ras-induced premature senescence. Genes Cells. 2013;18(1):32–41. doi: 10.1111/gtc.12015. [DOI] [PubMed] [Google Scholar]
- 21.Lener B, et al. The NADPH oxidase Nox4 restricts the replicative lifespan of human endothelial cells. Biochem J. 2009;423(3):363–374. doi: 10.1042/BJ20090666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reliene R, Fischer E, Schiestl RH. Effect of N-acetyl cysteine on oxidative DNA damage and the frequency of DNA deletions in atm-deficient mice. Cancer Res. 2004;64(15):5148–5153. doi: 10.1158/0008-5472.CAN-04-0442. [DOI] [PubMed] [Google Scholar]
- 23.Schubert R, et al. Cancer chemoprevention by the antioxidant tempol in Atm-deficient mice. Hum Mol Genet. 2004;13(16):1793–1802. doi: 10.1093/hmg/ddh189. [DOI] [PubMed] [Google Scholar]
- 24.Nisimoto Y, Jackson HM, Ogawa H, Kawahara T, Lambeth JD. Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochemistry. 2010;49(11):2433–2442. doi: 10.1021/bi9022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ambasta RK, et al. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279(44):45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 26.Boudreau HE, Emerson SU, Korzeniowska A, Jendrysik MA, Leto TL. Hepatitis C virus (HCV) proteins induce NADPH oxidase 4 expression in a transforming growth factor beta-dependent manner: A new contributor to HCV-induced oxidative stress. J Virol. 2009;83(24):12934–12946. doi: 10.1128/JVI.01059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sedelnikova OA, et al. Role of oxidatively induced DNA lesions in human pathogenesis. Mutat Res. 2010;704(1-3):152–159. doi: 10.1016/j.mrrev.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Wong PK. Oxidative stress is linked to ERK1/2-p16 signaling-mediated growth defect in ATM-deficient astrocytes. J Biol Chem. 2009;284(21):14396–14404. doi: 10.1074/jbc.M808116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito K, et al. Regulation of reactive oxygen species by Atm is essential for proper response to DNA double-strand breaks in lymphocytes. J Immunol. 2007;178(1):103–110. doi: 10.4049/jimmunol.178.1.103. [DOI] [PubMed] [Google Scholar]
- 30.Bagley J, Singh G, Iacomini J. Regulation of oxidative stress responses by ataxia-telangiectasia mutated is required for T cell proliferation. J Immunol. 2007;178(8):4757–4763. doi: 10.4049/jimmunol.178.8.4757. [DOI] [PubMed] [Google Scholar]
- 31.Barascu A, et al. Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation. EMBO J. 2012;31(5):1080–1094. doi: 10.1038/emboj.2011.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang R, et al. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev Cell. 2005;8(1):19–30. doi: 10.1016/j.devcel.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 33.Lee AC, et al. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J Biol Chem. 1999;274(12):7936–7940. doi: 10.1074/jbc.274.12.7936. [DOI] [PubMed] [Google Scholar]
- 34.Barzilai A, Rotman G, Shiloh Y. ATM deficiency and oxidative stress: A new dimension of defective response to DNA damage. DNA Repair (Amst) 2002;1(1):3–25. doi: 10.1016/s1568-7864(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 35.Alexander A, et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci USA. 2010;107(9):4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barlow C, et al. Loss of the ataxia-telangiectasia gene product causes oxidative damage in target organs. Proc Natl Acad Sci USA. 1999;96(17):9915–9919. doi: 10.1073/pnas.96.17.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guemez-Gamboa A, Morán J. NOX2 mediates apoptotic death induced by staurosporine but not by potassium deprivation in cerebellar granule neurons. J Neurosci Res. 2009;87(11):2531–2540. doi: 10.1002/jnr.22079. [DOI] [PubMed] [Google Scholar]
- 38.Barlow C, et al. Atm-deficient mice: A paradigm of ataxia telangiectasia. Cell. 1996;86(1):159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 39.Xu Y, et al. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 1996;10(19):2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- 40.Vallet P, et al. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132(2):233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 41.Dickey JS, et al. Susceptibility to bystander DNA damage is influenced by replication and transcriptional activity. Nucleic Acids Res. 2012;40(20):10274–10286. doi: 10.1093/nar/gks795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rotman G, Shiloh Y. ATM: From gene to function. Hum Mol Genet. 1998;7(10):1555–1563. doi: 10.1093/hmg/7.10.1555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.